Immunohistochemical Expression of Neurokinin-A and Interleukin-8 in the Bronchial Epithelium of Horses with Severe Equine Asthma Syndrome during Asymptomatic, Exacerbation, and Remission Phase
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
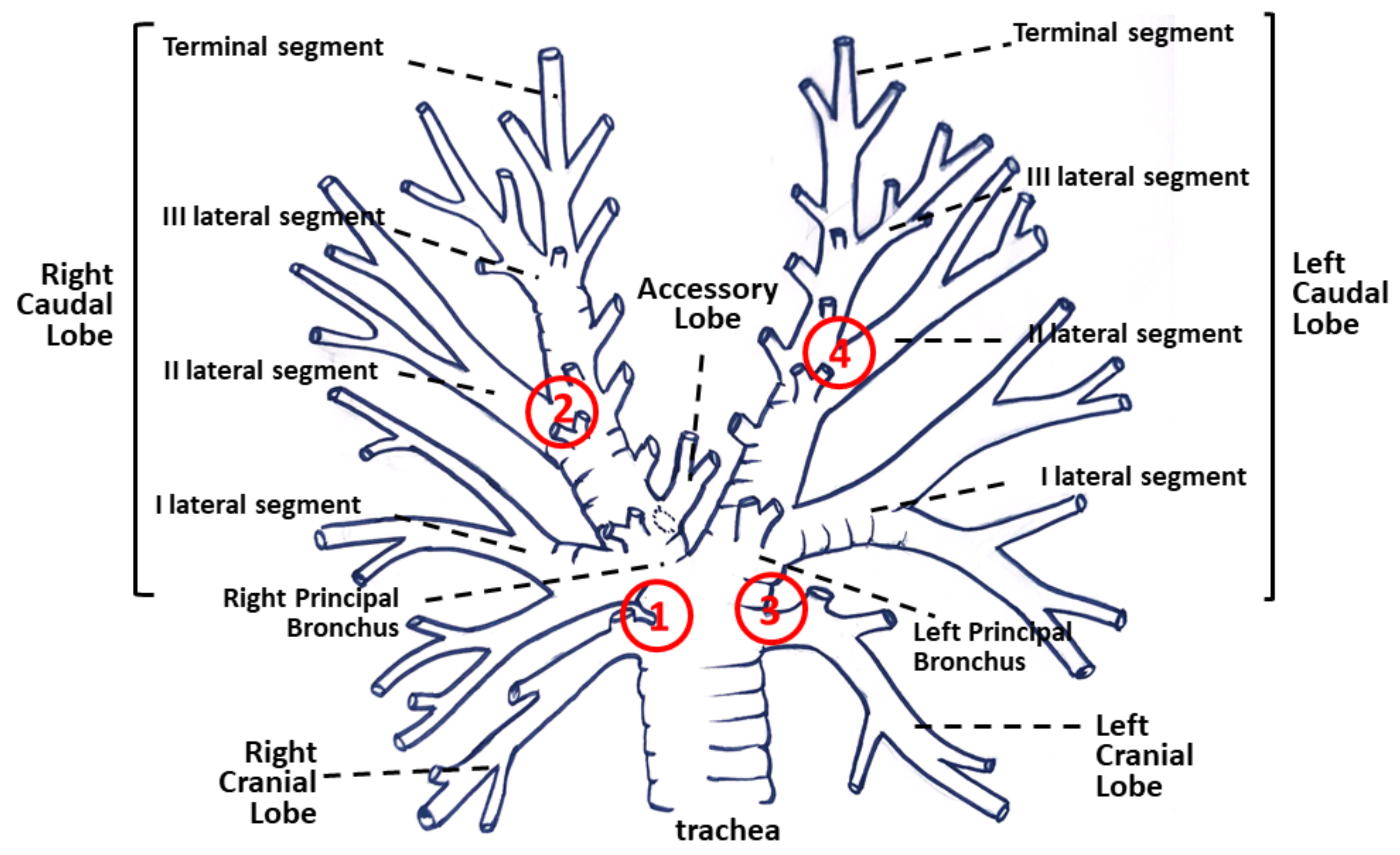
2.1. Horses
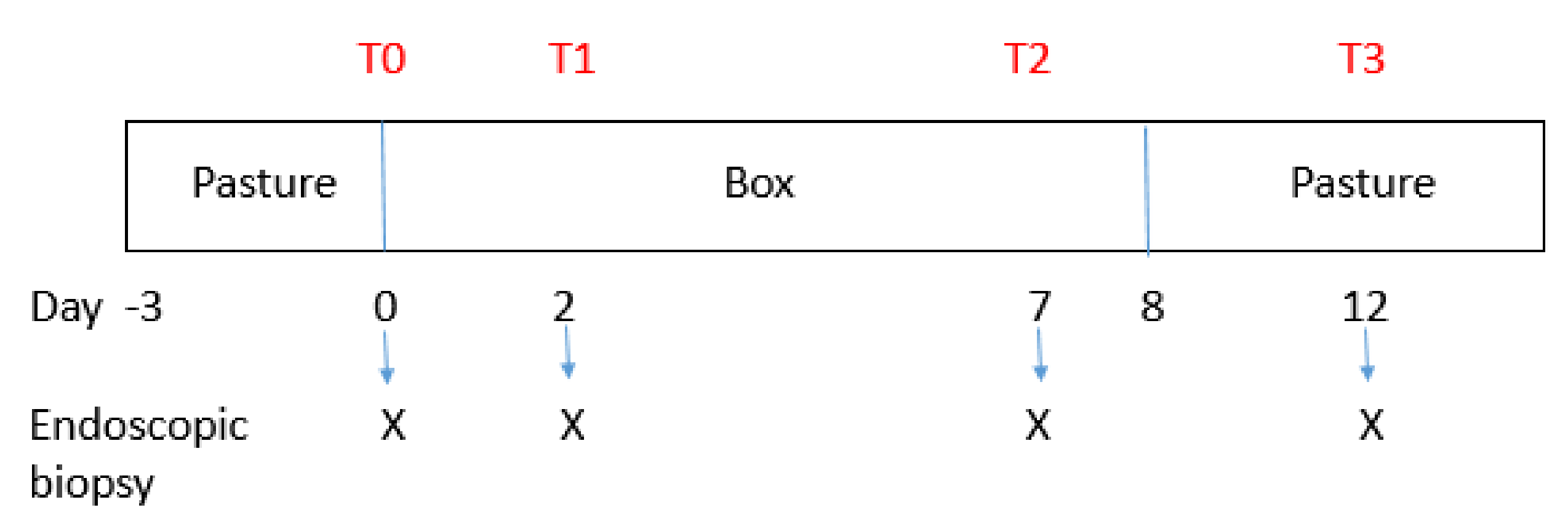
2.2. Experimental Design
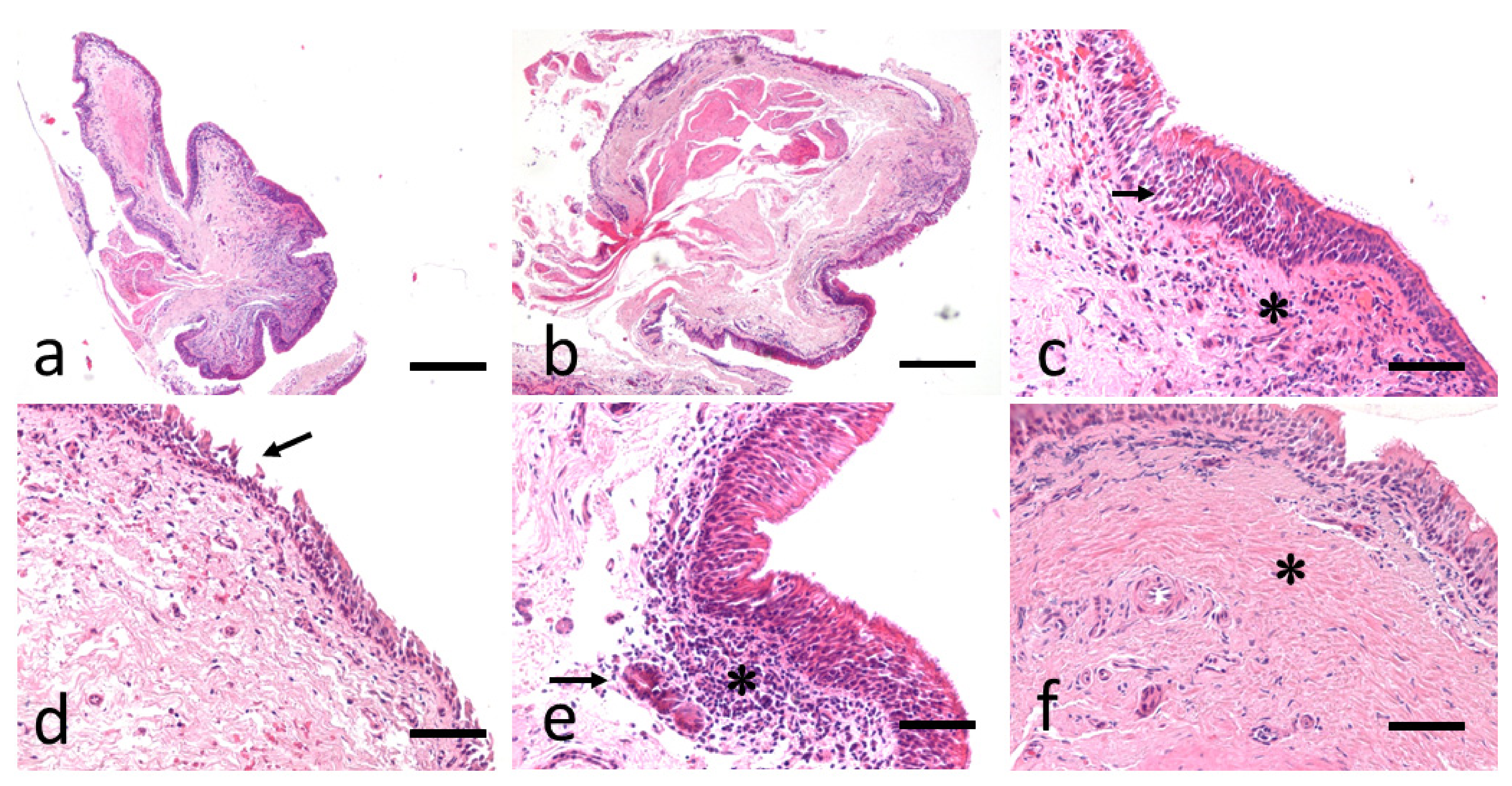
2.3. Bronchial Biopsy Histology
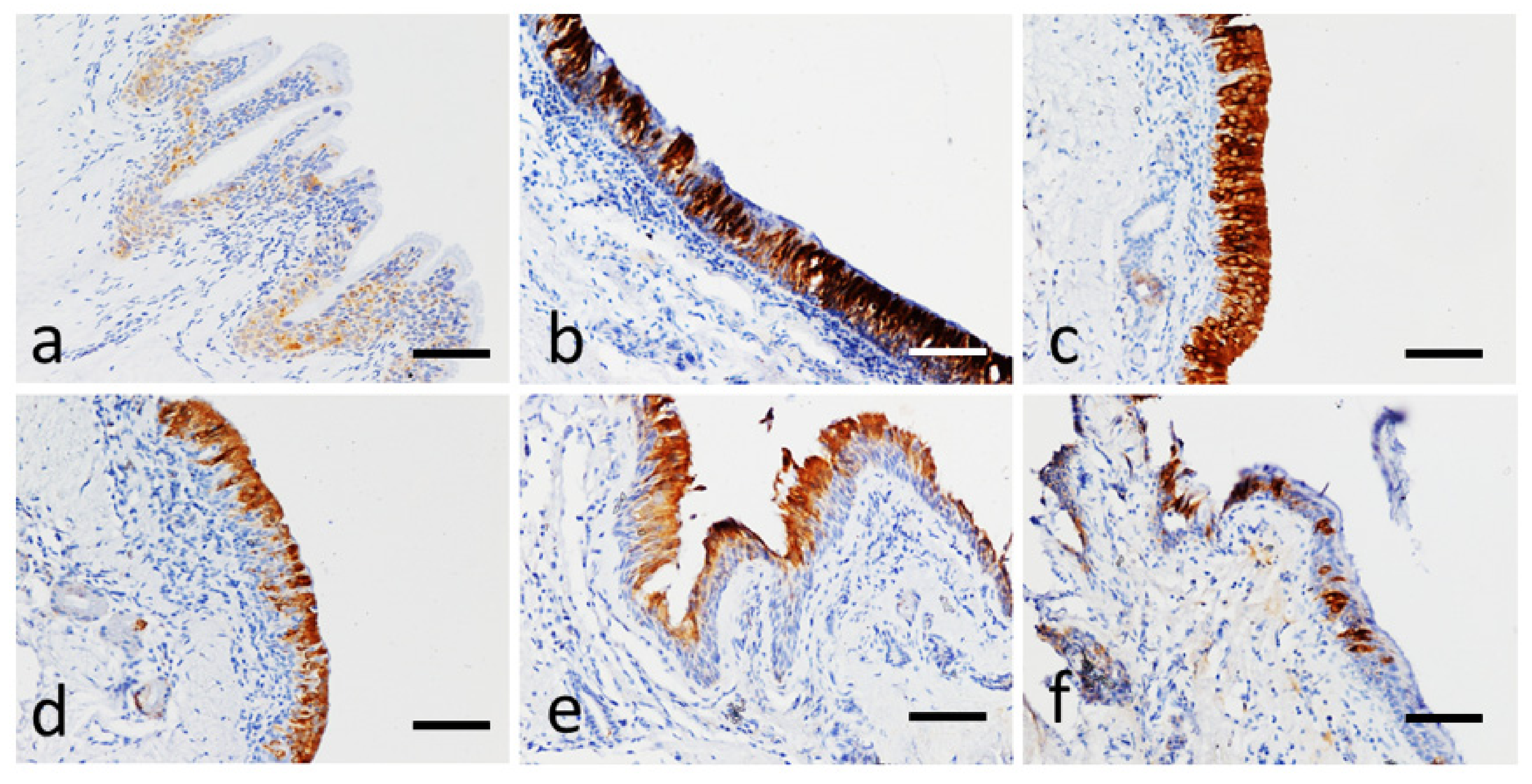
2.4. Immunohistochemical Analysis for Neurokinin A and IL-8
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, B.B., Jr.; Reef, V.B.; Parente, E.J.; Sage, A.D. Causes of poor performance of horses during training, racing, or showing: 348 cases (1992–1996). J. Am. Vet. Med. Assoc. 2000, 216, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Bullone, M.; Lavoie, J.P. Science-in-brief: Equine asthma diagnosis: Beyond bronchoalveolar lavage cytology. Equine Vet. J. 2017, 49, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450. [Google Scholar] [CrossRef]
- Couetil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.P.; Leguillette, R.; Richard, E.A. Inflammatory airway disease of horses–revised Consensus statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Tessier, L.; Côté, O.; Clark, M.E.; Viel, L.; Diaz-Méndez, A.; Anders, S.; Bienzle, D. Impaired response of the bronchial epithelium to inflammation characterizes severe equine asthma. BMC Genom. 2017, 18, 708. [Google Scholar] [CrossRef]
- Bond, S.; Léguillette, R.; Richard, E.A.; Couetil, L.; Lavoie, J.P.; Martin, J.G.; Pirie, R.S. Equine asthma: Integrative biologic relevance of a recently proposed nomenclature. J. Vet. Int. Med. 2018, 32, 2088–2098. [Google Scholar] [CrossRef]
- Seahorn, T.L.; Beadle, R.E. Summer pasture-associated obstructive pulmonary disease in horses: 21 cases (1983–1991). J. Am. Vet. Med. Assoc. 1993, 202, 779–782. [Google Scholar]
- McGorum, B.C.; Ellison, J.; Cullen, R.T. Total and respirable airborne dust endotoxin concentrations in three management systems. Equine Vet. J. 1998, 30, 430–434. [Google Scholar] [CrossRef]
- Vandenput, S.; Votion, D.; Duvivier, D.H.; van Erck, E.; Anciaux, N.; Art, T.; Lekeux, P. Effect of a set stabled environmental control on pulmonary function and airway reactivity of COPD affected horses. Vet. J. 1998, 155, 189–195. [Google Scholar] [CrossRef]
- Tremblay, G.M.; Ferland, C.; Lapointe, J.M.; Vrins, A.; Lavoie, J.P.; Cormier, Y. Effect of stabling on bronchoalveolar cells obtained from normal al COPD horses. Equine Vet. J. 1993, 25, 194–197. [Google Scholar] [CrossRef]
- Pirie, R.S. Recurrent airway obstruction: A review. Equine Vet. J. 2014, 46, 276–288. [Google Scholar] [CrossRef]
- Fogarty, U.; Buckley, T. Bronchoalveolar lavage findings in horses with exercise intolerance. Equine Vet. J. 1991, 23, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, S.J.; Jackson, C.; Gerber, V.; Jefcoat, A.; Berney, C.; Eberhardt, S.; Robinson, N.E. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet. J. 2001, 33, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Jean, D.; Vrins, A.; Beauchamp, G.; Lavoie, J.P. Evaluation of variations in bronchoalveolar lavage fluid in horses with recurrent airway obstruction. Am. J. Vet. Res. 2011, 72, 838–842. [Google Scholar] [CrossRef]
- Hoffman, A.M. Bronchoalveolar lavage: Sampling technique and guidelines for cytologic preparation and interpretation. Vet. Clin. N. Am. Equine Pract. 2008, 24, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.L.; Thompson, C.A. Airway Diagnostics: Bronchoalveolar Lavage, Tracheal Wash, and Pleural Fluid. Vet. Clin. N. Am. Equine Pract. 2020, 36, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Bullone, M.; Helie, P.; Joubert, P.; Lavoie, J.P. Development of a Semiquantitative Histological Score for the Diagnosis of Heaves Using Endobronchial Biopsy Specimens in Horses. J. Vet. Intern. Med. 2016, 30, 1739–1746. [Google Scholar] [CrossRef]
- Niedzwiedz, A.; Mordak, R.; Jaworski, Z.; Nicponc, J. Utility of the Histological Examination of the Bronchial Mucosa in the Diagnosis of Severe Equine Asthma Syndrome in Horses. J. Equine Vet. Sci. 2018, 67, 44–49. [Google Scholar] [CrossRef]
- Ainsworth, D.M.; Grünig, G.; Matychak, M.B.; Young, J.; Wagner, B.; Erb, H.N.; Antczak, D.F. Recurrent airway obstruction (RAO) in horses is characterized by IFN-γ and IL-8 production in bronchoalveolar lavage cells. Vet. Immun. Immunopathol. 2003, 96, 83–91. [Google Scholar] [CrossRef]
- Venugopal, C.S.; Holmes, E.P.; Polikepahad, S.; Laborde, M.K.; Moore, R.M. Neurokinin receptors in recurrent airway obstruction; a comparative study of affected and unaffected horses. Can. J. Vet. Res. 2009, 73, 25–33. [Google Scholar]
- Buechner-Maxwell, V. Airway hyperresponsiveness. Compend. Contin. Educ. Pract. Vet. 1993, 15, 1379–1389. [Google Scholar]
- Brazil, T.J.; McGorum, B.C. Molecules and inflammation in equine heaves: Mechanism and markers of disease. Equine Vet. J. 2001, 33, 113–115. [Google Scholar] [CrossRef]
- Calzetta, L.; Rogliani, P.; Pistocchini, E.; Mattei, M.; Cito, G.; Alfonsi, P.; Page, C.; Matera, M.G. Effect of lipopolysaccharide on the responsiveness of equine bronchial tissue. Pulm. Pharmacol. Ther. 2018, 49, 88–94. [Google Scholar] [CrossRef]
- Wada, R.; Aida, H.; Kaneko, M.; Oikawa, M.; Yoshihara, T.; Tomioka, Y.; Nitta, M. Identification of the bronchi for bronchoscopy in the horse and segmentation of the horse lung. Jpn. J. Equine Sci. 1992, 3, 37–43. [Google Scholar] [CrossRef]
- Rush, B.R.; Raub, E.S.; Rhoads, W.S.; Flaminio, M.J.; Matson, C.J.; Hakala, J.E.; Gillespie, J.R. Pulmonary function in horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am. J. Vet. Res. 1998, 59, 1039–1043. [Google Scholar]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.P. Heaves, an asthma-like disease of horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef]
- Ferrari, C.R.; Cooley, J.; Mujahid, N.; Costa, L.R.; Wills, R.W.; Johnson, M.E.; Swiderski, C.E. Horses with pasture asthma have airway remodelling that is characteristic of human asthma. Vet. Pathol. 2018, 55, 144–158. [Google Scholar] [CrossRef]
- Bullone, M.; Lavoie, J.P. Asthma “of horses and men”—How can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol. Immunol. 2015, 66, 97–105. [Google Scholar] [CrossRef]
- Woort, F.T.; Caswell, J.L.; Arroyo, L.G.; Viel, L. Histologic investigation of airway inflammation in postmortem lung samples from racehorses. Am. J. Vet. Res. 2018, 79, 342–347. [Google Scholar] [CrossRef]
- Giguère, S.; Prescott, J.F. Quantitation of equine cytokine mRNA expression by transcription-competitive polymerase chain reaction. Vet. Immunol. Immunopathol. 1999, 67, 1–15. [Google Scholar] [CrossRef]
- Swiderski, C.E.; Klei, T.R.; Horohov, D.W. Quantitative measurement of equine cytokine mRNA expression by polymerase chain reaction using target-specific standard curves. J. Immunol. Methods 1999, 222, 155–169. [Google Scholar] [CrossRef]
- Joubert, P.; Silversides, D.W.; Lavoie, J.P. Equine neutrophils express mRNA for tumor necrosis factor-α, interleukin (IL)-1β, IL-6, IL-8, macrophage-inflammatory-protein-2 but not for IL-4, IL-5 and interferon-γ. Equine Vet. J. 2001, 33, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Beadle, R.E.; Horohov, D.W.; Gaunt, S.D. Interleukin-4 and interferon-gamma gene expression in summer pasture-associated obstructive pulmonary disease affected horses. Equine Vet. J. 2002, 34, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P.; Cordeau, M.E.; Boyer, A.; Silversides, D.W.; Lavoie, J.P. Quantification of mRNA expression by peripheral neutrophils in an animal model of asthma. Am. J. Respir. Crit. Care Med. 2002, 165, A317. [Google Scholar]
- Horohov, D.W.; Beadle, R.E.; Mouch, S.; Pourciau, S.S. Temporal regulation of cytokine mRNA expression in equine recurrent airway obstruction. Vet. Immunol. Immunopathol. 2005, 108, 237–245. [Google Scholar] [CrossRef]
- Laan, T.T.; Bull, S.; Pirie, R.S.; Fink-Gremmels, J. Evaluation of cytokine production by equine alveolar macrophages exposed to lipopolysaccharide, Aspergillus fumigatus, and a suspension of hay dust. Am. J. Vet. Res. 2005, 66, 1584–1589. [Google Scholar] [CrossRef]
- Ainsworth, D.M.; Wagner, B.; Franchini, M.; Grünig, G.; Erb, H.N.; Tan, J.Y. Time-dependent alterations in gene expression of interleukin-8 in the bronchial epithelium of horses with recurrent airway obstruction. Am. J. Vet. Res. 2006, 67, 669–677. [Google Scholar] [CrossRef]
- Riihimäki, M.; Raine, A.; Art, T.; Lekeux, P.; Couëtil, L.; Pringle, J. Partial divergence of cytokine mRNA expression in bronchial tissues compared to bronchoalveolar lavage cells in horses with recurrent airway obstruction. Vet. Immunol. Immunopathol. 2008, 122, 256–264. [Google Scholar] [CrossRef]
- Klukowska-Rotzler, J.; Swinburne, J.E.; Drogemuller, C.; Dolf, G.; Janda, J.; Leeb, T.; Gerber, V. The interleukin 4 receptor gene and its role in recurrent airway obstruction in Swiss Warmblood horses. Anim. Genet. 2012, 43, 450–453. [Google Scholar] [CrossRef]
- Padoan, E.; Ferraresso, S.; Pegolo, S.; Castagnaro, M.; Barnini, C.; Bargelloni, L. Real time RT-PCR analysis of inflammatory mediator expression in recurrent airway obstruction-affected horses. Vet. Immunol. Immunopathol. 2013, 156, 190–199. [Google Scholar] [CrossRef]
- Barton, A.K.; Gehlen, G. Pulmonary Remodelling in Equine Asthma: What Do We Know about Mediators of Inflammation in the Horse? Mediat. Inflamm. 2016, 2016, 5693205. [Google Scholar] [CrossRef]
- Kraneveld, A.D.; Nijkamp, F.P.; Van Oosterhout, A.J. Role for neurokinin-2 receptor in interleukin-5-induced airway hyperresponsiveness but not eosinophilia in guinea pigs. Am. J. Respir. Crit. Care Med. 1997, 156, 367–374. [Google Scholar] [CrossRef]
- Fattori, D.; Altamura, M.; Maggi, C.A. Small molecule antagonists of the tachykinin NK 2 receptor. Mini-Rev. Med. Chem. 2004, 4, 331–340. [Google Scholar] [CrossRef]
- Pennefather, J.N.; Lecci, A.; Candenas, M.L.; Patak, E.; Pinto, F.M.; Maggi, C.A. Tachykinins and tachykinin receptors: A growing family. Life Sci. 2004, 74, 1445–1463. [Google Scholar] [CrossRef]
- Maggi, C.A.; Patacchini, R.; Santicioli, P.; Giuliani, S. Tachykinin antagonists and capsaicin-induced contraction of the rat isolated urinary bladder: Evidence for tachykinin-mediated cotransmission. Br. J. Pharmacol. 1991, 103, 1535–1541. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Holmes, E.P.; Moore, R.M.; Kappel, L.; Venugopal, C.S. Non-adrenergic non-cholinergic excitatory innervation in the airways: Role of neurokinin-2 receptors. Auton. Autacoid Pharmacol. 2002, 22, 215–224. [Google Scholar] [CrossRef]
- Schelfhout, V.; Van De Velde, V.; Maggi, C.; Pauwels, R.; Joo, G. The effect of the tachykinin NK2 receptor antagonist MEN11420 (nepadutant) on neurokinin A induced bronchoconstriction in asthmatics. Ther. Adv. Respir. Dis. 2009, 3, 219–226. [Google Scholar] [CrossRef]
- Calzetta, L.; Luongo, L.; Cazzola, M.; Page, C.; Rogliani, P.; Facciolo, F.; Maione, S.; Capuano, A.; Rinaldi, B.; Matera, M.G. Contribution of sensory nerves to LPS-induced hyperresponsiveness of human isolated bronchi. Life Sci. 2015, 131, 44–50. [Google Scholar] [CrossRef]
- Advenier, C.; Lagente, V.; Boichot, E. The role of tachykinin receptor antagonists in the prevention of bronchial hyperresponsiveness, airway inflammation and cough. Eur. Respir. J. 1997, 10, 1892–1906. [Google Scholar] [CrossRef]
- Solinger, N.; Sonea, I.M. Distribution of the neurokinin-1 receptor in equine intestinal smooth muscle. Equine Vet. J. 2008, 40, 321–325. [Google Scholar] [CrossRef]
- Lavoie, J.P.; Maghni, K.; Desnoyers, M.; Rame, T.; Martin, J.G.; Hamid, Q.A. Neutrophilic airway inflammation in horses with heaves is characterized by a Th2-type cytokine profile. Am. J. Respir. Crit. Care Med. 2001, 164, 1410–1413. [Google Scholar] [CrossRef]
- Giguere, S.; Viel, L.; Lee, E.; MacKay, R.J.; Hernandez, J.; Franchini, M. Cytokine induction in pulmonary airways of horses with heaves and effect of therapy with inhaled fluticasone propionate. Vet. Immunol. Immunopathol. 2002, 85, 147–158. [Google Scholar] [CrossRef]
- Cordeau, M.E.; Joubert, P.; Dewachi, O.; Hamid, Q.; Lavoie, J.P. IL-4, IL-5 and IFNgamma mRNA expression in pulmonary lymphocytes in equine heaves. Vet. Immunol. Immunopathol. 2004, 97, 87–96. [Google Scholar] [CrossRef]
- Hansen, S.; Otten, N.D.; Bircha, K.; Skovgaard, K.; Hopster-Iversen, C.; Fjeldborg, J. Bronchoalveolar lavage fluid cytokine, cytology and IgE allergen in horses with equine asthma. Vet. Immunol. Immunopathol. 2020, 220, 109976. [Google Scholar] [CrossRef]
- Moran, G.; Folch, H. Recurrent airway obstruction in horses—An allergic inflammation: A review. Vet. Med. 2011, 56, 1–13. [Google Scholar] [CrossRef]
- Leguillette, R. Recurrent airway obstruction—Heaves. Vet. Clin. Equine 2003, 19, 63–86. [Google Scholar] [CrossRef]
- Laan, T.T.J.M.; Bull, S.; Pirie, R.S.; Fink-Gremmels, J. The role of alveolar macrophages in the pathogenesis of recurrent airway obstruction in horses. J. Vet. Intern. Med. 2006, 20, 167–174. [Google Scholar] [CrossRef]
- Franchini, M.; Gill, U.; Von Fellenberg, R.; Bracher, V.D. Interleukin-8 concentration and neutrophil chemotactic activity in bronchoalveolar lavage fluid of horses with chronic obstructive pulmonary disease following exposure to hay. Am. J. Vet. Res. 2000, 61, 1369–1374. [Google Scholar] [CrossRef]
- Ainsworth, D.M.; Matychak, M.; Reyner, C.L.; Erb, H.N.; Young, J.C. Effects of in vitro exposure to hay dust on the gene expression of chemokines and cell-surface receptors in primary bronchial epithelial cell cultures established from horses with chronic recurrent airway obstruction. Am. J. Vet. Res. 2009, 70, 365–372. [Google Scholar] [CrossRef]
- Ainsworth, D.M.; Wagner, B.; Erb, H.N.; Young, J.C.; Retallick, D.E. Effects of in vitro exposure to hay dust on expression of interleukin-17, -23, -8, and -1beta and chemokine (C-X-C motif) ligand 2 by pulmonary mononuclear cells isolated from horses chronically affected with recurrent airway disease. Am. J. Vet. Res. 2007, 68, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Derksen, F.J.; Venta, P.J.; Ewart, S.; Yuzbasiyan-Gurkan, V.; Robinson, N.E. Elevated amount of Toll-like receptor 4 mRNA in bronchial epithelial cells is associated with airway inflammation in horses with recurrent airway obstruction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L936–L943. [Google Scholar] [CrossRef] [PubMed]
- Reyner, C.L.; Wagner, B.; Young, J.C.; Ainsworth, D.M. Effects of in vitro exposure to hay dust on expression of interleukin-23, -17, -8, and -1beta and chemokine (C-X-C motif) ligand 2 by pulmonary mononuclear cells from horses susceptible to recurrent airway obstruction. Am. J. Vet. Res. 2009, 70, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Pietra, M.; Peli, A.; Bonato, A.; Ducci, A.; Cinotti, S. Equine bronchoalveolar lavage cytokines in the development of Recurrent Airway Obstruction. Vet. Res. Commun. 2007, 31, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Pietra, M.; Cinotti, S.; Ducci, A.; Giunti, M.; Peli, A. Time-dependent changes of cytokines mRNA in bronchoalveolar lavage fluid from symptomatic recurrent airway obstruction-affected horses. Pol. J. Vet. Sci. 2011, 14, 343–351. [Google Scholar] [CrossRef][Green Version]
- Hansen, S.; Baptiste, K.E.; Fjeldborg, J.; Betancourt, A.; Horohov, D.W. A comparison of pro-inflammatory cytokine mRNA expression in equine bronchoalveolar lavage (BAL) and peripheral blood. Vet. Immunol. Immunopathol. 2014, 158, 238–243. [Google Scholar] [CrossRef]

| Control | EA-Affected | EA-Affected | EA-Affected | EA-Affected | |
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| Histological score (0–10) | 3 | 5.5 | 5 | 6.5 | 7.5 |
| median (min-max) | (2–4) | (4–6) | (4–8) | (5–10) | (5–9) |
| p | p = 0.92 | p = 0.48 | p = 0.16 | p = 0.63 | p = 0.81 |
| NKA immunoreactivity score | |||||
| Intensity of positivity (0–4) | 2 | 3 | 3 | 3 | 4 |
| median (min-max) | (2–3) | (1–4) | (2–4) | (2–4) | (2–4) |
| p | p = 0.5 | p = 0.88 | p = 0.49 | p = 0.83 | p = 0.06 |
| Signal distribution (1–2) | 1 | 1 | 2 | 1 | 1 |
| median (min-max) | (1–1) | (1–2) | (1–2) | (1–2) | (1–2) |
| p | p = 0.27 | p = 0.99 | p = 0.54 | p = 0.91 | |
| Cell localization (1–3) | 1 | 1.5 | 1 | 1.5 | 2 |
| median (min-max) | (1–1) | (1–3) | (1–3) | (1–3) | (1–3) |
| p | p = 0.58 | p = 0.96 | p = 0.41 | p = 0.85 | |
| IL-8 immunoreactivity score | |||||
| Intensity of positivity (0–4) | 2 | 3 | 3 | 3 | 3 |
| median (min-max) | (1–3) | (3–4) | (2–4) | (2-4) | (2-4) |
| p | p = 0.07 | p = 0.65 | p = 0.87 | p = 0.35 | p = 0.34 |
| Signal distribution (1–2) | 2 | 1 | 2 | 1 | 1 |
| median (min-max) | (1–2) | (1–2) | (1–2) | (1–2) | (1–2) |
| p | p = 0.5 | p = 0.56 | p = 0.63 | p = 0.48 | p = 0.41 |
| Control | EA-Affected | EA-Affected | EA-Affected | EA-Affected | |
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| Histological score (0–10) | 3 | 5.5 | 5 | 6.5 | 7.5 |
| median (min-max) | (2–4) A | (4–6) B | (4–8) B | (5–10) B | (5–9) B |
| NKA immunoreactivity score | |||||
| Intensity of positivity (0–4) | 2 | 3 | 3 | 3 | 4 |
| median (min-max) | (2–3) a | (1–4) | (2–4) | (2–4) b | (2–4) b |
| Signal distribution (1–2) | 1 | 1 | 2 | 1 | 1 |
| median (min-max) | (1–1) | (1–2) | (1–2) | (1–2) | (1–2) |
| Cell localization (1–3) | 1 | 1.5 | 1 | 1.5 | 2 |
| median (min-max) | (1–1) | (1–3) | (1–3) | (1–3) | (1–3) |
| IL-8 immunoreactivity score | |||||
| Intensity of positivity (0–4) | 2 | 3 | 3 | 3 | 3 |
| median (min-max) | (1–3) | (3–4) | (2–4) | (2–4) | (2–4) |
| Signal distribution (1–2) | 2 | 1 | 2 | 1 | 1 |
| median (min-max) | (1–2) | (1–2) | (1–2) | (1–2) | (1–2) |
| EA-Affected | EA-Affected | EA-Affected | EA-Affected | |
|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |
| Histological score (0–10) | 5.5 | 5 | 6.5 | 7.5 |
| median (min-max) | (4–6) a | (4–8) | (5–10) | (5–9) b |
| NKA immunoreactivity score | ||||
| Intensity of positivity (0–4) | 3 | 3 | 3 | 4 |
| median (min-max) | (1–4) | (2–4) | (2–4) | (2–4) |
| Signal distribution (1–2) | 1 | 2 | 1 | 1 |
| median (min-max) | (1–2) | (1–2) | (1–2) | (1–2) |
| Cell localization (1–3) | 1.5 | 1 | 1.5 | 2 |
| median (min-max) | (1–3) | (1–3) | (1–3) | (1–3) |
| IL-8 immunoreactivity score | ||||
| Intensity of positivity (0–4) | 3 | 3 | 3 | 3 |
| median (min-max) | (3–4) | (2–4) | (2–4) | (2–4) |
| Signal distribution (1–2) | 1 | 2 | 1 | 1 |
| median (min-max) | (1–2) | (1–2) | (1–2) | (1–2) |
| NKA | EA-Case 1 | EA-Case 2 | EA-Case 3 | EA-Case 4 | EA-Case 5 | EA-Case 6 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B Right | B Left | B Right | B Left | B Right | B Left | B Right | B Left | B Right | B Left | B Right | B Left | |||||||||||||
| P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | |
| T0 | n.e. | n.e. | n.e. | n.e. | 2, N, diffuse | 2, C, diffuse | 3, N, diffuse | 2, C, diffuse | n.e. | n.e. | n.e. | n.e. | 2, C, diffuse | 3, CN, diffuse | n.e. | 2, N, diffuse | 3, C, focal | n.e. | n.e. | 3, CN, diffuse | 1, C, focal | n.e. | 1, C, diffuse | n.e. |
| T1 | n.e. | 2, C, diffuse | n.e. | 2, C, diffuse | 1, C, diffuse | 3, N, diffuse | 2, C, diffuse | 3, CN, diffuse | 2, CN, diffuse | 3, CN, diffuse | 2, CN, diffuse | 3, CN, diffuse | 1, C, focal | 1, C, focal | 1, C, focal | 1, C, focal | n.e. | 3, C, focal | 3, C, focal | 3, C, focal | 1, CN, focal | 2, CN, focal | 3, CN, focal | 2, C, focal |
| T2 | n.e. | 2, C, diffuse | n.e. | 3, CN, diffuse | 2, C, diffuse | 2, C, diffuse | 2, C, diffuse | 1, C, diffuse | 2, C, diffuse | 3, CN, diffuse | 2, C, diffuse | 3, CN, diffuse | 1, C, focal | 2, CN, diffuse | 2, C, diffuse | 3, CN, diffuse | 2, CN, focal | 3, CN, diffuse | 2, C, focal | 3, N, focal | 2, CN, focal | 1, CN, focal | 1, C, focal | 1, C, focal |
| T3 | 3, N, diffuse | 3, N, diffuse | 3, N, diffuse | 2, N, diffuse | n.e. | 3, CN, diffuse | 3, CN, diffuse | 1, C, diffuse | 3, CN, diffuse | 3, CN, diffuse | 3, C, diffuse | 3, C, diffuse | 3, CN, focal | n.e. | 3, CN, diffuse | 3, CN, diffuse | 3, CN, diffuse | 3, CN, focal | 3, N, focal | 2, CN, focal | 1, C, focal | n.e. | n.e. | 1, C, focal |
| IL-8 | EA-Case 1 | EA-Case 2 | EA-Case 3 | EA- Case 4 | EA- Case 5 | EA- Case 6 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B Right | B Left | B Right | B Left | B Right | B Left | B Right | B Left | B Right | B Left | B Right | B Left | |||||||||||||
| P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | P | D | |
| T0 | 3, focal | n.e. | 3, focal | n.e. | 3, diffuse | 4, diffuse | 4, diffuse | 2, diffuse | n.e. | n.e. | n.e. | n.e. | 3, focal | 4, diffuse | n.e. | 3, diffuse | 4, diffuse | 3, focal | n.e. | 3, diffuse | n.e. | n.e. | n.e. | n.e. |
| T1 | n.e. | 2, focal | n.e. | 3, focal | 3, diffuse | 3, focal | 3, focal | 4, diffuse | n.e. | 3, diffuse | 4, diffuse | 3, focal | 2, focal | 3, focal | 2, focal | 3, focal | 4, diffuse | 4, diffuse | 4, diffuse | 4, diffuse | 4, diffuse | 4, focal | 3, focal | 4, diffuse |
| T2 | n.e. | 4, diffuse | 3, diffuse | 3, focal | 3, diffuse | 3, diffuse | 3, focal | 4, diffuse | 2, focal | 2, diffuse | 2, focal | 2, diffuse | 2, focal | 3, diffuse | 3, diffuse | 3, focal | 3, diffuse | 3, diffuse | 4, focal | 2, diff | 2, focal | 4, focal | 3, diffuse | 2, focal |
| T3 | 4, diffuse | 4, diffuse | 3, diffuse | 3, focal | n.e. | n.e. | 4, diffuse | 4, diffuse | 3, diffuse | 4, diffuse | 3, diffuse | 4, diffuse | 4, diffuse | n.e. | 3, diffuse | 3, diffuse | 4, diffuse | 4, focal | 4, focal | n.e. | n.e. | 3, focal | 2, focal | 2, focal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morini, M.; Peli, A.; Rinnovati, R.; Magazzù, G.; Romagnoli, N.; Spadari, A.; Pietra, M. Immunohistochemical Expression of Neurokinin-A and Interleukin-8 in the Bronchial Epithelium of Horses with Severe Equine Asthma Syndrome during Asymptomatic, Exacerbation, and Remission Phase. Animals 2021, 11, 1376. https://doi.org/10.3390/ani11051376
Morini M, Peli A, Rinnovati R, Magazzù G, Romagnoli N, Spadari A, Pietra M. Immunohistochemical Expression of Neurokinin-A and Interleukin-8 in the Bronchial Epithelium of Horses with Severe Equine Asthma Syndrome during Asymptomatic, Exacerbation, and Remission Phase. Animals. 2021; 11(5):1376. https://doi.org/10.3390/ani11051376
Chicago/Turabian StyleMorini, Maria, Angelo Peli, Riccardo Rinnovati, Giuseppe Magazzù, Noemi Romagnoli, Alessandro Spadari, and Marco Pietra. 2021. "Immunohistochemical Expression of Neurokinin-A and Interleukin-8 in the Bronchial Epithelium of Horses with Severe Equine Asthma Syndrome during Asymptomatic, Exacerbation, and Remission Phase" Animals 11, no. 5: 1376. https://doi.org/10.3390/ani11051376
APA StyleMorini, M., Peli, A., Rinnovati, R., Magazzù, G., Romagnoli, N., Spadari, A., & Pietra, M. (2021). Immunohistochemical Expression of Neurokinin-A and Interleukin-8 in the Bronchial Epithelium of Horses with Severe Equine Asthma Syndrome during Asymptomatic, Exacerbation, and Remission Phase. Animals, 11(5), 1376. https://doi.org/10.3390/ani11051376






