Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Fluorescent Immunostaining
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Animals
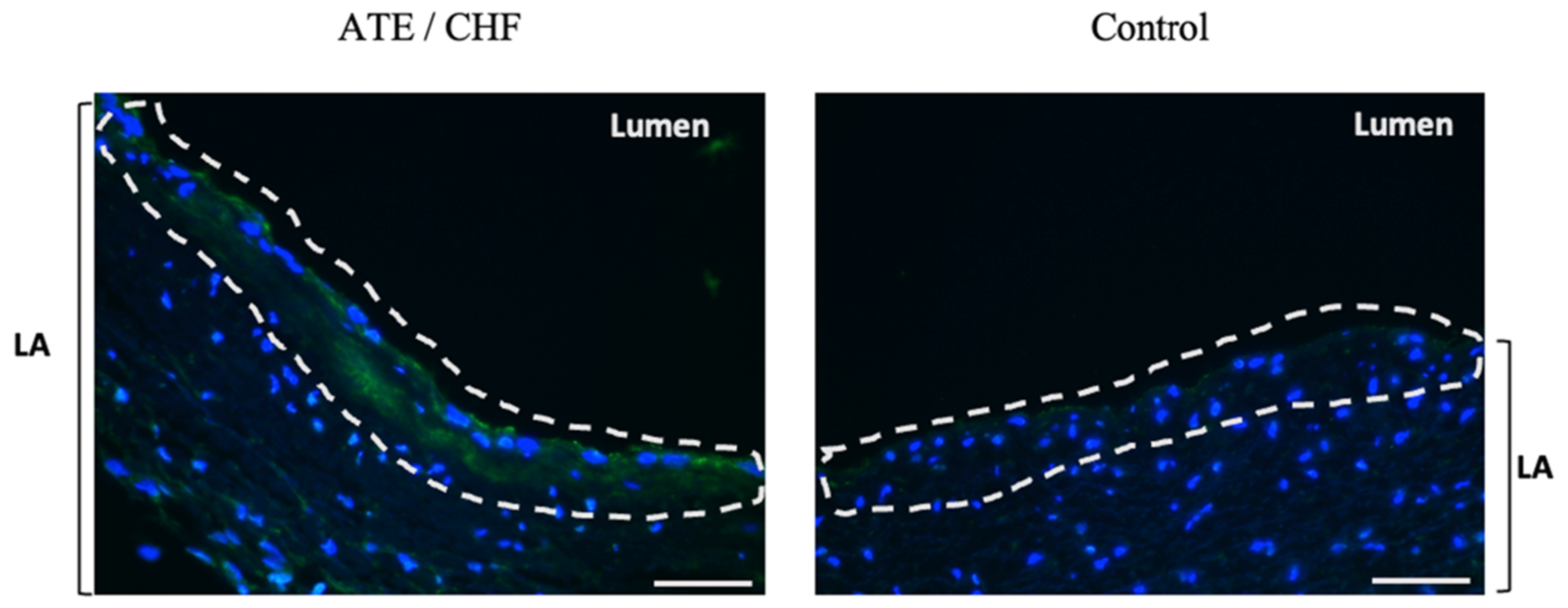
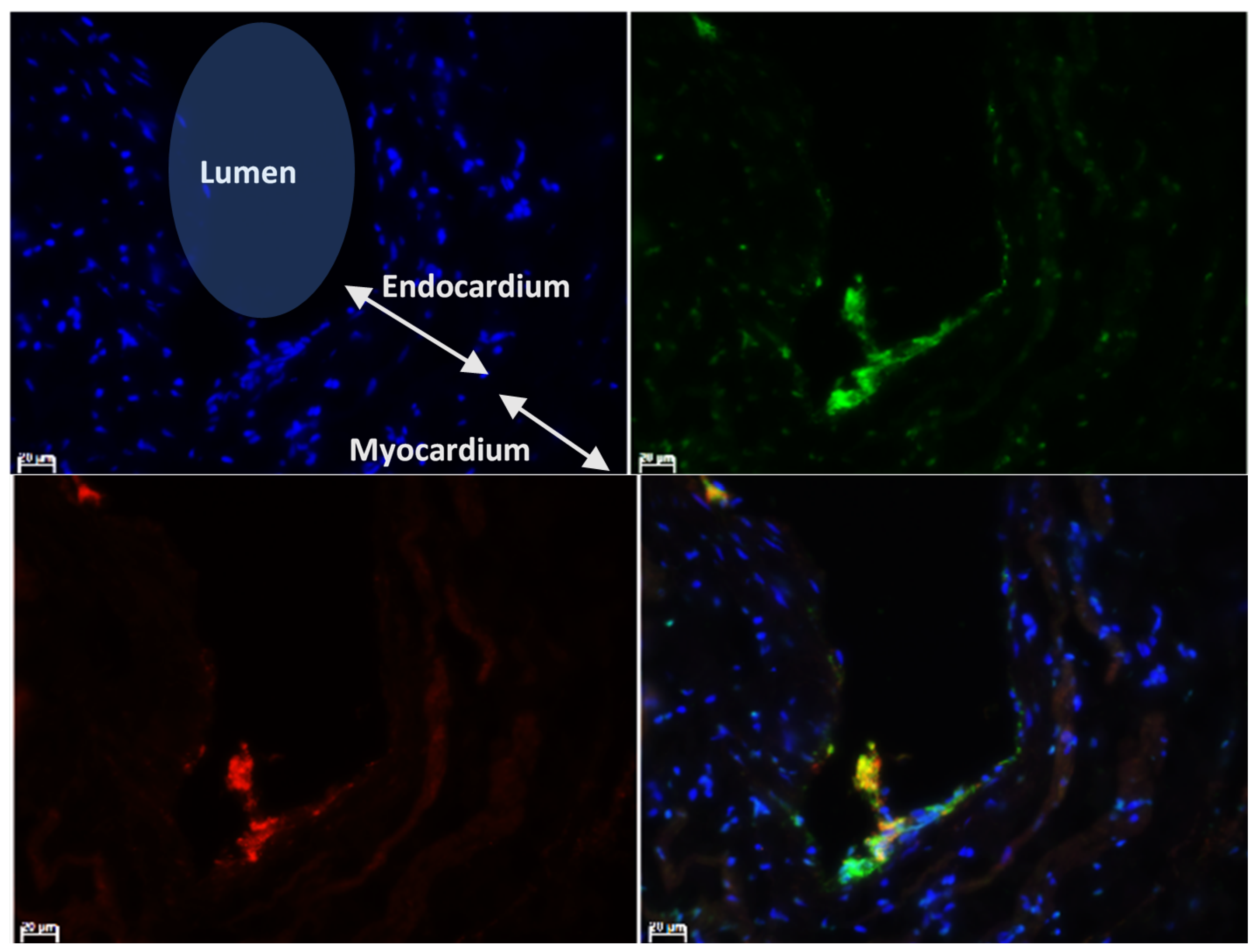
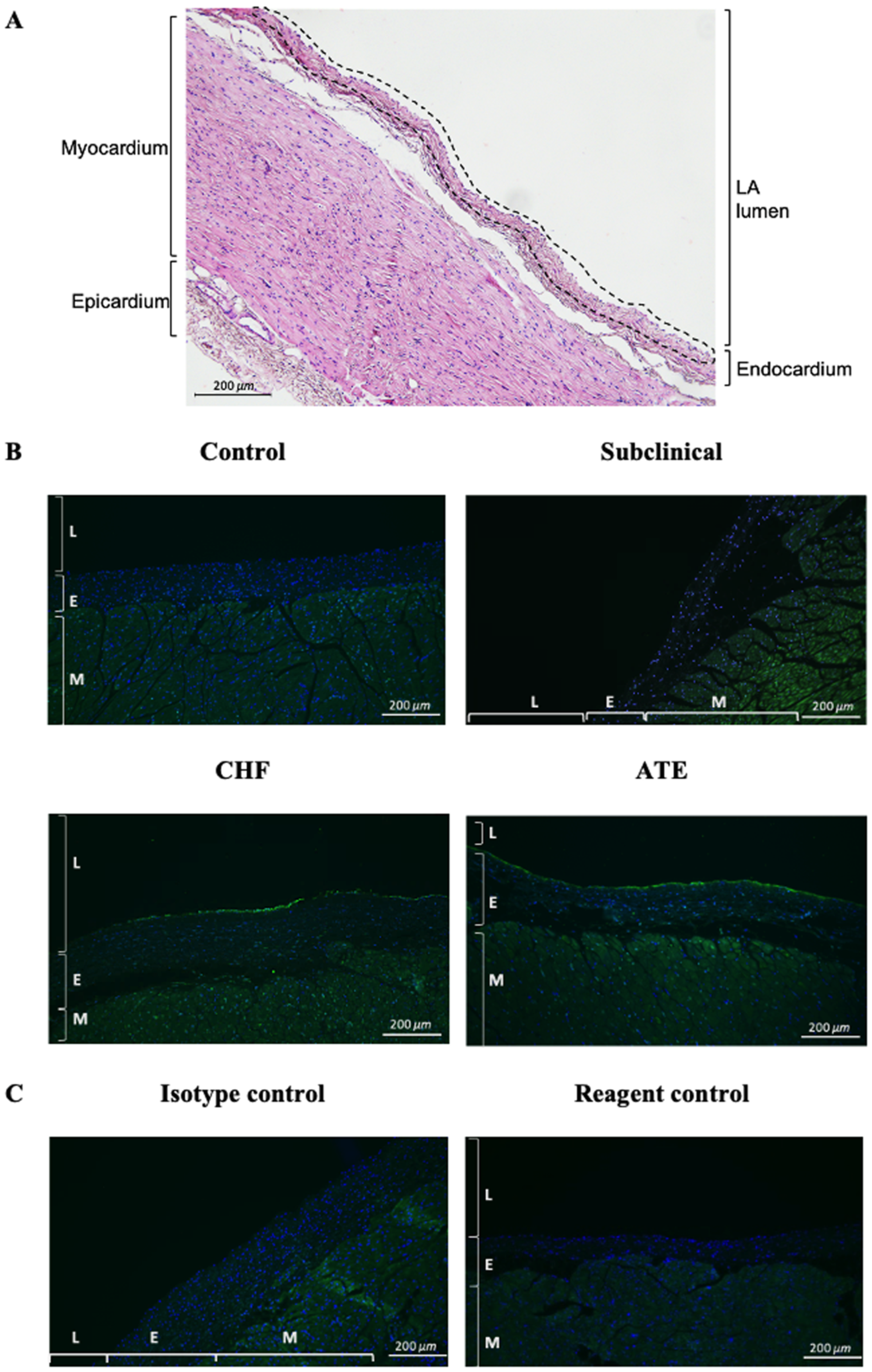
3.2. Localisation of vWF Protein in the Left Atrial Samples
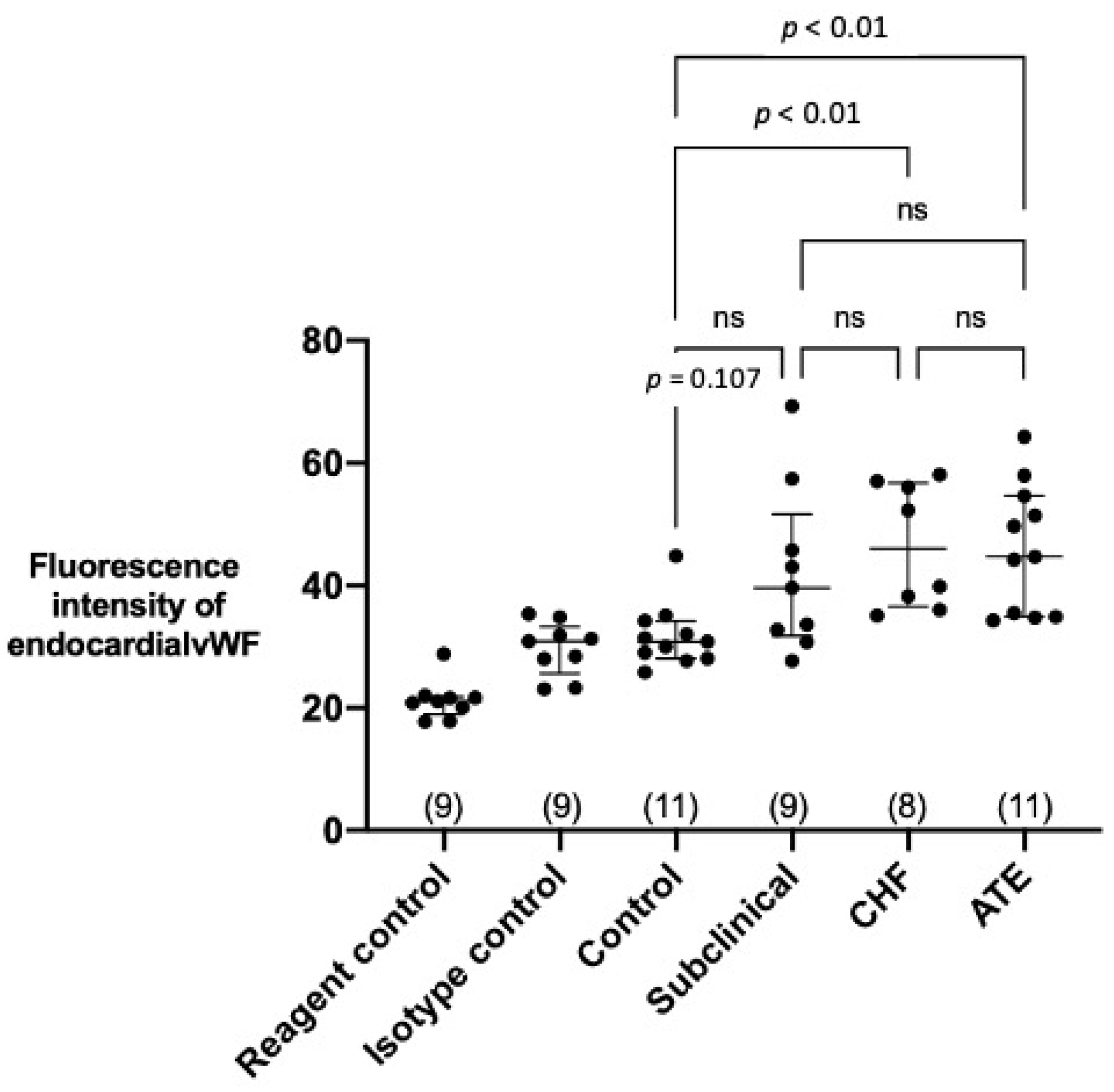
3.3. Quantification of Endocardial vWF Expression in Left Atrial Samples
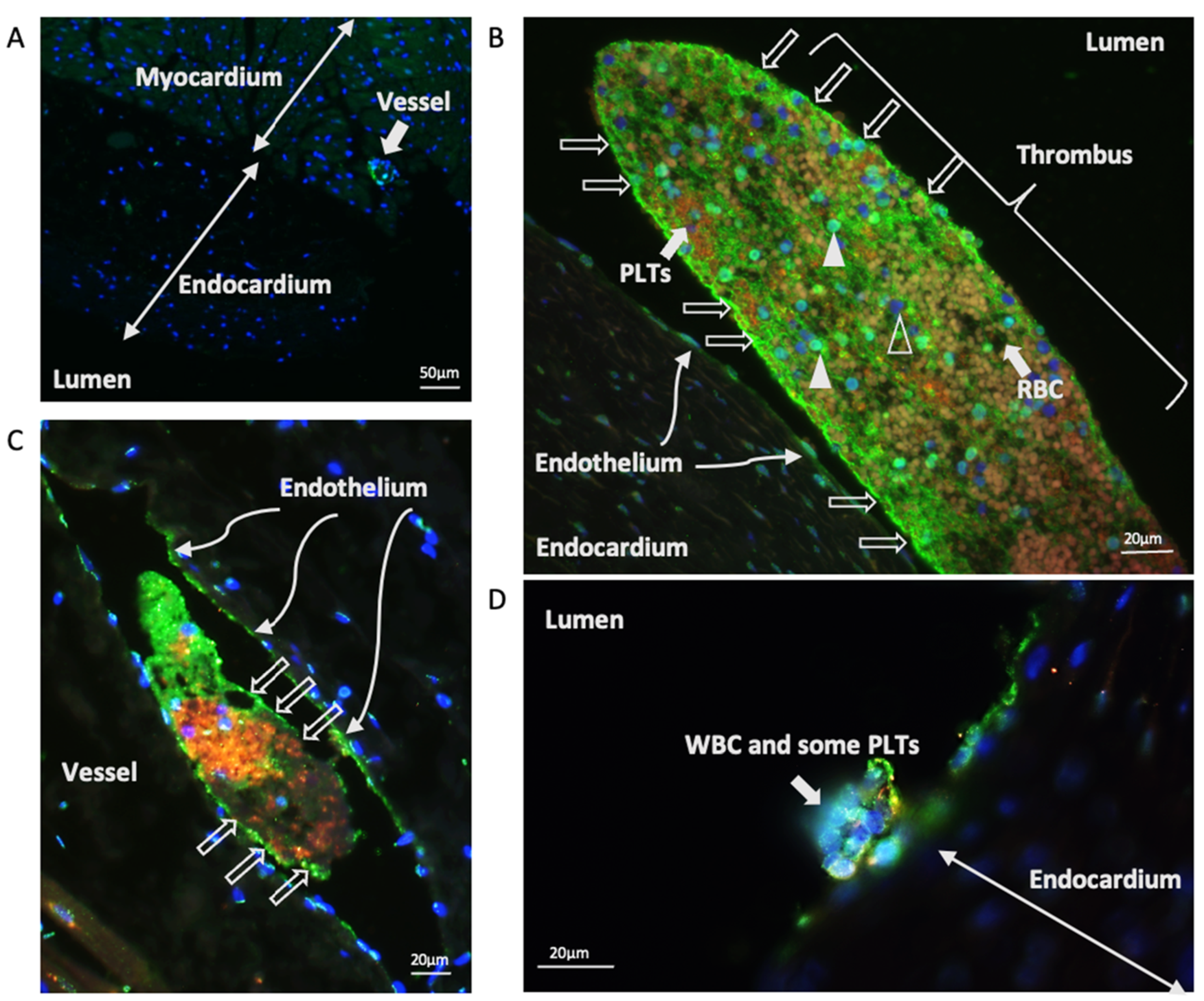
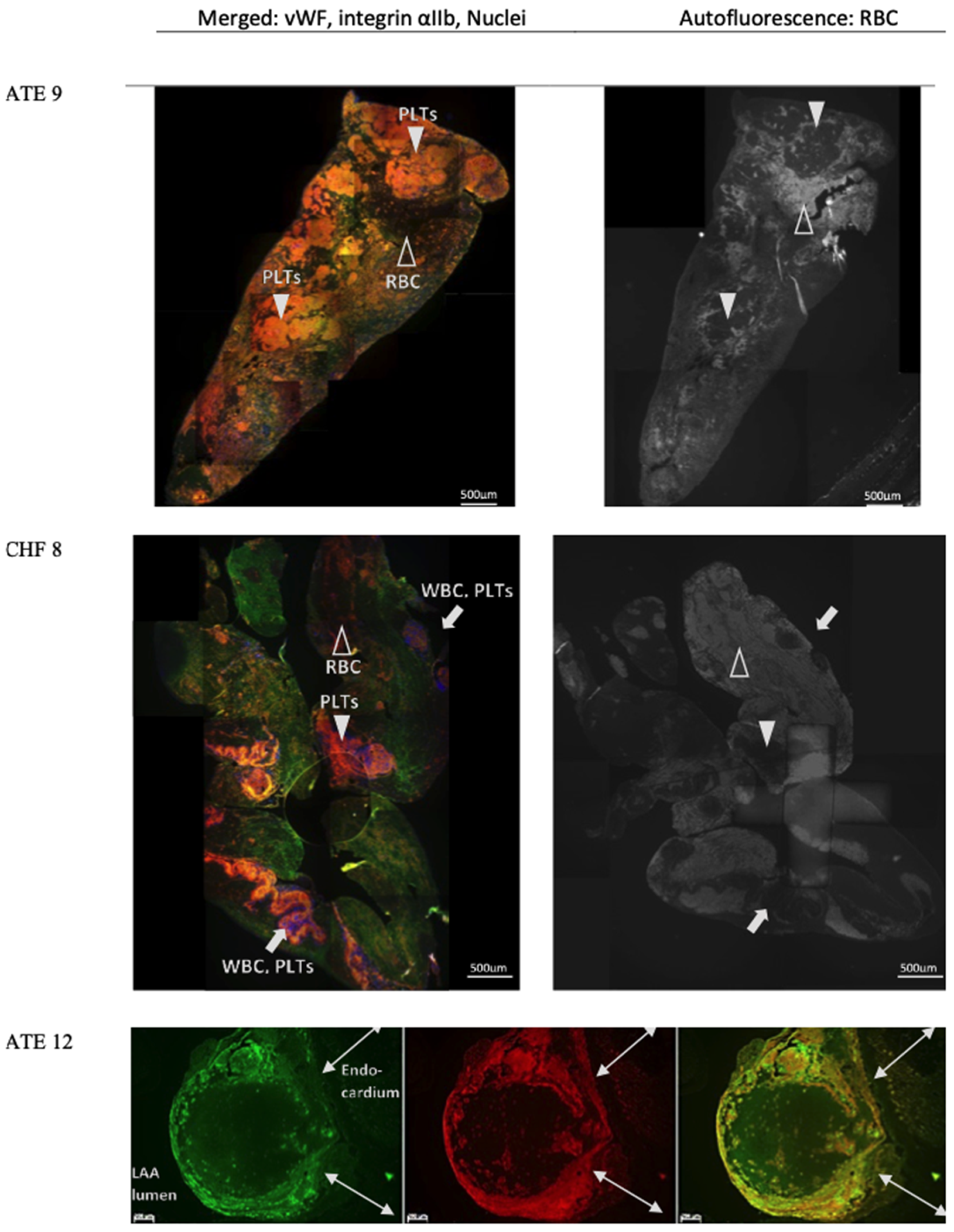
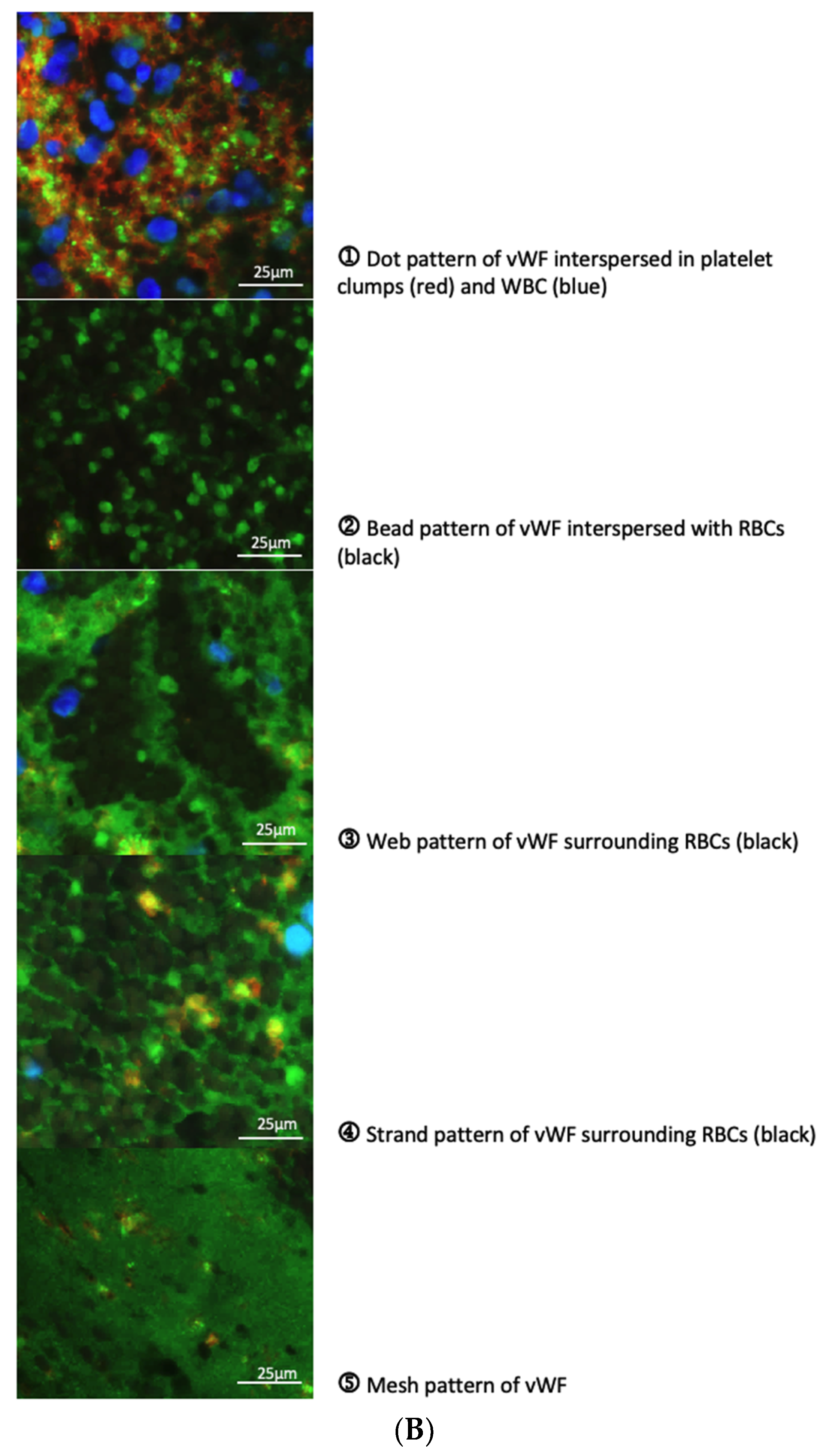
3.4. Characterisation of vWF in Thrombi Obtained from LA and LAA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.A.; Tobias, A.H.; Jacob, K.A.; Fine, D.M.; Grumbles, P.L. Arterial Thromboembolism in Cats: Acute Crisis in 127 Cases (1992–2001) and Long-Term Management with Low-Dose Aspirin in 24 Cases. J. Vet. Intern. Med. 2003, 17, 73–83. [Google Scholar] [CrossRef]
- Laste, N.J.; Harpster, N.K. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993. J. Am. Anim. Hosp. Assoc. 1995, 31, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.F. Feline Cardiogenic Arterial Thromboembolism. Vet. Clin. N. Am. Small Anim. Pr. 2017, 47, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.R.; Keene, B.W.; Lamb, K.; Schober, K.A.; Chetboul, V.; Fuentes, V.L.; Wess, G.; Payne, J.R.; Hogan, D.F.; Motsinger-Reif, A.; et al. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study. J. Vet. Intern. Med. 2018, 32, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Fuentes, V.L.; Boswood, A.; Connolly, D.; Koffas, H.; Brodbelt, D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J. Small Anim. Pr. 2010, 51, 540–547. [Google Scholar] [CrossRef]
- Fox, P.R.; Liu, S.-K.; Maron, B.J. Echocardiographic Assessment of Spontaneously Occurring Feline Hypertrophic Cardiomyopathy: An animal model of human disease. Circulation 1995, 92, 2645–2651. [Google Scholar] [CrossRef]
- Borgeat, K.; Wright, J.; Garrod, O.; Payne, J.; Fuentes, V.L. Arterial Thromboembolism in 250 Cats in General Practice: 2004–2012. J. Veter Intern. Med. 2013, 28, 102–108. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Schober, K.E.; Maerz, I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J. Vet. Intern. Med. 2006, 20, 120–130. [Google Scholar] [CrossRef]
- Stokol, T.; Brooks, M.; Rush, J.; Rishniw, M.; Erb, H.; Rozanski, E.; Kraus, M.; Gelzer, A. Hypercoagulability in Cats with Cardiomyopathy. J. Veter Intern. Med. 2008, 22, 546–552. [Google Scholar] [CrossRef]
- Liu, S.K.; Maron, B.J.; Tilley, L.P. Feline hypertrophic cardiomyopathy: Gross anatomic and quantitative histologic features. Am. J. Pathol. 1981, 102, 388–395. [Google Scholar]
- McMichael, M.; Freeman, L.; Selhub, J.; Rozanski, E.; Brown, D.; Nadeau; Rush, J. Plasma Homocysteine, B Vitamins, and Amino Acid Concentrations in Cats with Cardiomyopathy and Arterial Thromboembolism. J. Vet. Intern. Med. 2000, 14, 507–512. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. The role of von Willebrand factor in thrombus formation. Thromb. Res. 2007, 120, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F.; Eng, E.T.; Zhu, J.; Lu, C.; Walz, T.; Springer, T.A. Sequence and structure relationships within von Willebrand factor. Blood 2012, 120, 449–458. [Google Scholar] [CrossRef]
- Reinders, J.H.; De Groot, P.G.; Sixma, J.J.; Van Mourik, J.A. Storage and Secretion of von Willebrand Factor by Endothelial Cells. Pathophysiol. Haemost. Thromb. 1988, 18, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Li, C.Q.; Cao, Z.; Wang, H.; Wardell, M.R.; Sadler, J. Interaction of von Willebrand Factor Domain A1 with Platelet Glycoprotein Ibα-(1–289). J. Biol. Chem. 2000, 275, 7539–7546. [Google Scholar] [CrossRef]
- Cosemans, J.M.E.M.; Schols, S.E.M.; Stefanini, L.; De Witt, S.; Feijge, M.A.H.; Hamulyák, K.; Deckmyn, H.; Bergmeier, W.; Heemskerk, J.W.M. Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow. Blood 2011, 117, 651–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lip, G.Y. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Fukuchi, M.; Watanabe, J.; Kumagai, K.; Katori, Y.; Baba, S.; Fukuda, K.; Yagi, T.; Iguchi, A.; Yokoyama, H.; Miura, M.; et al. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J. Am. Coll. Cardiol. 2001, 37, 1436–1442. [Google Scholar] [CrossRef]
- Kumagai, K.; Fukuchi, M.; Ohta, J.; Baba, S.; Oda, K.; Akimoto, H.; Kagaya, Y.; Watanabe, J.; Tabayashi, K.; Shirato, K. Expression of the von Willebrand Factor in Atrial Endocardium is Increased in Atrial Fibrillation Depending on the Extent of Structural Remodeling. Circ. J. 2004, 68, 321–327. [Google Scholar] [CrossRef][Green Version]
- Ammash, N.; Konik, E.A.; McBane, R.D.; Chen, D.; Tange, J.I.; Grill, D.E.; Herges, R.M.; McLeod, T.G.; Friedman, P.A.; Wysokinski, W.E. Left Atrial Blood Stasis and Von Willebrand Factor–ADAMTS13 Homeostasis in Atrial Fibrillation. Arter. Thromb. Vasc. Biol. 2011, 31, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.; Shantsila, E.; Lip, G.Y. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef]
- Ding, W.Y.; Gupta, D.; Lip, G.Y.H. Atrial fibrillation and the prothrombotic state: Revisiting Virchow’s triad in 2020. Hear. 2020, 106, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Spiel, A.O.; Gilbert, J.C.; Jilma, B. Von Willebrand Factor in Cardiovascular Disease: Focus on acute coronary syndromes. Circulation 2008, 117, 1449–1459. [Google Scholar] [CrossRef]
- Sanders, Y.V.; Eikenboom, J.; De Wee, E.M.; Van Der Bom, J.G.; Cnossen, M.H.; Degenaar-Dujardin, M.E.L.; Fijnvandraat, K.; Kamphuisen, P.W.; Gorkom, B.A.P.L.-V.; Meijer, K.; et al. Reduced prevalence of arterial thrombosis in von Willebrand disease. J. Thromb. Haemost. 2013, 11, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Fuchs, T.A.; Chauhan, A.K.; Yang, J.J.; De Meyer, S.F.; Koellnberger, M.; Wakefield, T.W.; Laemmle, B.; Massberg, S.; Wagner, D.D. von Willebrand factor–mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2011, 117, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Connell, S.; Miltenberger-Miltenyi, G.; Pereira, S.V.; Tavares, A.; Ariëns, R.A.S.; Santos, N.C. Atomic Force Microscopy-Based Molecular Recognition of a Fibrinogen Receptor on Human Erythrocytes. ACS Nano 2010, 4, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.; de Almeida, V.V.; Calado, A.; Rosário, H.; Saldanha, C. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim. et Biophys. Acta (BBA) Biomembr. 2012, 1818, 481–490. [Google Scholar] [CrossRef]
- Aleman, M.M.; Walton, B.L.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and red blood cells in venous thrombosis. Thromb. Res. 2014, 133, S38–S40. [Google Scholar] [CrossRef]
- Walton, B.L.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J. Thromb. Haemost. 2015, 13, S208–S215. [Google Scholar] [CrossRef] [PubMed]
- Smeets, M.W.J.; Mourik, M.J.; Niessen, H.W.M.; Hordijk, P.L. Stasis Promotes Erythrocyte Adhesion to von Willebrand Factor. Arter. Thromb. Vasc. Biol. 2017, 37, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, L.; Smith, K.; Fuentes, V.L. Cardiac pathology findings in 252 cats presented for necropsy; a comparison of cats with unexpected death versus other deaths. J. Veter Cardiol. 2015, 17, S329–S340. [Google Scholar] [CrossRef]
- Wilkie, L.J. Phenotypic progression and cardiac remodelling in feline cardiomyopathies. Ph.D. Thesis, Royal Veterinary College, University of London, Hatfield, UK, 2017. [Google Scholar]
- Payne, J.R.; Brodbelt, D.C.; Fuentes, V.L. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J. Veter Cardiol. 2015, 17, S244–S257. [Google Scholar] [CrossRef]
- Fuentes, V.L.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Veter Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Côté, E.; Macdonald, K.A.; Meurs, K.M.; Sleeper, M.M. Feline Cardiology, 1st ed.; John Wiley & Sons, Inc.: West Sussex, UK, 2011; ISBN 9781118785782. [Google Scholar]
- Seo, J.; Payne, J.R.; Matos, J.N.; Fong, W.W.; Connolly, D.J.; Fuentes, V.L. Biomarker changes with systolic anterior motion of the mitral valve in cats with hypertrophic cardiomyopathy. J. Veter Intern. Med. 2020, 34, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.; Haggstrom, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and m-mode echocardiography in cavalier king charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef] [PubMed]
- März, I.; Wilkie, L.J.; Harrington, N.; Payne, J.R.; Muzzi, R.A.L.; Häggström, J.; Smith, K.; Fuentes, V.L. Familial cardiomyopathy in Norwegian Forest cats. J. Feline Med. Surg. 2014, 17, 681–691. [Google Scholar] [CrossRef]
- Fuentes, V.L. Arterial Thromboembolism: Risks, realities and a rational first-line approach. J. Feline Med. Surg. 2012, 14, 459–470. [Google Scholar] [CrossRef]
- Whittington, N.C.; Wray, S. Suppression of Red Blood Cell Autofluorescence for Immunocytochemistry on Fixed Embryonic Mouse Tissue. Curr. Protoc. Neurosci. 2017, 81, 2.28.1–2.28.12. [Google Scholar] [CrossRef]
- Yamamoto, K.; De Waard, V.; Fearns, C.; Loskutoff, D.J. Tissue Distribution and Regulation of Murine von Willebrand Factor Gene Expression In Vivo. Blood 1998, 92, 2791–2801. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakamura, K.; Fukushima-Kusano, K.; Ohta, K.; Matsubara, H.; Hamuro, T.; Yutani, C.; Ohe, T. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: Possible involvement in intracardiac thrombogenesis. Thromb. Res. 2003, 111, 137–142. [Google Scholar] [CrossRef]
- Payne, J.; Borgeat, K.; Brodbelt, D.; Connolly, D.; Fuentes, V.L. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J. Veter Cardiol. 2015, 17, S318–S328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chung, D.W. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018, 132, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Whincup, P.H.; Papacosta, O.; Lennon, L.; Lowe, G.D. Associations between blood coagulation markers, NT-proBNP and risk of incident heart failure in older men: The British Regional Heart Study: JACC State-of-the-Art Review. Int. J. Cardiol. 2017, 230, 567–571. [Google Scholar] [CrossRef]
- Nanchen, D.; Stott, D.J.; Gussekloo, J.; Mooijaart, S.P.; Westendorp, R.G.; Jukema, J.W.; Macfarlane, P.W.; Cornuz, J.; Rodondi, N.; Buckley, B.M.; et al. Resting heart rate and incident heart failure and cardiovascular mortality in older adults: Role of inflammation and endothelial dysfunction: The PROSPER study. Eur. J. Hear. Fail. 2013, 15, 581–588. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Thrombus formation in vivo. J. Clin. Investig. 2005, 115, 3355–3362. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. New Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef]
- Tan, K.T.; Lip, G.Y.H. Red vs White Thrombi: Treating the Right Clot Is Crucial. Arch. Intern. Med. 2003, 163, 2534–2535. [Google Scholar] [CrossRef]
- Tsai, H.-M. Shear Stress and von Willebrand Factor in Health and Disease. Semin. Thromb. Hemost. 2003, 29, 479–488. [Google Scholar] [CrossRef]
- Tsai, H.-M. von Willebrand Factor, Shear Stress, and ADAMTS13 in Hemostasis and Thrombosis. ASAIO J. 2012, 58, 163–169. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Control | Subclinical |
| Cats that died of non-cardiac disease with no cardiac related abnormalities detected by clinical exam, gross and histopathology ± complete or POC echocardiography. | Cats that showed no clinical signs of heart disease. Cardiomyopathy confirmed on gross and histopathology + complete or POC echocardiography. | |
| CHF | ATE | |
| Cats that showed clinical signs of CHF. Cardiomyopathy and LAE confirmed on gross pathology and histopathology + complete or POC echocardiography. | Cats that showed clinical signs compatible with ATE (irrespective of CHF). Cardiomyopathy and LAE confirmed on gross pathology and histopathology + complete or POC echocardiography. | |
| Exclusion Criteria | For all cats with cardiomyopathy | |
| Documented chronic renal disease with hypertension, hyperthyroidism, and other uncontrolled systemic diseases that may induce structural cardiac changes. | ||
| See supplementary material Tables S1 and S2 for diagnostic criteria for cardiomyopathies by histopathology and echocardiography. | ||
| Group | Number of Cats | Median Age (Range) (Years) | Gender Male:Female | Breed (Number of Cats) |
|---|---|---|---|---|
| Control | 11 | 2.0 (0.2–7.5) | 4:7 | DSH (11) |
| Subclinical | 9 | 7.5 (2.5–11.0) | 6:3 | DSH (6) DLH (1) BSH (1) Bengal (1) |
| CHF | 8 | 7.5 (2.0–17.3) | 6:2 | DSH (6) BSH (1) Siamese (1) |
| ATE | 11 | 7 (1.8–11.0) | 11:0 | DSH (7) DLH (1) BSH (2) Siamese (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-C.; Wilkie, L.; Kurosawa, T.A.; Dobromylskyj, M.; Priestnall, S.L.; Luis Fuentes, V.; Connolly, D.J. Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy. Animals 2021, 11, 1240. https://doi.org/10.3390/ani11051240
Cheng W-C, Wilkie L, Kurosawa TA, Dobromylskyj M, Priestnall SL, Luis Fuentes V, Connolly DJ. Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy. Animals. 2021; 11(5):1240. https://doi.org/10.3390/ani11051240
Chicago/Turabian StyleCheng, Wan-Ching, Lois Wilkie, Tsumugi Anne Kurosawa, Melanie Dobromylskyj, Simon Lawrence Priestnall, Virginia Luis Fuentes, and David J. Connolly. 2021. "Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy" Animals 11, no. 5: 1240. https://doi.org/10.3390/ani11051240
APA StyleCheng, W.-C., Wilkie, L., Kurosawa, T. A., Dobromylskyj, M., Priestnall, S. L., Luis Fuentes, V., & Connolly, D. J. (2021). Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy. Animals, 11(5), 1240. https://doi.org/10.3390/ani11051240







