Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample and Data Collection
2.3. Determination of Gastrointestinal Peptides
2.4. DNA Extraction and PCR Amplification
2.5. Illumina MiSeq Sequencing
2.6. Processing of Sequencing Data
2.7. Determination of SCFAs
2.8. Determination of Serum Biochemical Parameters
2.9. Statistical Analysis
3. Results
3.1. Effect of High Temperature on Performance
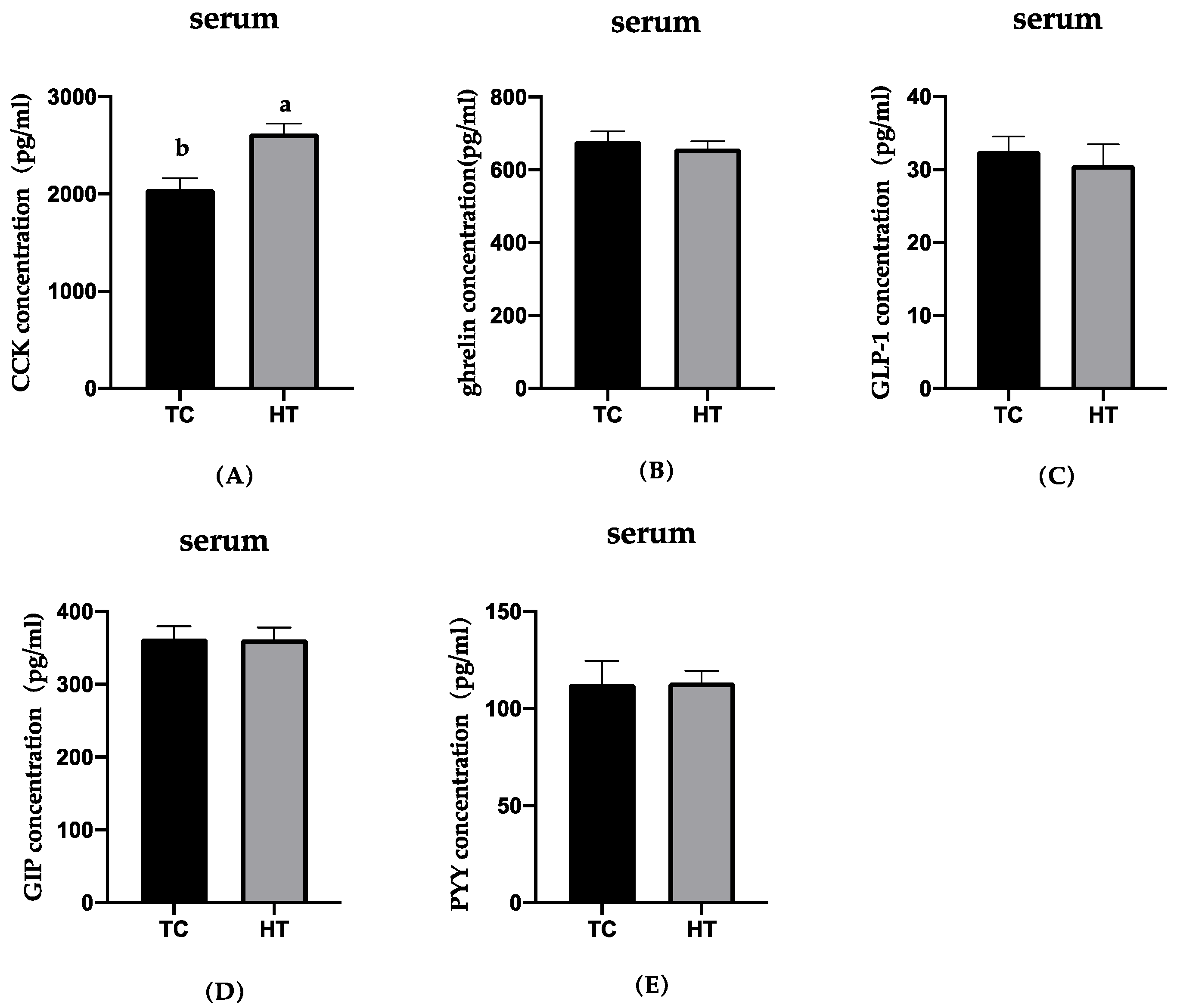
3.2. Effects of High Temperature on Gastrointestinal Peptide
3.3. Effects of High Temperature on Cecal Microbial Composition
3.4. Effects of High Temperature on SCFAs Concentration
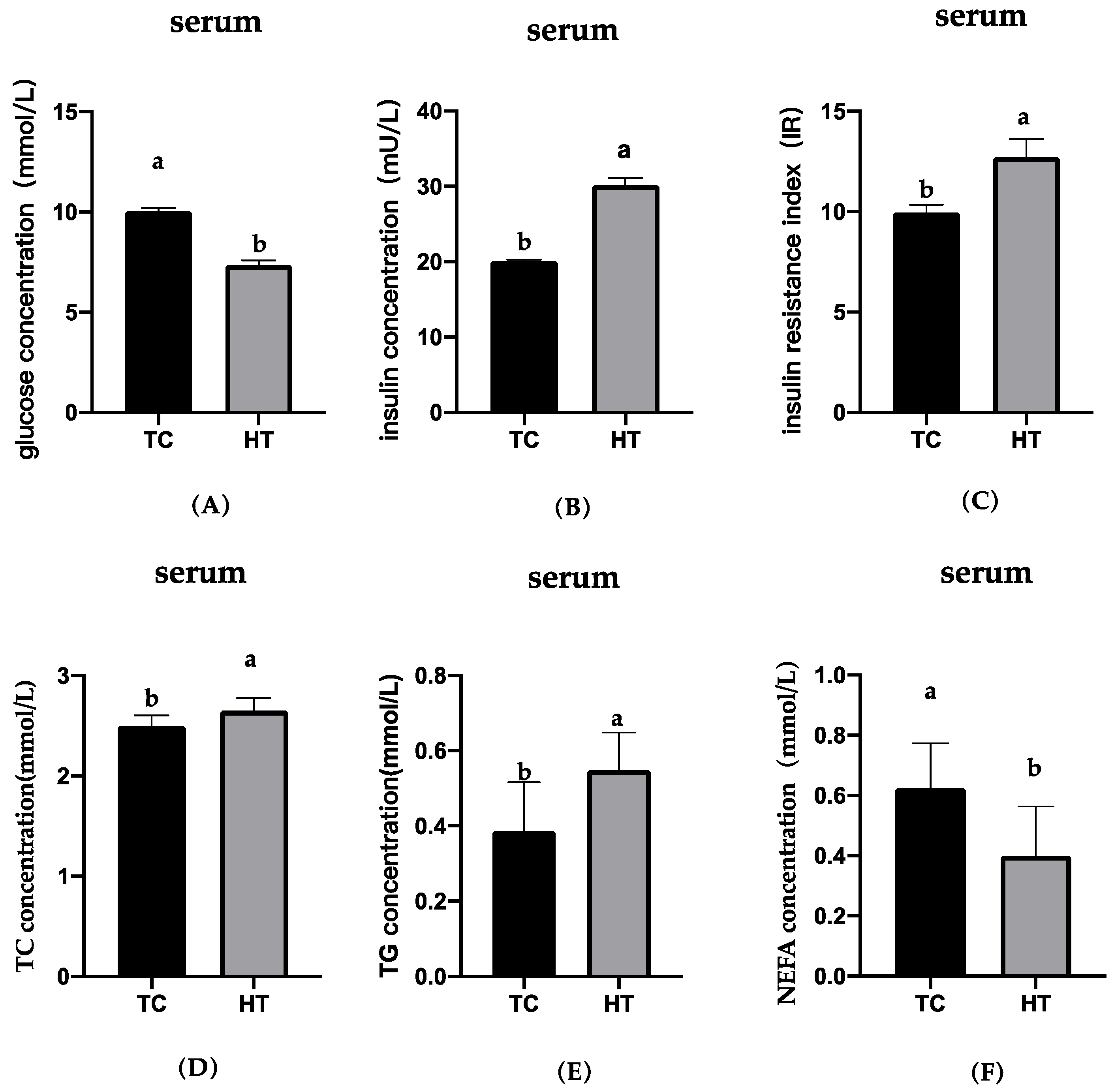
3.5. Effects of High Temperatures on Serum Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Hosseini-Vashan, S.; Golian, A.; Yaghobfar, A.; Zarban, A.; Afzali, N.; Esmaeilinasab, P. Antioxidant Status, Immune System, Blood Metabolites and Carcass Characteristic of Broiler Chickens Fed Turmeric Rhizome Powder under Heat Stress. Afr. J. Biotechnol. 2012, 11, 16118–16125. [Google Scholar]
- Garriga, C.; Hunter, R.R.; Amat, C.; Planas, J.M.; Mitchell, M.A.; Moretó, M. Heat Stress Increases Apical Glucose Transport in the Chicken Jejunum. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R195–R201. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Croom, J.; Christensen, V.; Black, B.; Bird, A.; Daniel, L.; Mcbride, B.; Eisen, E. Jejunal Glucose Uptake and Oxygen Consumption in Turkey Poults Selected for Rapid Growth. Poult. Sci. 1997, 76, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Al-Harthi, M.A.; Sh Elnaggar, A. Productive, Physiological and Immunological Responses of Two Broiler Strains Fed Different Dietary Regimens and Exposed to Heat Stress. Ital. J. Anim. Sci. 2018, 17, 686–697. [Google Scholar] [CrossRef]
- Afsal, A.; Sejian, V.; Madiajagan, B.; Krishnan, G. Heat Stress and Livestock Adaptation: Neuro-Endocrine Regulation. Int. J. Vet. Anim. Med. 2018, 1, 1–8. [Google Scholar]
- Xiong, Y.; Yi, H.; Wu, Q.; Jiang, Z.; Wang, L. Effects of Acute Heat Stress on Intestinal Microbiota in Grow-finishing Pigs, and Associations with Feed Intake and Serum Profile. J. Appl. Microbiol. 2020, 128, 840–852. [Google Scholar] [CrossRef]
- Akşit, M.; Yalçin, S.; Ozkan, S.; Metin, K.; Ozdemir, D. Effects of Temperature During Rearing and Crating on Stress Parameters and Meat Quality of Broilers. Poult. Sci. 2006, 85, 1867. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute Heat Stress Induces Oxidative Stress in Broiler Chickens. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased Fat Synthesis and Limited Apolipoprotein B Cause Lipid Accumulation in the Liver of Broiler Chickens Exposed to Chronic Heat Stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Sands, J.; Smith, M. Effects of Dietary Manganese Proteinate or Chromium Picolinate Supplementation on Plasma Insulin, Glucagon, Glucose and Serum Lipids in Broiler Chickens Reared under Thermoneutral or Heat Stress Conditions. Int. J. Poult. Sci. 2002, 1, 145–149. [Google Scholar]
- Geraert, P.A.; Padilha, J.C.F.; Guillaumin, S. Metabolic and Endocrine Changes Induced by Chronic Heatexposure in Broiler Chickens: Growth Performance, Body Composition and Energy Retention. Br. J. Nutr. 1996, 75, 195–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a Probiotic Mixture on Intestinal Microflora, Morphology, and Barrier Integrity of Broilers Subjected to Heat Stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef]
- Park, S.-O.; Hwangbo, J.; Ryu, C.-M.; Park, B.-S.; Chae, H.-S.; Choi, H.-C.; Seo, O.-S.; Choi, Y.-H. Effects of Extreme Heat Stress on Growth Performance, Lymphoid Organ, IgG and Cecum Microflora of Broiler Chickens. Int. J. Agric. Biol. 2013, 15, 5. [Google Scholar]
- Ramiah, S.K.; Atta Awad, E.; Hemly, N.I.M.; Ebrahimi, M.; Joshua, O.; Jamshed, M.; Saminathan, M.; Soleimani, A.F.; Idrus, Z. Effects of Zinc Oxide Nanoparticles on Regulatory Appetite and Heat Stress Protein Genes in Broiler Chickens Subjected to Heat Stress. J. Anim. Sci. 2020, 98, skaa300. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Morrison, E.; Vodyanoy, V.; Sorokulova, I. Yeast Fermentate Prebiotic Improves Intestinal Barrier Integrity during Heat Stress by Modulation of the Gut Microbiota in Rats. J. Appl. Microbiol. 2019, 127, 1192–1206. [Google Scholar] [CrossRef]
- Giles, K.; Pluvinage, B.; Boraston, A.B. Structure of a Glycoside Hydrolase Family 50 Enzyme from a Subfamily That Is Enriched in Human Gut Microbiome Bacteroidetes: Structure of a Bacteroides GH50. Proteins Struct. Funct. Bioinform. 2017, 85, 182–187. [Google Scholar] [CrossRef]
- Cani, P.D.; Dewever, C.; Delzenne, N.M. Inulin-Type Fructans Modulate Gastrointestinal Peptides Involved in Appetite Regulation (Glucagon-like Peptide-1 and Ghrelin) in Rats. Br. J. Nutr. 2004, 92, 521–526. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Højlund, K. Metabolism and Insulin Signaling in Common Metabolic Disorders and Inherited Insulin Resistance. Dan. Med. J. 2014, 61, B4890. [Google Scholar] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S RRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Smith, M.O. Effects of Different Levels of Zinc on the Performance and Immunocompetence of Broilers Under Heat Stress. Poult. Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Liu, F.Z.; Yan, Q.L.; Li, W.C. Effects of Different Levels of Vitamin E on Growth Performance and Immune Responses of Broilers under Heat Stress. Poult. Sci. 2009, 88, 2101–2107. [Google Scholar] [CrossRef]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of Gut Microbiota Structure in Heat-Stressed Broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic Heat Stress Impairs the Quality of Breast-Muscle Meat in Broilers by Affecting Redox Status and Energy-Substance Metabolism. J. Agric. Food Chem. 2017, 65, 11251–11258. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Yoshiki, Y.; Akiba, Y.; Toyomizu, M. Superoxide Radical Production in Chicken Skeletal Muscle Induced by Acute Heat Stress. Poult. Sci. 2005, 84, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.; Ferreira, A.; Palermo-Neto, J. Heat Stress Impairs Performance Parameters, Induces Intestinal Injury, and Decreases Macrophage Activity in Broiler Chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Zeferino, C.P.; Komiyama, C.M.; Pelícia, V.C.; Fascina, V.B.; Aoyagi, M.M.; Coutinho, L.L.; Sartori, J.R.; Moura, A.S.A.M.T. Carcass and Meat Quality Traits of Chickens Fed Diets Concurrently Supplemented with Vitamins C and E under Constant Heat Stress. Animal 2016, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Yang, H.; Wang, Y.; Haraguchi, S.; Miyazaki, T.; Bungo, T.; Tashiro, K.; Furuse, M.; Chowdhury, V.S. L-Leucine Increases the Daily Body Temperature and Affords Thermotolerance in Broiler Chicks. Asian-Australas. J. Anim. Sci. 2019, 32, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, L.; Sheikhahmadi, A.; Jiao, H.; Lin, H. Effect of Heat Exposure on Gene Expression of Feed Intake Regulatory Peptides in Laying Hens. J. Biomed. Biotechnol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Yunianto, V.D.; Hayashit, K.; Kaiwda, S.; Ohtsuka, A.; Tomita, Y. Effect of Environmental Temperature on Muscle Protein Turnover and Heat Production in Tube-Fed Broiler Chickens. Br. J. Nutr. 1997, 77, 897–909. [Google Scholar] [CrossRef]
- Lu, Q.; Wen, J.; Zhang, H. Effect of Chronic Heat Exposure on Fat Deposition and Meat Quality in Two Genetic Types of Chicken. Poult. Sci. 2007, 86, 1059–1064. [Google Scholar] [CrossRef]
- Toghyani, M.; Toghyani, M.; Shivazad, M.; Gheisari, A.; Bahadoran, R. Chromium Supplementation Can Alleviate the Negative Effects of Heat Stress on Growth Performance, Carcass Traits, and Meat Lipid Oxidation of Broiler Chicks without Any Adverse Impacts on Blood Constituents. Biol. Trace Elem. Res. 2012, 146, 171–180. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, H.; Jiang, K.J.; Jiao, H.C.; Song, Z.G. Corticosterone Administration and High-Energy Feed Results in Enhanced Fat Accumulation and Insulin Resistance in Broiler Chickens. Br. Poult. Sci. 2008, 49, 487–495. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Serum Metabolomics Study of Nutrient Metabolic Variations in Chronic Heat-Stressed Broilers. Br. J. Nutr. 2018, 119, 771–781. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial Effect of Butyrate-producing Lachnospiraceae on Stress-induced Visceral Hypersensitivity in Rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Demidova, T.Y.; Lobanova, K.G.; Oynotkinova, O.S. Gut Microbiota Is an Endocrine Organ. Obes. Metab. 2020, 17, 299–306. [Google Scholar] [CrossRef]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic Heat Stress Alters Hypothalamus Integrity, the Serum Indexes and Attenuates Expressions of Hypothalamic Appetite Genes in Broilers. J. Therm. Biol. 2019, 81, 110–117. [Google Scholar] [CrossRef]
- Melo-Duran, D.; Gonzalez-Ortiz, G.; Sola-Oriol, D.; Martinez-Mora, M.; Perez, J.F.; Bedford, M.R. Relationship between Peptide YY, Cholecystokinin and Fermentation Products in Fasted, Re-Fed and Ad Libitum Fed Broiler Chickens. Anim. Feed Sci. Technol. 2019, 247, 141–148. [Google Scholar] [CrossRef]
- Louie, D.S.; May, D.; Miller, P.; Owyang, C. Cholecystokinin Mediates Feedback Regulation of Pancreatic Enzyme Secretion in Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 1986, 250, G252–G259. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Tachibana, T.; Ohgushi, A.; Ando, R.; Yoshimatsu, T.; Denbow, D.M. Intracerebroventricular Injection of Ghrelin and Growth Hormone Releasing Factor Inhibits Food Intake in Neonatal Chicks. Neurosci. Lett. 2001, 301, 123–126. [Google Scholar] [CrossRef]
- Xu, P.; Siegel, P.B.; Denbow, D.M. Genetic Selection for Body Weight in Chickens Has Altered Responses of the Brain’s AMPK System to Food Intake Regulation Effect of Ghrelin, but Not Obestatin. Behav. Brain Res. 2011, 221, 216–226. [Google Scholar] [CrossRef]
- Liu, L.; Song, Z.; Jiao, H.; Lin, H. Glucocorticoids Increase NPY Gene Expression via Hypothalamic AMPK Signaling in Broiler Chicks. Endocrinology 2014, 155, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S. Evaluation of the Chronological Impact Heat Stress Has on Swine Intestinal Function and Integrity. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Ding, K.-H.; Shi, X.-M.; Zhong, Q.; Kang, B.; Xie, D.; Bollag, W.B.; Bollag, R.J.; Hill, W.; Washington, W.; Mi, Q.-S.; et al. Impact of Glucose-Dependent Insulinotropic Peptide on Age-Induced Bone Loss. J. Bone Miner. Res. 2007, 23, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut Microbiota Fermentation of Prebiotics Increases Satietogenic and Incretin Gut Peptide Production with Consequences for Appetite Sensation and Glucose Response after a Meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Klosterbuer, A.; Sanders, L.; Potter, S.; Thomas, W.; Slavin, J. A Blend of Soluble Fiber and Resistant Starch Promotes Feelings of Fullness in Humans. FASEB J. 2010, 24, 220–224. [Google Scholar]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Zhang, X.; Miyamoto, J.; Kimura, I.; Taknaka, T.; Furusawa, K.; Jomori, T.; Fujimoto, K.; Uematsu, S.; Miki, T. Gut Carbohydrate Inhibits GIP Secretion via a Microbiota/SCFA/FFAR3 Pathway. J. Endocrinol. 2018, 239, 267–276. [Google Scholar] [CrossRef]


| Items | Content (%) |
|---|---|
| Ingredients | |
| Corn | 56.51 |
| Soybean meal | 35.52 |
| Soybean oil | 4.50 |
| Na Cl | 0.30 |
| Limestone | 1.00 |
| Ca HPO4 | 1.78 |
| d L-Met | 0.11 |
| Premix (1) | 0.28 |
| Total | 100.00 |
| Nutrient levels (2) | |
| ME/(MJ/Kg) | 12.73 |
| CP | 20.07 |
| Ca | 0.90 |
| AP | 0.40 |
| Lys | 1.00 |
| Met | 0.42 |
| Met + Cys | 0.78 |
| Treatments | ||||
|---|---|---|---|---|
| Item | TC | HT | SEM | p Value |
| IABW (g) | 1427.31 | 1432.10 | 19.35 | >0.05 |
| FABW (g) | 2840.22 a | 2567.78 b | 24.32 | <0.05 |
| ADG (g/d) | 80.58 a | 70.70 b | 5.03 | <0.05 |
| ADFI (g/d) | 157.51 a | 148.95 b | 7.16 | <0.05 |
| FCR (g/g) | 1.95 b | 2.11b a | 0.11 | <0.05 |
| CT (°C) | 41.49 b | 42.66 a | 1.28 | <0.05 |
| Level | Species Name | Treatments | SEM | p Value | |
|---|---|---|---|---|---|
| TC | HT | ||||
| Firmicutes (%) | 89.33 | 87.49 | 2.18 | >0.05 | |
| phylum | Bacteroides (%) | 5.69 | 5.18 | 0.74 | >0.05 |
| Actinomycetes (%) | 3.78 | 6.50 | 1.12 | >0.05 | |
| family | Ruminococcaceae (%) | 51.32 | 51.14 | 1.25 | >0.05 |
| Lachnospiraceae (%) | 18.68 a | 10.15 b | 0.80 | <0.05 | |
| Christensenellaceae (%) | 1.20 a | 0.51 b | 0.07 | <0.05 | |
| Peptococcaceae (%) | 0.21 b | 0.54 a | 0.05 | <0.05 | |
| Faecalibacterium (%) | 28.32 | 31.63 | 1.18 | >0.05 | |
| genus | Romboutsia (%) | 6.91 | 11.29 | 0.45 | >0.05 |
| Ruminococcus (%) | 0.08 b | 0.15 a | 0.02 | <0.05 | |
| Clostridium (%) | 2.55 b | 5.16 a | 0.40 | <0.05 | |
| Faecalibacterium (%) | 28.32 | 31.63 | 1.18 | >0.05 | |
| Item | Treatments | SEM | p Value | |
|---|---|---|---|---|
| TC | HT | |||
| acetate (μg/mL) | 572.9 b | 741.1 a | 16.79 | <0.05 |
| propionic acids (μg/mL) | 56.45 | 63.96 | 5.89 | >0.05 |
| butyric acids (μg/mL) | 262.9 | 252.7 | 29.71 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Li, X.; Zhou, Y.; Feng, J.; Zhang, M. Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers. Animals 2021, 11, 1286. https://doi.org/10.3390/ani11051286
Wang G, Li X, Zhou Y, Feng J, Zhang M. Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers. Animals. 2021; 11(5):1286. https://doi.org/10.3390/ani11051286
Chicago/Turabian StyleWang, Guangju, Xiumei Li, Ying Zhou, Jinghai Feng, and Minhong Zhang. 2021. "Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers" Animals 11, no. 5: 1286. https://doi.org/10.3390/ani11051286
APA StyleWang, G., Li, X., Zhou, Y., Feng, J., & Zhang, M. (2021). Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers. Animals, 11(5), 1286. https://doi.org/10.3390/ani11051286





