1. Introduction
Poultry nutrition is aiming at improving meat production in a cost-effective way while adhering to global strategies, such as animal welfare and reduction in feed antibiotics [
1,
2]. To that end, the development of appropriate interventions is performed in a collaborative way by producers, industry, and academia. Feed supplementation with enzymes has attracted attention since enzymes active on non-starch polysaccharides (NSP) are claimed to offset the anti-nutritive effect of dietary NSP from cereals and legumes [
3,
4,
5].
NSP are an indispensable part of poultry diets. Once ingested, NSP can be partially soluble or insoluble, depending on their botanical source, chemical structure, chain length, and association degree with the other cell wall components [
5,
6,
7]. Although not digestible by endogenous enzymes, NSP can influence feed use throughout the gastrointestinal tract (GIT) [
8,
9]. The anti-nutritive effect of soluble NSP (arabinoxylan: AX, β-glucan) has been attributed to their ability to increase digesta viscosity, thereby limiting the diffusion of digestive enzymes and nutrients [
10,
11,
12]. Furthermore, increased digesta viscosity may promote pathogen growth in the GIT [
10,
13]. In addition, insoluble NSP (AX, cellulose) can limit the accessibility of the host’s enzymes to nutrients enveloped by the cell wall and hinder digestion. NSP can potentially exert prebiotic properties, as they can be fermented by microbiota in the ceca into short-chain fatty acids (SCFA). SCFA can promote gut health and provide additional energy to the host, among others [
14,
15].
Several animal studies have demonstrated that exogenous NSP-degrading enzymes (NSPases) improved broiler performance [
12,
16,
17,
18,
19,
20,
21]. For instance, xylanases (EC 3.2.1.8) are hydrolytic enzymes that split the β-(1→4) bonds between unsubstituted xylosyl residues of the xylan backbone [
22]. The enzymatic conversion of AX to oligosaccharides (AXOS) with prebiotic potential finds various applications in the feed and food industry [
15,
22,
23]. Xylanases belonging to the glycosyl hydrolase (GH) families 10 and 11 are widely used to improve animal performance, alongside other NSPases, such as β-glucanases, mannanases, and galactosidases [
23,
24,
25]. Beta-glucanases target β-glucans and cellulose present in cereal, and their application in animal feed historically preceded that of xylanases [
4]. The enzymatic depolymerization of soluble AX and β-glucan has been linked with reduced intestinal viscosity and, consequently, improved animal performance [
12,
26]. Nevertheless, viscosity reduction is not the only mechanism involved.
NSPases have been reported to degrade NSP present within the intact cell wall. Such rupture of the cell wall may improve the digestibility of physically entrapped nutrients [
18,
27]. Additionally, the solubilization of polymeric AX and the release of arabinoxylan-oligosaccharides (AXOS) by NSPases has been linked with increased SCFA production in the broiler’s ceca and could contribute to the NSP’s prebiotic potential [
13,
15,
28,
29]. Yet, direct evidence of the in situ formation of potentially prebiotic AXOS remains elusive. To date, the potential of xylanase and glucanase to release entrapped nutrients and to form prebiotic oligosaccharides is still under investigation [
4]. Hence, further research is warranted to understand how the postulated prebiotic formation and encapsulated nutrients release may promote gut health and animal growth.
Therefore, it was hypothesized that dietary supplementation of broilers with carbohydrate-active enzymes would enhance carbohydrate fermentation and nutrient digestion. This research aims at determining the potential of dietary xylanase to form oligosaccharides in the upper gastrointestinal tract of broilers fed wheat- or maize-based finisher diets. We further aim to investigate how the enzymatic degradation of NSP may influence carbohydrate fermentation in the hindgut and nutrient digestion in the small intestine.
2. Materials and Methods
2.1. Diets
All experimental basal diets were manufactured by Research Diet Services B.V. (Wijk bij Duurstede, The Netherlands), as summarized in
Table 1. Acid-insoluble ash (Diamol; Franz Bertram GmbH, Hamburg, Germany) was added as a digestibility marker to the finisher diets.
The finisher diets consisted of two different basal diets (wheat or maize) and were provided in mash form as such (control treatment) or were supplemented with commercially available non-starch polysaccharide-degrading enzymes from
Trichoderma spp. (Huvepharma NV, Berchem, Belgium) (enzyme treatment). The enzymes present were a GH11 endo-1,4-β-xylanase (EC 3.2.1.8), added at 1500 EPU/kg feed (xylanase activity), and an endo-1,4-β-glucananase, added at 100 CU/kg feed (glucanase activity). EPU is defined as the amount of enzyme, which releases 0.0083 μmol of reducing sugars (xylose equivalent) per minute from oat spelt xylan at pH 4.7 and 50 °C. CU is defined as the amount of enzyme, which releases 0.128 μmol of reducing sugars (glucose equivalents) per minute from barley β-glucan at pH 4.5 and 30 °C. The above combinations resulted in four dietary treatments (DT); wheat control (WC), wheat enzyme (WE), maize control (MC), and maize enzyme (ME). The analyzed xylanase and glucanase activities of the enzyme-containing DT ranged between 1550 and 1740 EPU/kg feed and 190–240 CU/kg feed, respectively. The measured chemical composition of wheat-based and maize-based DT is shown in
Table 2.
All reagents used were of analytical grade. The water used throughout laboratory experiments was purified with a Milli-Q Integral 5 (Millipore Corp., Billerica, MN, USA) purification system.
2.2. Birds Management and Sample Collection
The study was performed at the facilities of the Laboratory for Animal Nutrition and Animal Product Quality (LANUPRO), Department of Animal Sciences and Aquatic Ecology, Ghent University (Belgium), and was conducted in accordance with the ethical standards and recommendations for accommodation and care of laboratory animals covered by the European Directive 2010/63/EU on the protection of animals used for scientific purposes and the Belgian royal decree KB29.05.13 on the use of animals for experimental studies. Birds were housed in one room throughout the study with 23L:1D and 18L:6D (18L from 4:00 a.m. to 10:00 p.m.) light schedule during day 0–7 and beyond, respectively. Room temperature was 34 °C at setting and linearly decreased to 22 °C by day 28. During the first 5 days, additional infra-red lamp heating (one per pen) was used. Ninety-six (96) one-day-old male broilers (Ross 308) (Vervaeke-Belavi; Tielt, Belgium) were wing-tagged and randomly assigned in two floor pens (48 birds/pen): one receiving wheat-based and one receiving maize-based diets, until day 20 of the experiment. The broilers were vaccinated on the first day of age against Newcastle disease and infectious bronchitis at the hatcheries facilities. At 18 days of age, the vaccination against Newcastle disease was repeated with Nobilis ND Clone 30 by spraying. After arrival, birds were fed the starter diets (day 0–10) and grower diets (day 10–20) ad libitum (
Table 1). On day 20, the birds were weighed and allocated according to body weight to pens with a wire floor so that the average body weight of birds in each pen was similar. The treatments (WC, WE, MC, and ME) were assigned to pens following a randomized block design. The blocking factor referred to the spatial organization in the facility. Each treatment consisted of 6 replicate pens, with each pen containing 4 birds. During the adaptation period (day 20–24), the birds received the finisher diets ad libitum. The birds were weighed in the morning of day 24 and then continued to be fed finisher diets until day 28. Feed intake was measured per pen and daily (morning of day 25, 26, 27, and 28). During this period, excreta were collected twice daily, homogenized, and an aliquot of a minimum of 250 g fresh material per pen was immediately stored at −20 °C. On day 28, all birds were weighed and euthanized by cervical dislocation followed by bleeding. The gizzard, ileum, and ceca contents were collected, pooled per pen, and frozen at −20 °C. Thawed aliquots were used for the determination of dry matter, ash, and acid-insoluble ash content. Frozen digesta were dried by lyophilization and homogenized with a MM 400 Mixer Mill (Retsch GmbH, Haan, Germany) prior to other chemical analyses. Feed samples were ground to pass a 0.7 mm sieve using a ZM200 mill (Retsch GmbH) prior to analysis.
2.3. Proximate Composition Analysis
2.3.1. Dry Matter, Ash, and Acid-Insoluble Ash Content
Feed samples and thawed aliquots from the gizzard, ileum, and excreta were dried in an air oven at 80 °C, overnight. Subsequently, the dry matter content was determined by drying at 103 °C, according to the AOAC 935.29 method [
30]. For that purpose, approximately 5 g feed samples and 1–2 g digesta were weighed in ceramic crucibles. Next, ash and acid-insoluble ash (AIA) contents were determined sequentially, according to the method described by Van Keulen and Young (1977) [
31] with certain modifications introduced by Montaño-Vargas et al. (2002) [
32], allowing the reduction in sample size. In brief, dried samples were incinerated at 575 °C, and the resulting ash was weighed and boiled with 10 mL 4 N HCl and filtered through ashless filter paper. The retentate was incinerated again at 575 °C, and the remaining AIA was weighed. The organic matter (OM) was calculated by subtracting ash from dry matter.
2.3.2. Cecal Dry Matter
Due to sample limitations, the dry matter and ash content of cecal digesta were determined gravimetrically using an XP6 Excellence Plus Micro Balance (5 decimals) Mettler-Toledo International Inc., Columbus, OH, USA). Approximately 2 mg of fresh cecal matter was weighed in Eco-Cup LF pyrolysis cups (Frontier Laboratories Ltd., Fukushima, Japan) and were incubated at 80 °C, overnight. Next, the samples were incubated at 103 °C for 4 h and weighed. The ash content was determined by incinerating the dried samples at 575 °C and weighing the remaining material.
2.3.3. Crude Protein Content
The nitrogen content of feed samples and digesta was determined according to the AOAC 990.03 method [
30] using a FlashEA
® 1112 NC Analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The nitrogen conversion factor used to estimate the crude protein was 6.25.
2.4. Carbohydrate Analysis
2.4.1. Sugar Composition
The total sugar composition of feed and digesta samples was determined according to Englyst and Cummings (1984) [
33]. Samples were pre-hydrolyzed in 72% (
w/
w) H
2SO
4 (30 °C, 1 h) and subsequently hydrolyzed in 1 M H
2SO
4 (100 °C, 3 h). Neutral monosaccharides released were derivatized to alditol acetates and analyzed by gas chromatography on a Trace 1300 GC system (Thermo Fisher Scientific Inc.) equipped with a DB-225 column (Agilent Technologies Inc., Santa Clara, CA, USA) and a flame/ionization detector (FID), using inositol as internal standard. Uronic acid content was determined by the colorimetric
m-hydroxyphenyl assay with an automated analyzer (Skalar Analytical B.V., Breda, The Netherlands), according to Blumenkrantz and Asboe-Hansen (1973) and Thibault and Robin (1975) [
34,
35].
2.4.2. Total Starch Content
The total starch content of feed and digesta samples was determined according to the AOAC Method 996.11 (KOH format) [
36] using the Total Starch Assay Kit (Megazyme, Bray, Ireland) as modified by Martens et al. (2018) [
37]. In brief, 25 μL supernatant of enzymatically treated samples was transferred in the wells of a 96 well plate followed by the addition of 225 μL glucose oxidase peroxidase (GOPOD) reagent (Megazyme). The reaction was performed in a shaking incubator at 50 °C for 20 min, and the absorbance at 520 nm was read against reagent blank using a Tecan Infinite
® F500 (Tecan Group Ltd., Männedorf, Switzerland) spectrophotometer. The glucose (Glc) content was determined using a Glc calibration curve (0.1–0.6 mg/mL).
2.4.3. Oligosaccharide Characterization by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS)
The structural characterization of oligosaccharides present in the ileum was performed according to Broxterman et al. (2017) [
38] on an ultrafleXtreme
TM MALDI-TOF/TOF mass spectrometer (Bruker Daltonics Inc., Billerica, MA, USA). The equipment was controlled with FlexControl 3.3 software and operated in positive mode. The mass spectrometer was calibrated with maltodextrins (Avebe, Veendam, The Netherlands) in a mass range of 500–3000 (
m/
z). Approximately 100 mg of dried ileal digesta was suspended in 1 mL water and incubated at 99 °C for 30 min. The supernatant was then separated by centrifugation at 20,000×
g for 10 min, diluted ten times with water, and desalted with Dowex 50W-X8 resin (Sigma-Aldrich, St. Louis, MO, USA). Next, an aliquot (100 μL) was removed, and NaCl was added at 1 μM to allow the formation of sodium adducts during ionization. Afterward, sample (1 μL) was co-crystallized with matrix solution (1 μL); 25 mg/mL dihydroxy-benzoic acid (Sigma-Aldrich) in 50% (
v/
v) acetonitrile (VWR International B.V., Amsterdam, The Netherlands) on a target plate under a stream of dry air.
2.5. Microbial Metabolites Analysis
2.5.1. Short-Chain Fatty Acids (SCFA)
The SCFA content of ileal and cecal digesta was determined by gas chromatography (GC-FID), as described by Logtenberg et al. (2020) [
39]. An aqueous solution of acetic, butyric, propionic, isobutyric, and isovaleric acids (Sigma-Aldrich) was prepared for quantification. The standard solution was diluted to obtain final concentrations in the range of 0.01–1.0 mg/mL and was treated similarly to the samples.
2.5.2. Lactic and Succinic Acids
The concentrations of lactic and succinic acids in ileal and cecal samples were determined by high-performance liquid chromatography (HPLC), according to Jonathan et al. (2012) [
40]. The samples were analyzed with an Ultimate 3000 HPLC System (Dionex Corp., Sunnyvale, CA, USA) equipped with an Aminex HPX-87 H column (Bio-Rad, Richmond, VA, USA) and a guard column. The HPLC system was coupled to a Shodex RI-101 refractive index detector (Showa Denko KK, Kawasaki, Japan). The samples (injection volume 10 μL) were run isocratically using 5 mM H
2SO
4 as eluent at 0.6 mL/min flow rate, with column temperature at 40 °C. A standard solution containing lactic and succinic acid (Sigma-Aldrich) was prepared for quantification and was diluted to obtain final concentrations in the range of 0.1–10.0 mg/mL.
2.6. Calculations
The apparent ileal digestibility (AID) and apparent total tract digestibility (ATTD) of organic matter, starch, and protein were estimated with Equation (1), using AIA as an indigestible marker [
20]:
where NT
d, NTi, NT
e is the measured nutrient content (% DM) in the diet, ileum, and excreta, respectively, and AIA
d, AIA
i, AIA
e is the measured marker content (% DM) in the diet, ileum, and excreta. NT
i and AIA
i, and NT
e and AIA
e were used to determine AID and ATTD, respectively.
The recovery of NSP in the ileum and excreta was determined through the constituting monosaccharides. For that reason, the recovery of xylose, arabinose, galactose, uronic acid, and non-glucosyl NSP (NGP) was estimated using Equation (2):
where M
d, Mi, M
e is the measured monosaccharide content (% DM) in the diet, ileum, and excreta, respectively, and AIA
d, AIA
i, AIA
e is the measured marker content (% DM) in the diet, ileum, and excreta. M
i and AIA
i, and M
e and AIA
e were used to determine recovery in the ileum and excreta, respectively.
2.7. Statistical Analysis
The obtained data were subjected to analysis of variance (ANOVA) using the R statistical software (R Core Team), with the pen being the experimental unit. The observations from wheat-based and maize-based DT were modeled separately. The effect of enzyme treatment (E; control vs. enzyme) on carbohydrate content and microbial metabolites was determined. Nutrient digestibility and NSP recovery were modeled using E and Sampling Site (S: ileum for AID and excreta for ATTD) as main effects, including their two-way interaction term. The blocking factor was considered as the main effect in the model. To test the significance of the differences between different treatments, Tukey’s post-hoc test was performed, with a significance threshold set at p < 0.05.
The data obtained for NSP content and recovery along the GIT, SCFA content in the ceca, nutrient digestibility, and animal growth were subjected to principal component analysis (PCA) using R. Next, the Pearson correlation coefficients of the aforementioned variables were calculated, and the corresponding correlation matrix was constructed to visualize the relations. Correlations with p < 0.05 were considered significant.
3. Results
3.1. Growth Parameters
The growth of broilers was recorded during the finisher period (day 24–28) in order to evaluate the possible effect of enzyme addition on the broiler’s nutritional responses (
Table A1). It should be noted that the first aim of this experiment was not to evaluate the effect of the enzyme on animal performance. Therefore, the measured growth parameters are approached with caution and only considered in the context of this study. The obtained values for body weight (BW), average daily gain (ADG), and average daily feed intake (ADFI) were lower, and the feed conversion ratio (FCR) was higher than breed performance objectives for 28-day-old male Ross 308 broilers [
41], mainly because broilers were fed mash diets. Overall, WE presented increased BW (6% higher;
p = 0.021), ADG (14% higher;
p = 0.059) and ADFI (6% higher;
p = 0.281) values compared to WC, while FCR decreased by 7% (
p = 0.018). ME presented numerically positive responses compared to MC, but not to the extent observed in the wheat-based DT. For example, ME presented increased BW (3% higher;
p = 0.136), ADG (6% higher;
p = 0.320) and ADFI (4% higher;
p = 0.285) values compared to MC, while FCR was decreased by 2% (
p = 0.498).
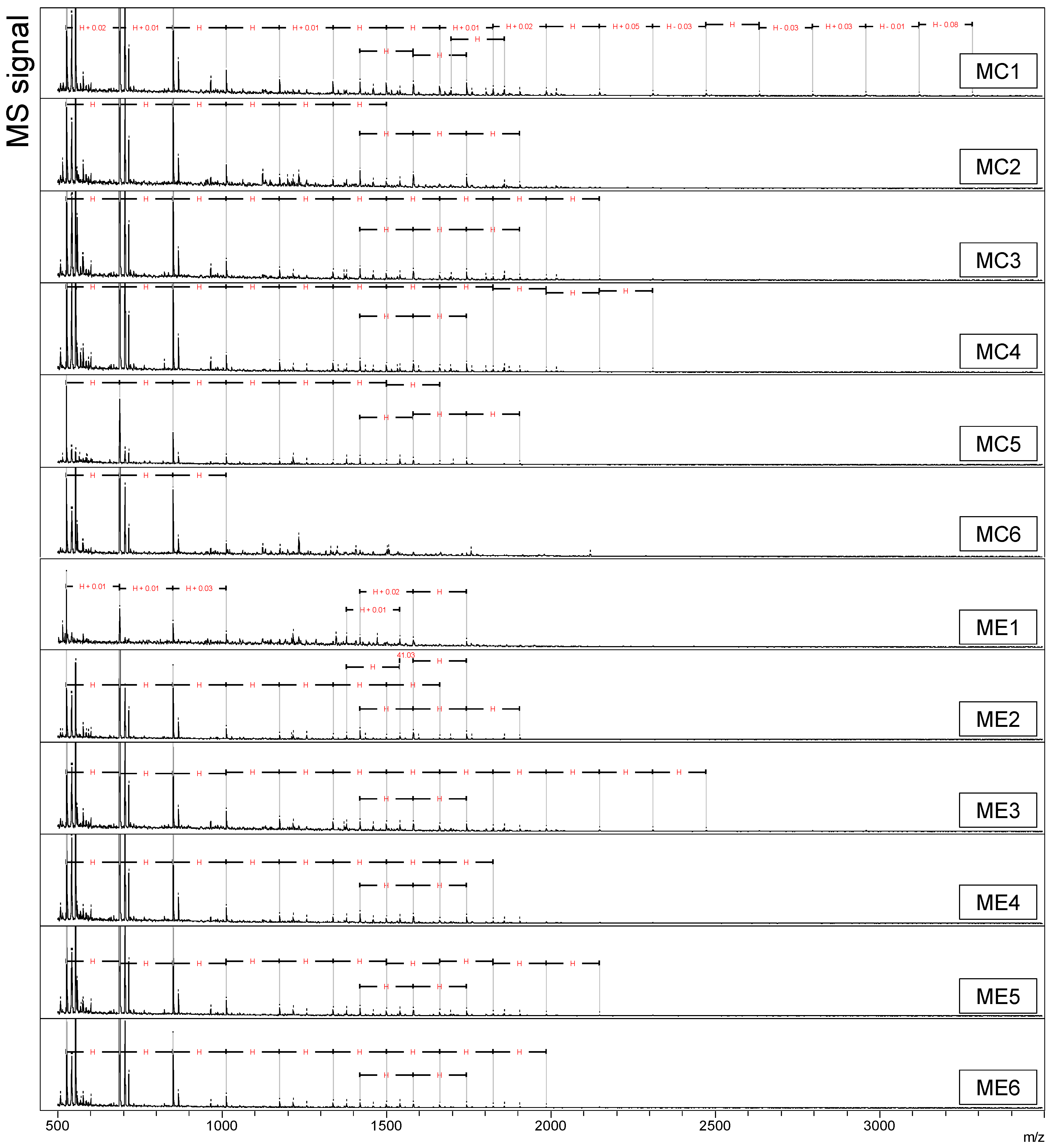
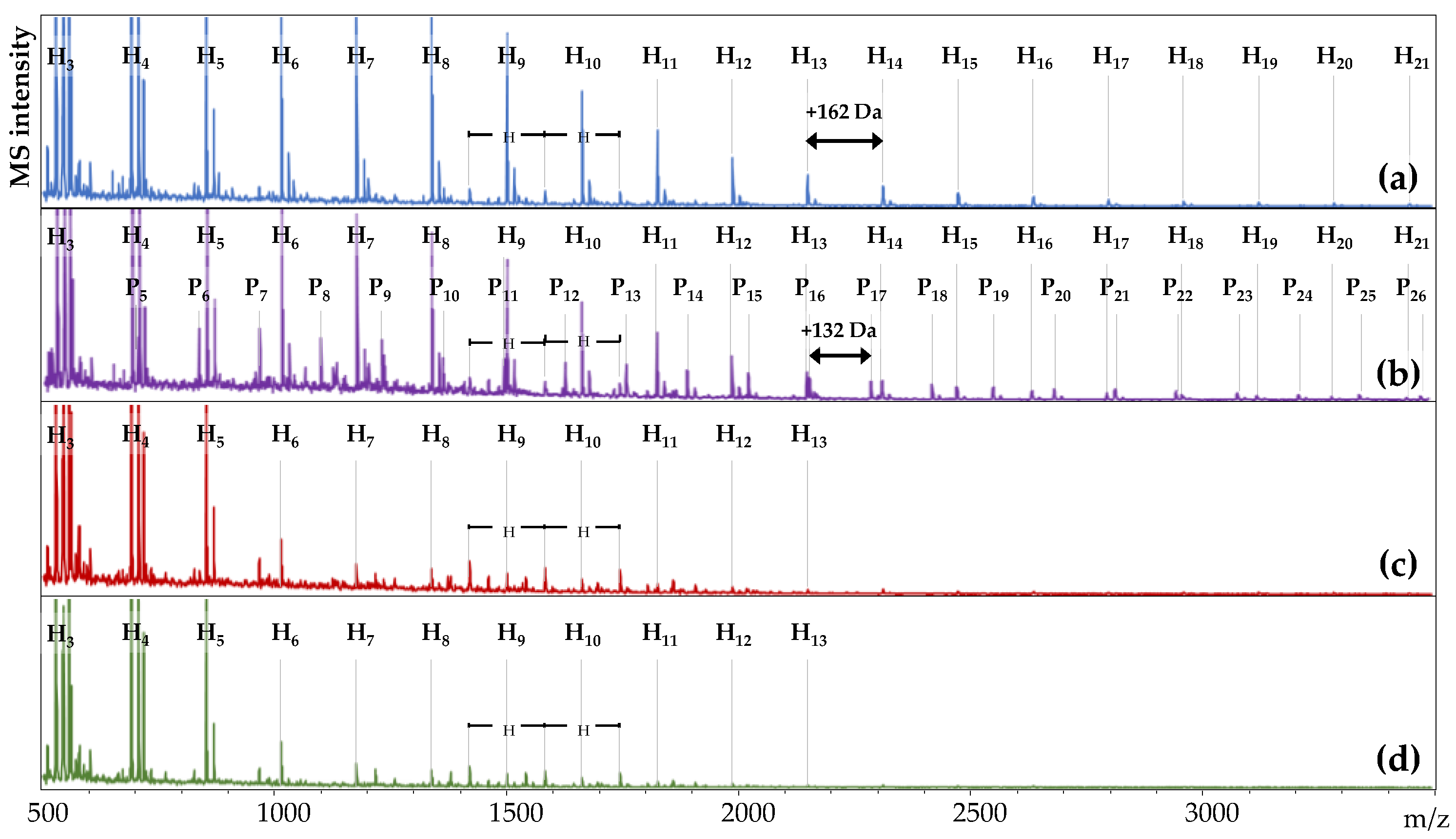
3.2. Oligosaccharide Profiles in Ileal Digesta
The addition of dietary xylanase is hypothesized to degrade polymeric arabinoxylan (AX) to oligosaccharides (AXOS) during feed digestion, and these products are expected to be released in solution. The ability of dietary xylanase to form oligosaccharides was determined by MALDI-TOF-MS analysis of the water-soluble fraction of ileal digesta from the four DT (
Figure 1,
Figure A1 and
Figure A2).
At first glance,
m/
z values corresponding to homologous series of hexose oligomers were abundantly detected in all samples (sequential increments of
m/
z 162). The hexose oligomers in both wheat-based DT had a polymerization degree (DP) of 3 to 21 (
Figure 1a,b). Hexose oligomers of DP 3 to 10 were detected in both maize-based DT (
Figure 1c,d). Another series of three oligomers with two consecutive
m/
z 162 increments (
m/
z 1419, 1581, and 1743) was present in all four DT. Alongside these compounds, a homologous series with increments of
m/
z 132 was detected in WE, representing pentose oligomers (
Figure 1b). The pentose oligomers were detected in all six replicate pens (
Figure A1) between
m/
z 701 and
m/
z 3444 and presented DP 5–26. The pentose oligomers were unique for the WE treatment and were absent in WC, MC, and ME.
3.3. Monosaccharide Contents in Digesta
In order to evaluate the effect of enzyme addition on the carbohydrate content present in digesta, the monosaccharide content after acid hydrolysis of all carbohydrates present in the finisher diets, gizzard, ileum, ceca, and excreta was determined (
Table 2,
Table 3 and
Table A2). Glucose (Glc) was the most abundant monosaccharide in all diets, followed by xylose (Xyl), arabinose (Ara), galactose (Gal), and uronic acids (UA) (
Table 2). Mannose (Man), rhamnose (Rha) and fucose (Fuc) were present in the diets at values lower than 0.6%, 0.1% and 0.2% (
w/
w), respectively (data not shown). Man, Rha and Fuc were taken into account when estimating the total carbohydrate contents but will not be further discussed due to their low amounts.
Gizzard: Glc was the main carbohydrate present in the gizzard and ranged between 33.7% (
w/
w) and 38.5% (
w/
w) (
Table 3). In wheat-based DT, Xyl was the second most abundant carbohydrate (7.8–8.9% (
w/
w)), followed by Ara, Gal and UA. WC presented significantly higher Glc content than WE (
p = 0.014). At the same time, WC presented lower Ara (
p = 0.043), Xyl (
p = 0.051), Gal (
p = 0.001), UA (
p = 0.010) and non-glucosyl NSP (NGP) (
p = 0.013) contents than WE. No differences in total carbohydrates (
p = 0.203) and A/X ratio (
p = 0.230) were observed between WC and WE. MC and ME presented similar monosaccharide contents in the gizzard (
p > 0.05).
Ceca: The Xyl content in the ceca significantly decreased upon enzyme addition (p = 0.036), from 0.6% (w/w) in WC to 0.2% (w/w) in WE. The Ara content showed a trend to decrease upon enzyme addition (p = 0.059) from 0.3% (w/w) in WC to 0.2% (w/w) in WE. The decrease in Ara and Xyl contents coincided with a significantly higher A/X ratio in WE (1.11) compared to WC (0.64) (p = 0.005). The Xyl content in the ceca for the maize-based DT was found to be lower than 0.1% (w/w), while higher A/X values (MC: 2.41, ME: 2.52) than in the wheat-based DT were obtained. ME presented significantly lower NGP content than MC (p = 0.036). It should be mentioned that the cecal samples contained 1.1–1.3% (w/w) rhamnose (data not shown). Since this monosaccharide was only present in trace amounts in the diets, it is suspected to originate from the bacterial cell wall.
Ileum and excreta: Carbohydrates accounted for approximately 44.9–48.8% (
w/
w) of the solids present in the ileum (
Table A2). Glc was the most abundant carbohydrate, followed by Xyl, Gal, Ara, and UA. The carbohydrate content in the excreta was somewhat lower than in the ileum (35.0–37.1% (
w/
w)) (
Table A2). Glc was the most abundant carbohydrate, followed by Xyl, Ara, Gal, and UA. To further investigate the transit and fermentability of NSP and individual polymers in the GIT, the recovery values of individual carbohydrates in the ileum and excreta (Equation (2)) were determined and are shown next.
3.4. Recovery of NSP in the Ileum and the Total Tract
The transit and fermentability of NSP and individual polymers in the GIT were studied by estimating the recovery values of Ara, Xyl Gal, UA, and NGP in the ileum and excreta (total tract) (
Table 4). The absence of significant interactions between enzyme (E) and sampling site (S: ileum, total tract) suggested that the effect of the enzyme was independent of the sampling site for both wheat-based and maize-based DT.
In the wheat-based DT, the sampling site significantly influenced the recovery values of all measured monosaccharides (p < 0.05). Significantly lower values were obtained in the total tract compared to the ileum in all cases (p < 0.05). Enzyme addition significantly affected the recovery of Ara (p = 0.022) and Xyl (p = 0.025). Ara recoveries in the ileum were close to 100% for both WC and WE. Furthermore, 94.8% and 88.5% of the Xyl present in the diet was recovered in the ileum for WC and WE, respectively. The differences observed between WC and WE regarding the Ara and Xyl recovery in the ileum were not significant (Ara: p = 0.130, Xyl: p = 0.214). Nevertheless, the Ara and Xyl values in WE tended to be lower than in WC by 6.3% and 6.7%, respectively. Similarly, the total tract recoveries of Ara and Xyl obtained in WE tended to be lower than the ones obtained in WC (4.3% and 5.9% lower, respectively) but not significantly different (p = 0.459 and p = 0.628, respectively).
In maize-based DT, the sampling site significantly influenced the Gal (p < 0.001) and NGP (p = 0.011) recovery values, with lower values being obtained in the total tract compared to the ileum. On the contrary, there was no significant effect of sampling site on Ara (p = 0.743), Xyl (p = 0.111) and UA (p = 0.912) recovery. The ileal and total tract recoveries of Xyl, Ara, and UA were similar, fluctuating around 100% of the constituent monosaccharides present in the diet. No significant effect of enzyme addition was observed in all cases (p > 0.05).
3.5. Lactate, Succinate, and Short-Chain Fatty Acids (SCFA) Contents in the Ileum and the Ceca
The formation of lactate, succinate, and SCFA in the broiler’s ileum and ceca was determined to monitor the effect of enzyme supplementation on the fermentation processes along the GIT (
Table 5). Lactate was the most abundant metabolite in the ileum (129.4–250.2 μmol/g dry matter basis), while acetate and succinate contents ranged between 2.5 and 9.9 μmol/g. Acetate (172.7–354.5 μmol/g) and butyrate (53.1–78.5 μmol/g) were the two most abundant SCFA in the ceca, followed by propionate (11.0–31.4 μmol/g). Isobutyrate and isovalerate were detected in the ceca in considerably lower amounts (1.3–4.3 μmol/g) for all DT.
In the wheat-based DT, enzyme addition significantly increased acetate (p = 0.004) and succinate (p = 0.013) contents in the ileum. However, it did not significantly affect lactate contents (p = 0.163), even though the value obtained in WE was 1.9 times higher than in WC. The reason behind the lack of significance could be the high variation in individual values. Furthermore, enzyme addition significantly increased the contents of acetate (p = 0.014), butyrate (p = 0.044) and total SCFA (p = 0.019) in the ceca. No significant influence of enzyme addition was observed in the contents of propionate (p = 0.906), isobutyrate (p = 0.728) and isovalerate (p = 0.881).
In the maize-based DT, enzyme addition showed a trend to decrease lactate formation in the ileum (p = 0.057), but did not impact acetate (p = 0.195) and succinate (p = 0.425) contents. In the ceca, enzyme addition was found to significantly decrease the contents of acetate (p = 0.037), butyrate (p = 0.010), propionate (p = 0.039) and total SCFA (p = 0.021), while it did not impact the contents of isobutyrate (p = 0.185) and isovalerate (p = 0.333).
Overall, enzyme addition was found to impact the bacterial metabolite formation differently in wheat-based and maize-based DT, highlighting the importance of the cereal type present for hindgut fermentation.
3.6. Nutrient Digestibility
The impact of enzyme action on nutrient (organic matter: OM, starch, and crude protein: CP) digestion in the small intestine and OM and starch fermentation in the hindgut is presented in
Table 6. The apparent ileal digestibility (AID) values obtained were between 72.2 and 75.3% for OM, 94.8–97.5% for starch, and 77.0–81.2% for CP. The apparent total tract digestibility (ATTD) values obtained ranged between 73.3 and 75.4% for OM and 96.0–98.2% for starch.
A significant enzyme (E) and sampling site (S) interaction (p = 0.036) was observed only for OM in the wheat-based DT. Firstly, the pair-wise comparison between the OM-AID and ATTD values of WC and WE revealed that WC-ATTD was significantly higher than WC-AID (p = 0.014). However, similar values between WE-AID and WE-ATTD were obtained (p = 0.995). Secondly, WE-AID was significantly higher than WC-AID (p < 0.001). Lastly, WE-ATTD showed a trend to be higher than WC-ATTD (p = 0.074).
Enzyme (E) significantly impacted starch digestibility (p = 0.001). In particular, starch WE-AID was significantly higher than WC-AID (p = 0.004). No significant differences were found between WE-AID and WE-ATTD (p = 0.998). Similarly, no significant differences were found between WC-AID and WC-ATTD (p = 0.255). Lastly, WC-ATTD was similar to WE-AID (p = 0.185) and WE-ATTD (p = 0.248) as well. The similarity of WE-AID and WE-ATTD with WC-ATTD, but not with WC-AID, suggests that the non-significant increase of 1.3% as observed in starch digestibility for WC between the ileum and the total tract could have biological relevance. Finally, enzyme addition significantly increased CP-AID (p = 0.002). The nitrogen content in excreta was not corrected for endogenous secretions, and the CP ATTD values were not estimated.
No significant E × S interactions were observed in the maize-based DT (p > 0.05). Moreover, enzyme addition did not affect significantly OM (p = 0.757), starch (p = 0.384) or CP (p = 0.102) digestibility. Although the sampling site had a significant effect on OM (p = 0.033) digestibility, the individual AID and ATTD values of MC and ME were similar (p > 0.05). Next, starch digestibility was significantly affected by the sampling site (p = 0.001), with higher values being obtained in the total tract compared to the ileum. In particular, MC-ATTD was significantly higher than MC-AID (p = 0.032). ME-AID was not significantly different from MC-AID (p = 0.766), but at the same time was similar to both MC-ATTD (p = 0.242) and ME-ATTD (p = 0.148) values. Hence, a subtle improvement in starch AID due to enzyme addition in maize-based DT could still be of biological importance.
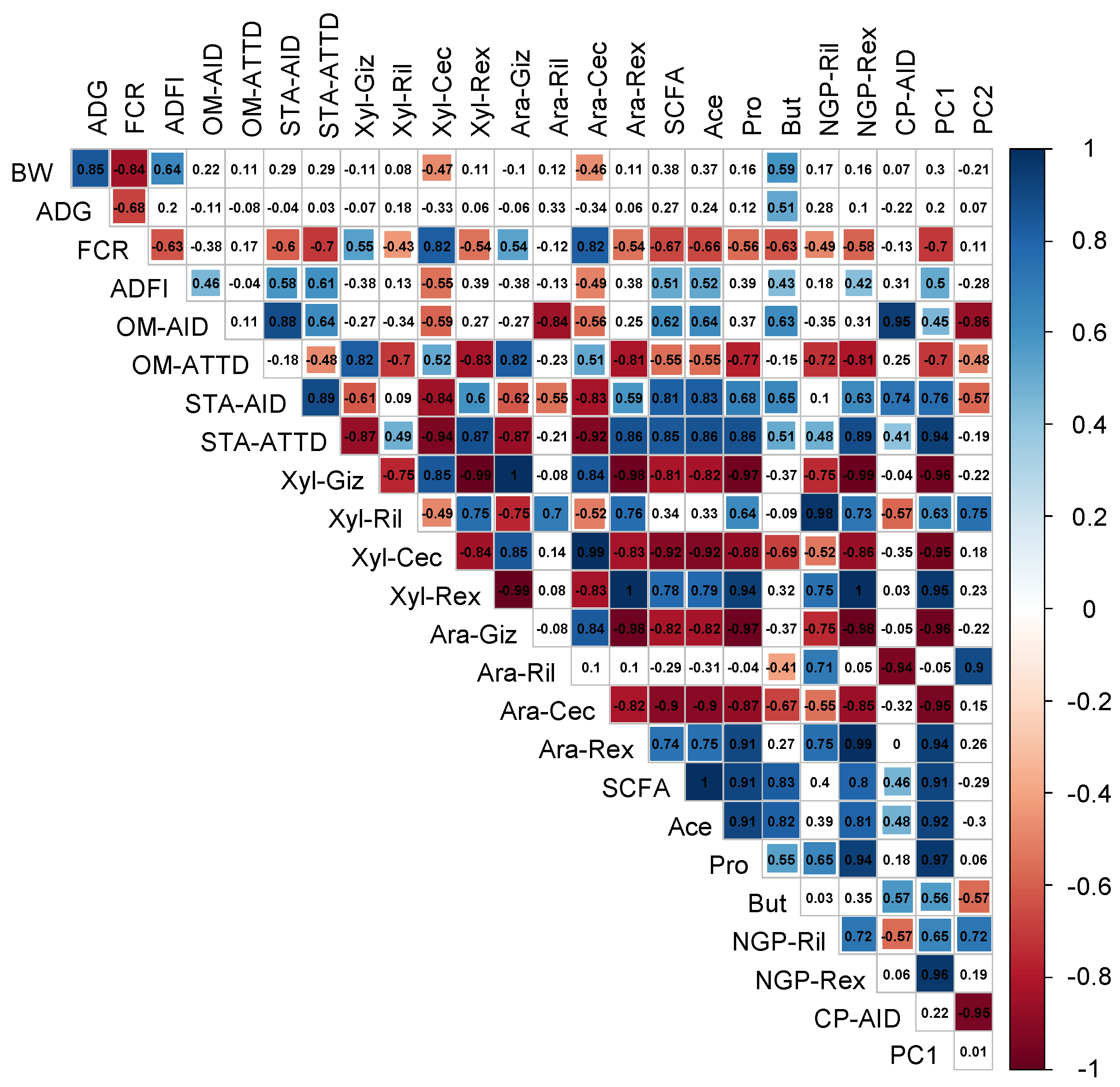
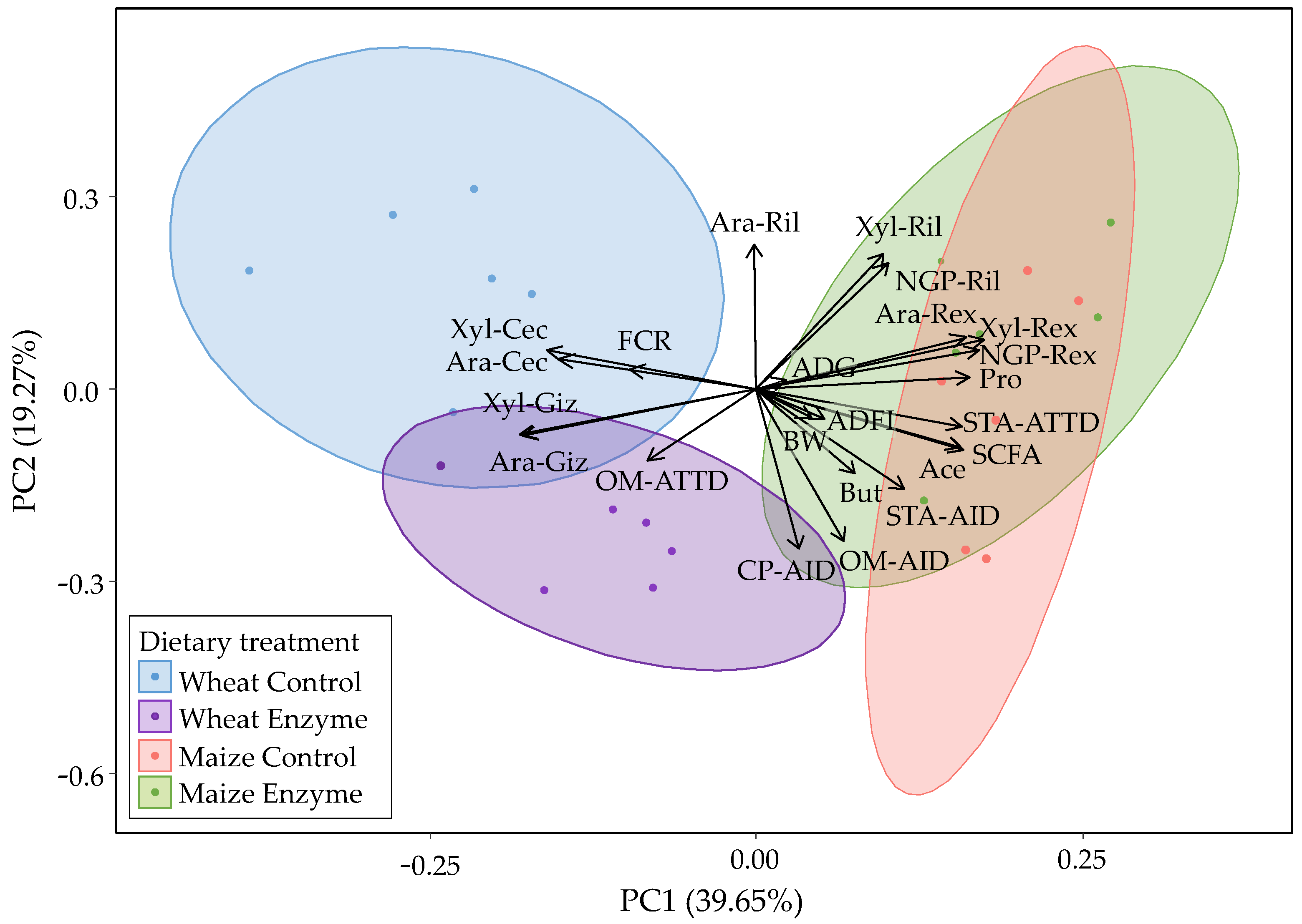
3.7. Interrelationships between Nutrient Digestibility, NSP Fermentation in the Hindgut and Growth Parameters
Principal component analysis was performed to obtain an overview of the response of the different dietary treatments to the investigated parameters (
Figure 2).
The first principal component (PC1) explained 39.65%, and the second principal component (PC2) explained 19.27% of the total variance. Overall, PC1 appeared to separate the wheat-based from the maize-based DT while WC and WE were further separated by PC2. WC presented high Ara and Xyl contents in the cecum (Ara-Cec, Xyl-Cec) and high FCR values. WE formed a separate cluster from WC mainly due to the higher Xyl-Giz, Ara-Giz, OM-AID, OM-ATTD, and CP-AID loadings. The maize-based DT were clustered together and presented differences compared to both WC and WE. Both MC and ME were characterized by high Ara, Xyl, and NGP recovery in the ileum (Ril) and excreta (Rex).
The potential interrelationships between the investigated parameters were then examined (
Figure 2 and
Figure A3). Organic matter (OM) AID was negatively correlated with Ara-Cec and Xyl-Cec and with Ara-Ril. On the contrary, OM-ATTD presented positive correlations with Ara and Xyl contents in the gizzard (Giz) and the ceca, while it was negatively correlated with their ileal and total tract recoveries. Starch AID and ATTD were negatively correlated with Ara and Xyl contents in the gizzard and the ceca and were positively correlated with Ara and Xyl recovery in the excreta. The SCFA were negatively correlated with Ara and Xyl contents in the gizzard and the ceca but were positively correlated with Ara, Xyl recovery in the excreta (Rex), and starch ATTD. While unexpected, the positive correlation between Ara and Xyl total tract recovery and SCFA content was due to the maize-based DT, as those treatments presented high values for both sets of parameters. High SCFA loadings were positively correlated with improved animal growth. Improved animal growth was illustrated by high loadings of BW, ADFI, ADG, and low FCR loadings. Finally, the Ara and Xyl contents in the ceca were negatively correlated with animal growth.