The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Statistical Analysis
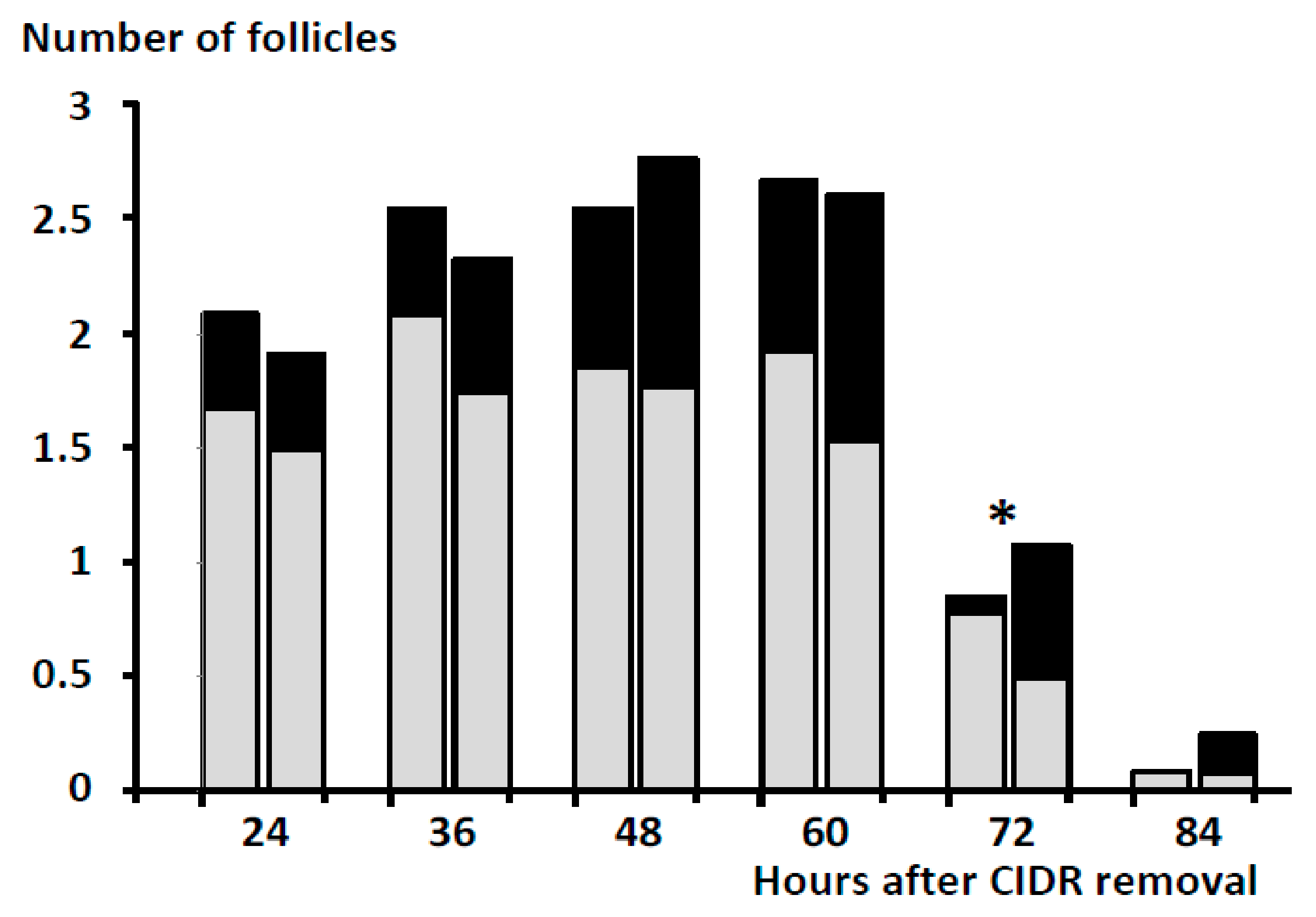
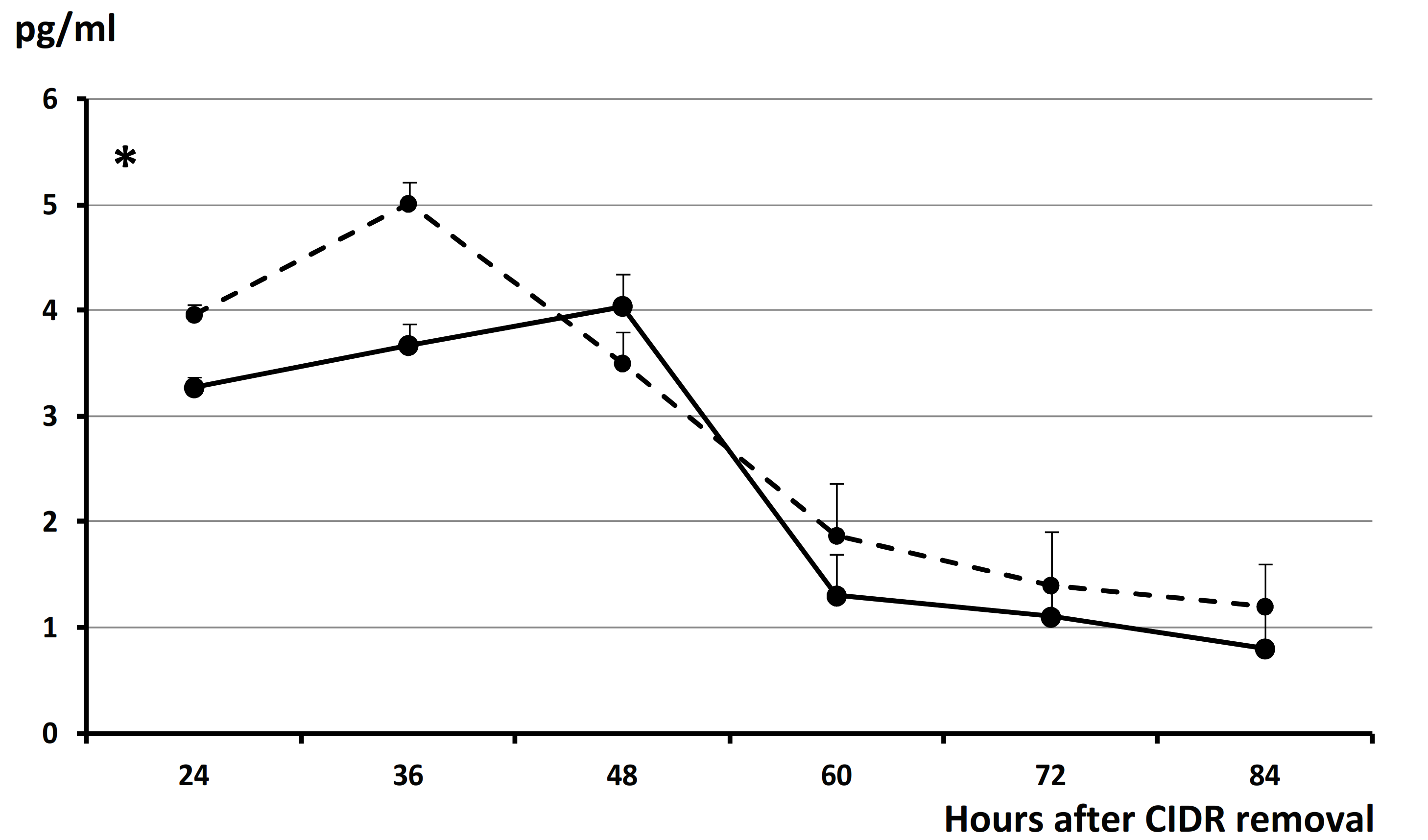
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abecia, J.A.; Forcada, F.; Gonzalez-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Menchaca, A.; Martin, G.B.; Martinez-Ros, P. Seventy years of progestagen treatments for management of the sheep oestrous cycle: Where we are and where we should go. Reprod. Fertil. Dev. 2020, 32, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ros, P.; Gonzalez-Bulnes, A. Efficiency of CIDR-based protocols including GnRH instead of eCG for estrus synchronization in sheep. Animals 2019, 9, 146. [Google Scholar] [CrossRef]
- Santos-Jimenez, Z.; Martinez-Herrero, C.; Encinas, T.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Comparative efficiency of oestrus synchronization in sheep with progesterone/eCG and progesterone/GnRH during breeding and non-breeding season. Reprod. Dom. Anim. 2020, 55, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Santos-Jimenez, Z.; Guillen-Gargallo, S.; Encinas, T.; Berlinguer, F.; Veliz-Deras, F.G.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Use of propylene-glycol as a cosolvent for GnRH in synchronization of estrus and ovulation in sheep. Animals 2020, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Lapthorn, A.J.; Harris, D.C.; Littlejohn, A.; Lustbader, J.W.; Canfield, R.E.; Machin, K.J.; Morgan, F.J.; Isaacs, N.W. Crystal structure of human chorionic gonadotropin. Nature 1994, 369, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kinser, A.R.; Gibson, M.F.; Vincent, D.L.; Scheffrahn, N.S.; Kesler, D.J. Ovarian responses of seasonally anestrous ewes administered progesterone, PMS, hCG and(or) GnRH. Theriogenology 1983, 19, 449–464. [Google Scholar] [CrossRef]
- Diaz, T.; Schmitt, E.J.; Sota, R.L.; Thatcher, M.J.; Thatcher, W.W. Human chorionic gonadotropin-induced alterations in ovarian follicular dynamics during the estrous cycle of heifers. J. Anim. Sci. 1998, 76, 1929–1936. [Google Scholar] [CrossRef]
- Zamiri, M.J.; Hosseini, M. Effects of human chorionic gonadotropin (hCG) and phenobarbital on the reproductive performance of fat-tailed Ghezel ewes. Small Rumin. Res. 1998, 30, 157–161. [Google Scholar] [CrossRef]
- Dias, L.M.K.; Sales, J.N.S.; Viau, P.; Barros, M.B.P.; Nicolau, S.S.; Simões, L.M.S.; Alves, N.G.; Alonso, M.A.; Valentim, R.; Oliveira, C.A. Although it induces synchronized ovulation, hCG reduces the fertility of Santa Ines ewes submitted to TAI. Arq. Bras. Med. Veterinária Zootec. 2018, 70, 122–130. [Google Scholar] [CrossRef]
- Martinez-Ros, P.; Rios-Abellan, A.; Gonzalez-Bulnes, A. Influence of progesterone-treatment length and eCG administration on appearance of estrus behavior, ovulatory success and fertility in sheep. Animals 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Uriol, M.; Martinez-Ros, P.; Rios, A.; Encinas, T.; Gonzalez-Bulnes, A. Onset of oestrus and periovulatory events in sheep exposed to 5 and 14 days of CIDR treatment with and without eCG. Reprod. Dom. Anim. 2019, 54, 1489–1492. [Google Scholar] [CrossRef]
- Baird, D.T. Factors regulating the growth of the preovulatory follicle in sheep and human. J. Reprod. Fert. 1983, 69, 343–352. [Google Scholar] [CrossRef] [PubMed]
- González-Bulnes, A.; García-García, R.M.; Castellanos, V.; Santiago-Moreno, J.; Ariznavarreta, C.; Domínguez, V.; López-Sebastián, A.; Tresguerres, J.A.; Cocero, M.J. Influence of maternal environment on the number of transferable embryos obtained in response to superovulatory FSH treatments in ewes. Reprod. Nutr. Dev. 2003, 43, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K.; Mann, G.E.; McNeilly, A.S.; Baird, D.T. The pattern of ovarian inhibin estradiol and androstenodione secretion during the estrous cycle of the ewe. Endocrinology 1990, 127, 227–235. [Google Scholar] [CrossRef]
- Ireland, J.J.; Roche, J.F. Development of nonovulatory antral follicles in heifers: Changes in steroids in follicular fluid and receptors for gonadotrophins. Endocrinology 1983, 112, 150–156. [Google Scholar] [CrossRef]
- Kruip, T.A.M.; Dieleman, S.J. Macroscopic classification of bovine follicles and its validation by micromorphological and steroid biochemical procedures. Reprod. Nutr. Dev. 1982, 22, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Rouillier, P.; Guilbault, L.A.; Lussier, J.G.; Matton, P. Changes in morphological appearance and functional capacity of recruited follicles in cows treated with FSH in the presence or absence of a dominant follicle. Theriogenology 1996, 46, 1053–1061. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Gonzalez-Bulnes, A.; Tresguerres, J.A.; Dominguez, V.; Ariznavarreta, C.; Cocero, M.J. Causes, characteristics and consequences of anovulatory follicles in superovulated sheep. Domest. Anim. Endocrinol. 2006, 30, 76–87. [Google Scholar] [CrossRef]
- Eppleston, J.; Evans, G.; Roberts, E.M. Effect of time of PMSG and GnRH on the time of ovulation, LH secretion and reproductive performance after intrauterine insemination with frozen ram semen. Anim. Reprod. Sci. 1991, 26, 227–237. [Google Scholar] [CrossRef]
- Sartori, R.; Fricke, P.M.; Ferreira, J.C.P. Follicular deviation and acquisition of ovulatory capacity in bovine follicles. Biol Reprod. 2001, 65, 1403–1409. [Google Scholar] [CrossRef]
- Revah, I.; Butler, W.R. Prolonged dominance of follicles and reduced viability of bovine oocytes. J. Reprod. Fertil. 1996, 106, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.; Curran, N.; Hyttel, P.; Knight, P.G.; Boland, M.P.; Roche, J.F. Effect of dominant follicle persistence on follicular fluid estradiol and inhibin and on oocyte maturation in heifers. J. Reprod. Fertil. 1999, 116, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Palencia, P.; Sanchez, M.A.; Nieto, A.; Vilar, M.P.; Gonzalez, M.; Veiga-Lopez, A.; Gonzalez-Bulnes, A.; Flores, J.M. Sex steroid receptor expression in the oviduct and uterus of sheep with estrus synchronized with progestagen or prostaglandin analogues. Anim. Reprod. Sci. 2007, 97, 25–35. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, I.; Sanchez, M.A.; Garcia-Fernandez, R.A.; Garcia-Palencia, P.; Sanchez, B.; Letelier, C.A.; Gonzalez-Bulnes, A.; Flores, J.M. Endometrial expression of IFNAR-1 and oxytocin receptor (OTR) is not improved by prostaglandin analogues when compared to progestagens in ewes. Reprod. Domest. Anim. 2012, 47, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gonzalez, I.; Sanchez, M.A.; Garcia-Fernandez, R.A.; Garcia-Palencia, P.; Sanchez, B.; Gonzalez-Bulnes, A.; Flores, J.M. Different influence of ovine estrus synchronization treatments on caruncular early angiogenesis. Histol. Histopathol. 2013, 28, 373–383. [Google Scholar]
| Parameter | Group eCG First Trial (n = 13) | Group eCG Second Trial (n = 8) | Group hCG First Trial (n = 13) | Group hCG Second Trial (n = 16) |
|---|---|---|---|---|
| Estrous behavior | ||||
| Occurrence (%) | 13/13 100% a | 8/8 100% a | 8/13 61.5% b | 7/16 43.8% b |
| Timing (h) | 46.1 ± 2.7 | 48.0 ± 4.7 | ||
| Ovulation | ||||
| Sheep bearing CL (%) | 12/13 92.3% | 8/8 100% | 11/13 84.6% | 15/16 93.7% |
| Mean number of CL | 1.8 ± 0.3 | 2.1 ± 0.3 | 1.9 ± 0.2 | 2.2 ± 0.3 |
| Mean P4 concentrations | 2.3 ± 0.4 | 1.8 ± 0.3 | 2.4 ± 0.5 | 2.1 ±0.5 |
| Sheep bearing FL (%) | 3/13 23.1% a | 2/8 25% a | 8/13 61.5% b | 8/16 50% b |
| Mean number of FL | 1.0 ± 0.0 a | 1.5 ± 0.1 | 1.8 ± 0.2 b | 1.3 ± 0.1 |
| Sheep bearing FC (%) | 1/13 7.7% a | 0/8 0.0% a | 3/13 23.1% b | 2/16 12.5% b |
| Mean number of FC | 1.0 | 0.0 a | 1.7 ± 0.2 | 1.5 ± 0.1 b |
| Pregnancy | ||||
| Occurrence (%) | 9/13 69.2% a | 5/13 38.5% b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno-Galarraga, M.; Cano-Moreno, V.; Lago-Cruz, B.; Encinas, T.; Gonzalez-Bulnes, A.; Martinez-Ros, P. The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility. Animals 2021, 11, 984. https://doi.org/10.3390/ani11040984
Bruno-Galarraga M, Cano-Moreno V, Lago-Cruz B, Encinas T, Gonzalez-Bulnes A, Martinez-Ros P. The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility. Animals. 2021; 11(4):984. https://doi.org/10.3390/ani11040984
Chicago/Turabian StyleBruno-Galarraga, Macarena, Virginia Cano-Moreno, Beatriz Lago-Cruz, Teresa Encinas, Antonio Gonzalez-Bulnes, and Paula Martinez-Ros. 2021. "The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility" Animals 11, no. 4: 984. https://doi.org/10.3390/ani11040984
APA StyleBruno-Galarraga, M., Cano-Moreno, V., Lago-Cruz, B., Encinas, T., Gonzalez-Bulnes, A., & Martinez-Ros, P. (2021). The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility. Animals, 11(4), 984. https://doi.org/10.3390/ani11040984






