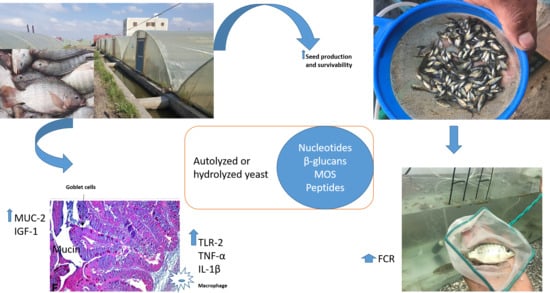
Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hilyses Composition and Diet Preparation
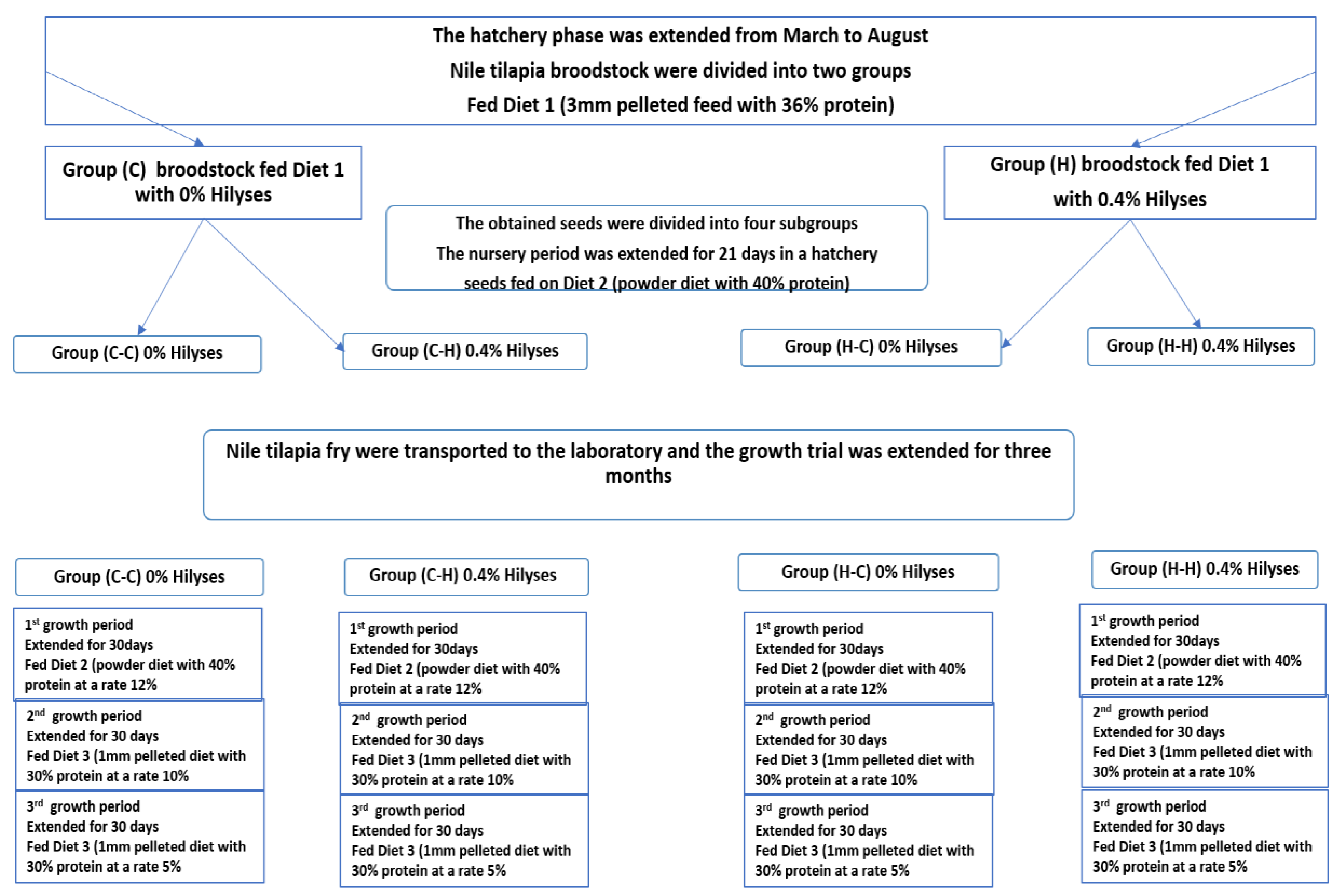
2.2. Experimental Design
2.2.1. Hatchery Trial: Evaluating the Effect of Dietary Inclusion of Hilyses on Production, Seed Mortality and Immune Response of Nile tilapia Broodstock
Blood and Tissue Samples
Hematological and Biochemical Analysis
Antioxidant Biomarkers, Lipid Peroxidation and Cortisol Levels Measurements
The Relative Expression of Immune- and Growth-Related Genes
Histological Examination
2.2.2. The Laboratory Experiment: Evaluating the Effect of Hilyses Treated (Diet and Broodstock) on the Growth Performance and Feed Utilization of Nile Tilapia Fry
2.3. Statistical Analyses
3. Results
3.1. Production of Nile Tilapia Broodstock and Percentage Mortality in Fry at the Hatchery during the Spawning Season
3.2. Hematological and Biochemical Parameters
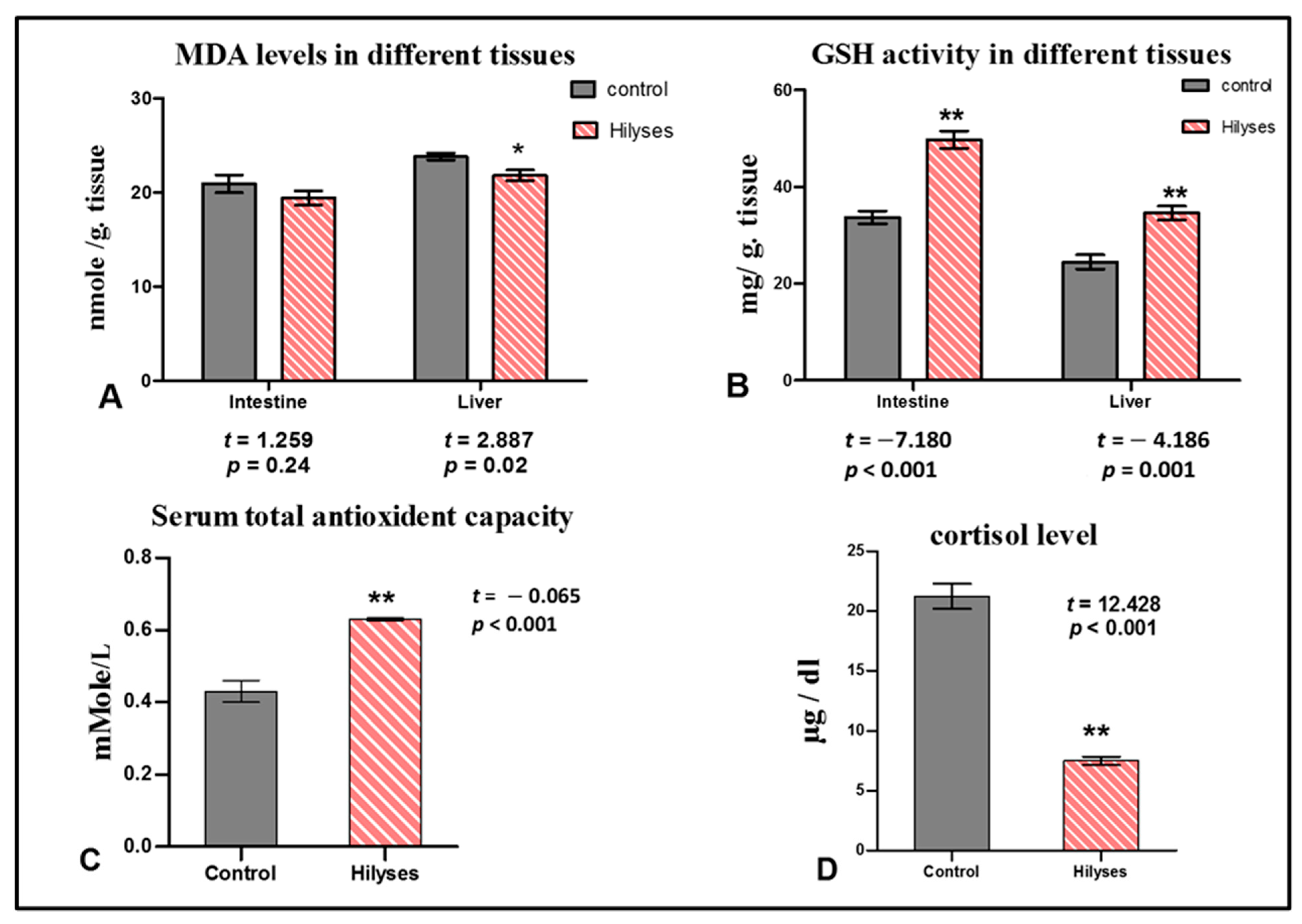
3.3. Oxidant and Antioxidant Biomarkers and Cortisol Level
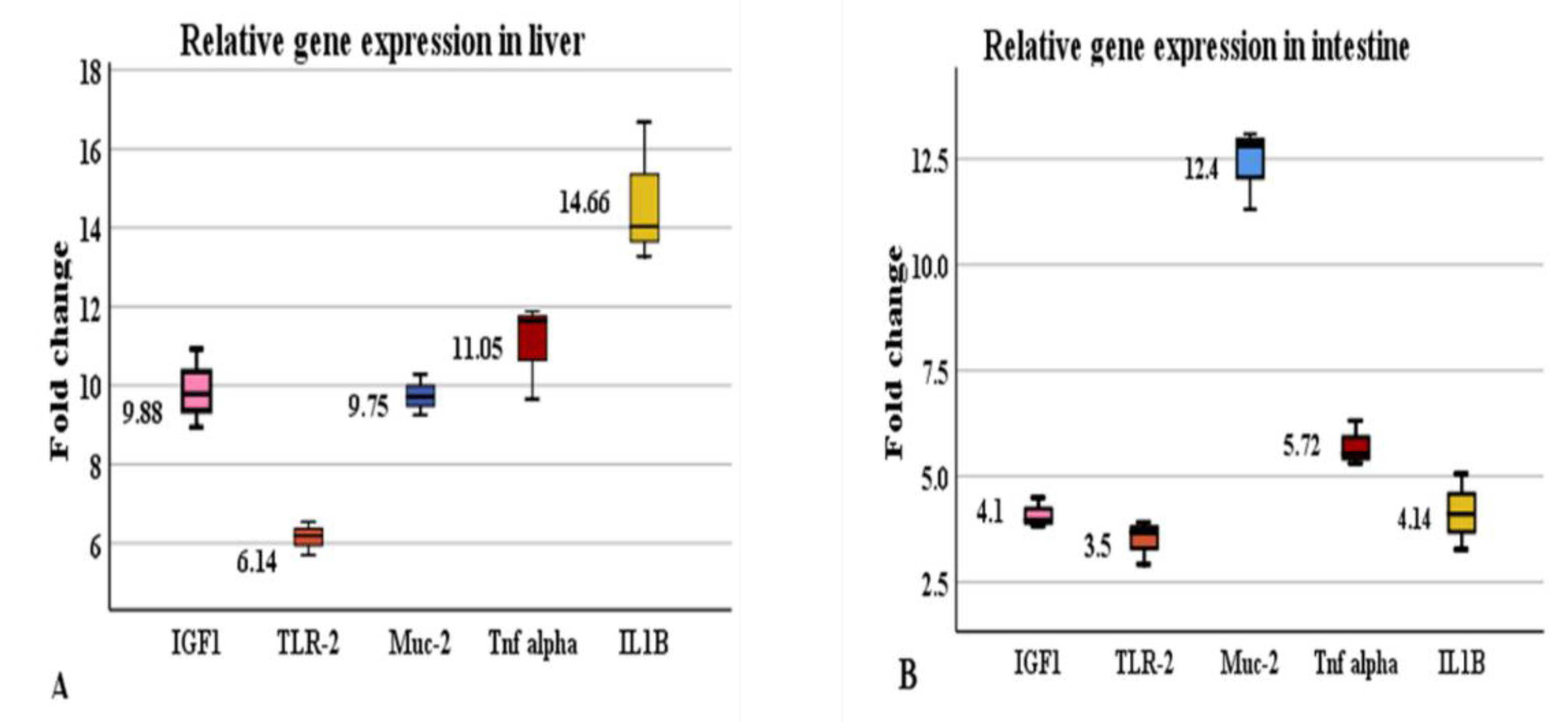
3.4. Effect of Hilyses on Immune-Related and Growth-Related Gene Expression
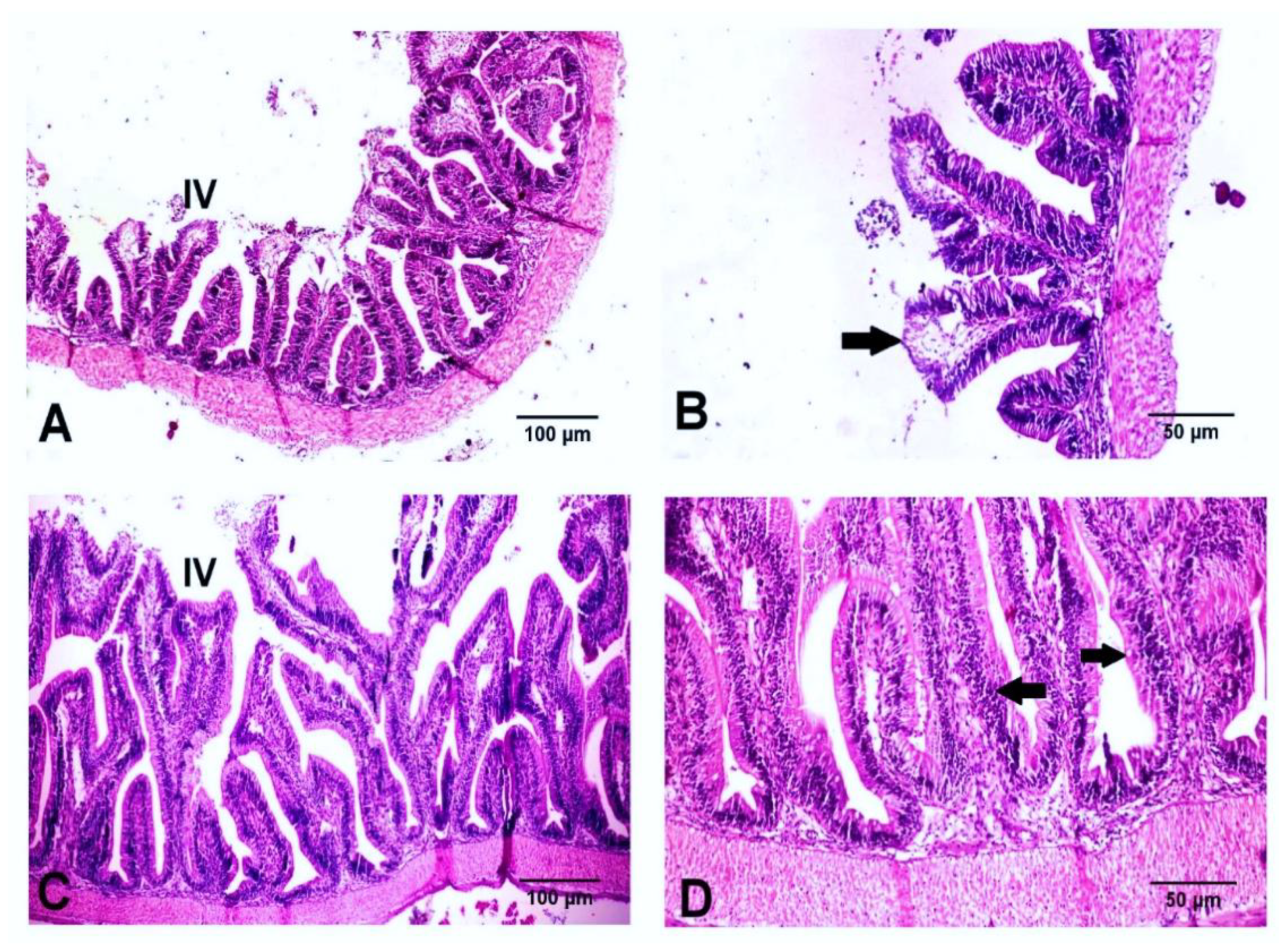
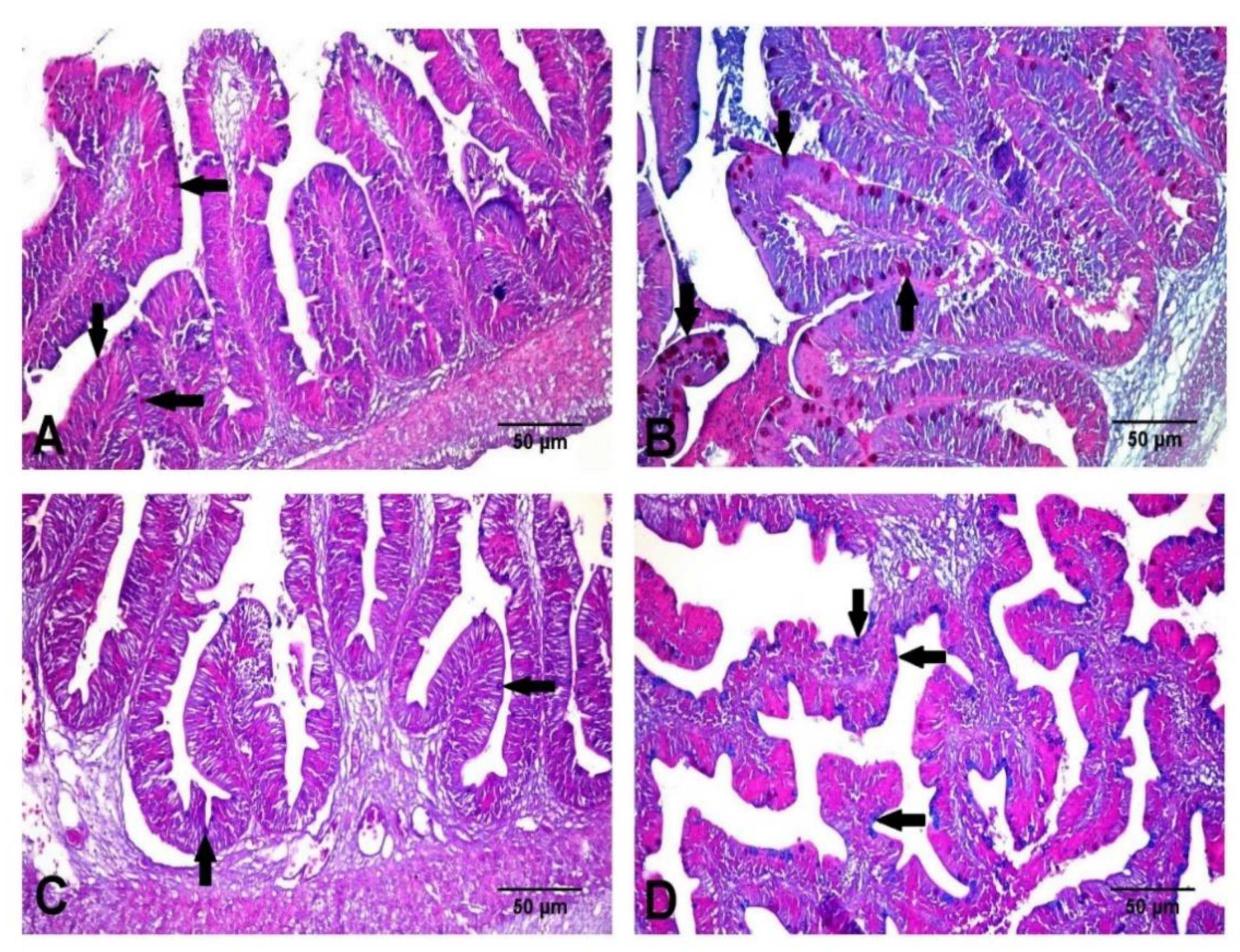
3.5. Histological Findings
3.6. Growth Performance and Feed Utilization in Nile Tilapia Fry and Fingerlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaalan, M.; El-Mahdy, M.; Saleh, M.; El-Matbouli, M. Aquaculture in Egypt: Insights on the Current Trends and Future Perspectives for Sustainable Development. Rev. Fish. Sci. Aquac. 2018, 26, 99–110. [Google Scholar] [CrossRef]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M.A. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Moffitt, C.M.; Cajas-Cano, L. Blue Growth: The 2014 FAO State of World Fisheries and Aquaculture. Fisheries 2014, 39, 552–553. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Aquaculture Department, the State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Abu-Elala, N.M.; Younis, N.A.; Abubakr, H.O.; Ragaa, N.M.; Borges, L.L.; Bonato, M.A. Influence of dietary fermented Saccharomyces cerevisiae on growth performance, oxidative stress parameters, and immune response of cultured Oreochromis niloticus. Fish Physiol. Biochem. 2020, 46, 533–545. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2017, 10, 950–974. [Google Scholar] [CrossRef]
- Abu Elala, N.M.; Galal, M.K.; Abd-Elsalam, R.M.; Mohey-Elsaeed, O.; Ragaa, N.M. Effects of Dietary Supplementation of Spirulina platensis and Garlic on the Growth Performance and Expression Levels of Immune-related Genes in Nile tilapia (Oreochromis niloticus). J. Aquac. Res. Dev. 2016, 7, 433–442. [Google Scholar] [CrossRef]
- Dawood, M.; El Basuini, M.; Zaineldin, A.; Yilmaz, S.; Hasan, T.; Ahmadifar, E.; El Asely, A.; Abdel-Latif, H.; Alagawany, M.; Abu-Elala, N.; et al. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, Y.; Gao, C.; Zhang, F.; Xia, R.; Li, D.; Hu, J.; Ran, C.; Zhang, Z.; Liu-Clarke, J.; et al. Surface display system for probiotics and its application in aquaculture. Rev. Aquac. 2020, 12, 2333–2350. [Google Scholar] [CrossRef]
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Hossain, S.; Koshio, S.; Kestemont, P. Recent advances of nucleotide nutrition research in aquaculture: A review. Rev. Aquac. 2019, 12, 1028–1053. [Google Scholar] [CrossRef]
- Dawood, M.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.; Hossain, M.; Nhu, T.; Moss, A.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2015, 23, 148–159. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Fadl, S.E.; Ahmed, H.A.; El Asely, A.; Abdel-Daim, M.M.; Alkahtani, S. The modulatory effect of mannanoligosaccharide on oxidative status, selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major). Aquac. Rep. 2020, 16, 100278. [Google Scholar] [CrossRef]
- Izquierdo, M.; Fernández-Palacios, H.; Tacon, A. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Mekawey, M. Incorporation of garlic meal (Allium sativum) as natural additive to enhance performance, immunity, gonad and larval survival of Nile tilapia (Oreochromis niloticus) broodstock. Afr. J. Biol. Sci. 2019, 15, 117–135. [Google Scholar] [CrossRef][Green Version]
- Freccia, A.; Sousa, S.M.D.N.; Meurer, F.; Butzge, A.J.; Mewes, J.K.; Bombardelli, R.A. Essential oils in the initial phase of broodstock diets of Nile tilapia. Rev. Bras. Zootec. 2014, 43, 1–7. [Google Scholar] [CrossRef][Green Version]
- Al-Feky, S.; El-Sayed, A.-F.; Ezzat, A. Dietary taurine improves reproductive performance of Nile tilapia (Oreochromis niloticus) broodstock. Aquac. Nutr. 2014, 22, 392–399. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Dacie, J.; Lewis, S. Practical Haematology, ELBS with Churchill Livingston; Churchil Livingstone: Edinburgh, England, 1991. [Google Scholar]
- Stoskopf, M.K. Fish Medicine Ed; WB Saunders Company: London, UK, 1993. [Google Scholar]
- Dacie, S.; Lewis, S. Practical Haematology, 11th ed.; Churchill Livingstone: London, UK, 2002. [Google Scholar]
- Shaw, A.F.B. A direct method for counting the leukocytes, thrombocytes and erythrocytes of birds’s blood. J. Pathol. Bacteriol. 1930, 33, 833–835. [Google Scholar] [CrossRef]
- Lopes-Virella, M.F.; Stone, P.; Ellis, S.; Colwell, J. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1977, 23, 882–884. [Google Scholar] [CrossRef]
- Tietz, N.W. Fundamentals of Clinical Chemistry Philadelphia; WB Saudners Co Ltd.: Philadelphia, PA, USA, 1976. [Google Scholar]
- Tietz, N. Clinical Guide to Laboratory Tests; WB Saudners Company: Philadelphia, PA, USA, 1990; Volume 554, p. 556. [Google Scholar]
- Coles, E. Veterinary Clinical Pathology, 2nd ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1974. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, M. Glutathione reagent and method-patent. J. Lab. Clin. Med. 1963, 61, 882. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Gröner, F.; Ziková, A.; Kloas, W. Effects of the pharmaceuticals diclofenac and metoprolol on gene expression levels of enzymes of biotransformation, excretion pathways and estrogenicity in primary hepatocytes of Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 167, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Midhun, S.J.; Neethu, S.; Arun, D.; Vysakh, A.; Divya, L.; Radhakrishnan, E.; Jyothis, M. Dietary supplementation of Bacillus licheniformis HGA8B improves growth parameters, enzymatic profile and gene expression of Oreochromis niloticus. Aquaculture 2019, 505, 289–296. [Google Scholar] [CrossRef]
- Standen, B.; Peggs, D.; Rawling, M.; Foey, A.; Davies, S.; Santos, G.; Merrifield, D. Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2016, 49, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Zou, J.; Secombes, C.J.; Martin, S.A. Cortisol modulates the induction of inflammatory gene expression in a rainbow trout macrophage cell line. Fish Shellfish. Immunol. 2011, 30, 215–223. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewartjr, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elala, N.M.; Abd-Elsalam, R.M.; Marzouk, M.S. Molecular and Immunohistochemical Diagnosis of Photobacterium damselae Subspecies piscicida During Naturally Occurring Disease in Egypt. J. World Aquac. Soc. 2015, 46, 583–595. [Google Scholar] [CrossRef]
- Abu Elala, N.M.; Ragaa, N.M. Eubiotic effect of a dietary acidifier (potassium diformate) on the health status of cultured Oreochromis niloticus. J. Adv. Res. 2015, 6, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Abo-State, H.A.; Tahoun, A.-A.M. Effect of Saccharomyces cerevisiae on seed production and fry performance of Oreochromis niloticus broodstock. Biosci. Res. 2017, 14, 1175–1181. [Google Scholar]
- Abasali, H.; Mohamad, S. Effect of Dietary Supplementation with Probiotic on Reproductive Performance of Female Livebearing Ornamental Fish. Res. J. Anim. Sci. 2010, 4, 103–107. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Gatlin, D.M. Nucleotide nutrition in fish: Current knowledge and future applications. Aquaculture 2006, 251, 141–152. [Google Scholar] [CrossRef]
- Guo, X.; Ran, C.; Zhang, Z.; He, S.; Jin, M.; Zhou, Z. The Growth-Promoting Effect of Dietary Nucleotides in Fish Is Associated with an Intestinal Microbiota-Mediated Reduction in Energy Expenditure. J. Nutr. 2017, 147, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Magouz, F.I.; Dawood, M.A.; Salem, M.F.; El-Ghandour, M.; Van Doan, H.; Mohamed, A.A. The role of a digestive enhancer in improving the growth performance, digestive enzymes activity, and health condition of Nile tilapia (Oreochromis niloticus) reared under suboptimal temperature. Aquaculture 2020, 526, 735388. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Dawood, M.A.; Dossou, S.; Yukun, Z.; Mzengereza, K. Singular effects of Bacillus subtilis C-3102 or Saccharomyces cerevisiae type 1 on the growth, gut morphology, immunity, and stress resistance of red sea bream (Pagrus major). Ann. Anim. Sci. 2020. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef]
- Xu, L.; Ran, C.; He, S.; Zhang, J.; Hu, J.; Yang, Y.; Du, Z.; Yang, Y.; Zhou, Z. Effects of dietary yeast nucleotides on growth, non-specific immunity, intestine growth and intestinal microbiota of juvenile hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Anim. Nutr. 2015, 1, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A.; El Asely, A.; Fadl, S.E.; Amin, A.A.; Paray, B.A.; Ahmed, H.A. Saccharomyces cerevisiae increases the acceptability of Nile tilapia (Oreochromis niloticus) to date palm seed meal. Aquac. Rep. 2020, 17, 100314. [Google Scholar] [CrossRef]
- Nieves-Rodríguez, K.N.; Álvarez-González, C.A.; Peña-Marín, E.S.; Vega-Villasante, F.; Martínez-García, R.; Camarillo-Coop, S.; Tovar-Ramírez, D.; Guzmán-Villanueva, L.T.; Andree, K.B.; Gisbert, E. Effect of β-Glucans in Diets on Growth, Survival, Digestive Enzyme Activity, and Immune System and Intestinal Barrier Gene Expression for Tropical Gar (Atractosteus tropicus) Juveniles. Fishes 2018, 3, 27. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Abu-Elala, N.; Marzouk, M.; Moustafa, M. Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int. J. Veter Sci. Med. 2013, 1, 21–29. [Google Scholar] [CrossRef]
- Mohapatra, S.; Chakraborty, T.; Kumar, V.; DeBoeck, G.; Mohanta, K.N. Aquaculture and stress management: A review of probiotic intervention. J. Anim. Physiol. Anim. Nutr. 2013, 97, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, R.T.; Washburn, P.C.; Wenning, R.J.; Winston, G.W.; Jewell, C.S. Biochemical responses in aquatic animals: A review of determinants of oxidative stress. Environ. Toxicol. Chem. 1989, 8, 1103–1123. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Veterinární Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Santacroce, M.P.; Merra, E.; Centoducati, G.; Zacchino, V.; Casalino, E. Effects of dietary yeast Saccaromyces cerevisiae on the antioxidant system in the liver of juvenile sea bass Dicentrarchus labrax. Fish Physiol. Biochem. 2012, 38, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, E.; Angienda, P.O.; Okoth, P.; Onyango, D. Stocking Density Induced Stress on Plasma Cortisol and Whole Blood Glucose Concentration in Nile Tilapia Fish (Oreochromis niloticus) of Lake Victoria, Kenya. Int. J. Zool. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Girao, P.M.; Pereira da Silva, E.M.; de Melo, M.P. Dietary lycopene supplementation on Nile tilapia (Oreochromis niloticus) juveniles submitted to confinement: Effects on cortisol level and antioxidant response. Aquac. Res. 2012, 43, 789–798. [Google Scholar] [CrossRef]
- Huynh, T.-G.; Shiu, Y.-L.; Nguyen, T.-P.; Truong, Q.-P.; Chen, J.-C.; Liu, C.-H. Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish. Immunol. 2017, 64, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Abo-Al-Ela, H.G.; Hasan, T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish. Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Chi, H.; Dalmo, R.A. Trained Innate Immunity of Fish Is a Viable Approach in Larval Aquaculture. Front. Immunol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Welker, T.L.; Lim, C.; Yildirim-Aksoy, M.; Klesius, P.H. Use of diet crossover to determine the effects of β-glucan supplementation on immunity and growth of Nile tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2012, 43, 335–348. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elala, N.M.; Younis, N.A.; AbuBakr, H.O.; Ragaa, N.M.; Borges, L.L.; Bonato, M.A. Efficacy of dietary yeast cell wall supplementation on the nutrition and immune response of Nile tilapia. Egypt. J. Aquat. Res. 2018, 44, 333–341. [Google Scholar] [CrossRef]
- Aramli, M.S.; Kamangar, B.; Nazari, R.M. Effects of dietary β-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish. Immunol. 2015, 47, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Ma, H.; Mai, K.; Zhang, W.; Bai, N.; Wang, X. Effects of dietary β-glucan, mannan oligosaccharide and their combinations on growth performance, immunity and resistance against Vibrio splendidus of sea cucumber, Apostichopus japonicus. Fish Shellfish. Immunol. 2011, 31, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ta’Ati, R.; Soltani, M.; Bahmani, M.; Zamini, A.A. Effects of the prebiotics Immunoster and Immunowall on growth performance of juvenile beluga (Huso huso). J. Appl. Ichthyol. 2011, 27, 796–798. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Ouraji, H.; Khalesi, M.K.; Sudagar, M.; Barari, A.; Zarei Dangesaraki, M.; Jani Khalili, K.H. Effects of a prebiotic, immunogen®, on feed utilization, body composition, immunity and resistance to Aeromonas hydrophila infection in the common carp Cyprinus carpio (linnaeus) fingerlings. J. Anim. Physiol. Anim. Nutr. 2012, 96, 591–599. [Google Scholar] [CrossRef] [PubMed]




| Ingredient % | Diet 1 (36% CP 5) | Diet 2 (40% CP) | Diet 3 (30% CP) |
|---|---|---|---|
| Fish meal (67%) | 21.8 | 23.55 | 16 |
| Soybean meal (48%) | 37.3 | 44.84 | 30.5 |
| Yellow corn | 29.18 | 21.33 | 38.22 |
| Wheat flour | 6.51 | 5.75 | 8.5 |
| Soybean oil | 3 | 3 | 3.5 |
| Mono calcium phosphate (23.7%) | 0.8 | 0.28 | 1.3 |
| Calcium carbonate | 0.42 | 0.34 | 0.87 |
| Common salt | 0.5 | 0.5 | 0.5 |
| Premix 1 | 0.3 | 0.3 | 0.3 |
| Vitamin C | 0.1 | 0.1 | 0.1 |
| DL-Methionine | 0.08 | 0 | 0.2 |
| BHT 2 | 0.01 | 0.01 | 0.01 |
| Total | 100 | 100 | 100 |
| Calculated Analysis (%) | |||
| Crude protein | 36.04 | 40 | 30.03 |
| Dry matter | 90.18 | 90.41 | 89.9 |
| Ash | 8.65 | 8.75 | 8.2 |
| Ether extract | 8.4 | 8.63 | 8.39 |
| Crude fiber | 3.58 | 3.95 | 3.26 |
| NFE 3 | 43.33 | 38.66 | 50.1 |
| Gross energy (kcal/100 g) 4 | 460.67 | 466.08 | 454.46 |
| Calcium | 1.22 | 1.19 | 1.23 |
| Total phosphorus | 1.06 | 1 | 1.01 |
| Methionine | 0.76 | 0.75 | 0.75 |
| Lysine | 2.25 | 2.54 | 1.79 |
| Threonine | 1.44 | 1.61 | 1.174 |
| Linoleic acid | 2.36 | 2.22 | 2.78 |
| Chemical Analysis (%) | |||
| Crude protein | 36.11 | 40.08 | 30.07 |
| Dry matter | 90.07 | 90.37 | 89.93 |
| Ash | 8.67 | 8.73 | 8.18 |
| Ether extract | 8.43 | 8.56 | 8.36 |
| Crude fiber | 3.61 | 3.98 | 3.37 |
| Calcium | 1.20 | 1.21 | 1.25 |
| Total phosphorus | 1.04 | 1.01 | 1.03 |
| Parameters | March | April | May | June | July | August |
|---|---|---|---|---|---|---|
| Temperature (°C) | 28 ± 1 | 28 ± 1 | 30 ± 1 | 30 ± 1 | 31 ± 1 | 32 ± 1 |
| Dissolved oxygen (mg/L) | 5.9 ± 0.2 | 6 ± 0.3 | 6.3 ± 0.2 | 6.2 ± 0.1 | 6.5 ± 0.2 | 6.5 ± 0.1 |
| pH | 8.3 ± 0.2 | 8.6 ± 0.2 | 8.6 ± 0.2 | 8.7 ± 0.1 | 8.7 ± 0.3 | 8.7 ± 0.2 |
| Total ammonia (mg/L) | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.01 |
| Genes | Primer Sequences | Reference |
|---|---|---|
| EF-1α (housekeeping) | CCTTCAACGCTCAGGTCATC | Gröner et al. [31] |
| TGTGGGCAGTGTGGCAATC | ||
| IGF1 | CATCGTGGACGAGTGCTG | Midhun et al. [32] |
| ACAGGTGCACAGTACATCTCAAG | ||
| TLR-2 | CCCACAATGGATTCACCAG | |
| AAAGATCAAGACTCAAGGCACTG | ||
| MUC-2 | CAACTGTTTTTGAGACAACTTCAGA | |
| CTGAAGTGACCGTGGAAGG | ||
| TNF alpha | CCAGAAGCACTAAAGGCGAAGA | Standen et al. [33] |
| CCTTGGCTTTGCTGCTGATC | ||
| IL-1β | GCTGGAGAGTGCTGTGGAAGAACATATAG | Castro et al. [34] |
| CCTGGAGCATCATGGCGTG |
| Parameters | Control | Hilyses | t-Value | p-Value |
|---|---|---|---|---|
| RBCs count (×106 cell/mm3) | 1.52 ± 0.02 | 1.68 ± 0.05 | −2.836 | 0.02 * |
| Hematocrit value (%) | 30.80 ± 1.39 | 38.80 ± 1.62 | −3.738 | 0.006 ** |
| Hb (g/dL) | 5.79 ± 0.15 | 6.93 ± 0.17 | −4.950 | 0.001 ** |
| MCV (fl) | 202.95 ± 7.83 | 230.71 ± 3.14 | −3.288 | 0.01 ** |
| MCH (pg) | 38.22 ± 0.85 | 41.29 ± 0.48 | −3.134 | 0.01 ** |
| MCHC (%) | 18.92 ± 0.66 | 17.92 ± 0.45 | 1.241 | 0.25 |
| WBCs count (×103 cell/mm3) | 61.60 ± 1.43 | 63.80 ± 0.80 | −1.339 | 0.2 |
| Granulocyte % | 38.00 ± 0.45 | 35.40 ± 0.68 | 3.200 | 0.01 ** |
| Lymphocyte % | 58.00 ± 0.71 | 60.80 ± 0.86 | −2.514 | 0.04 * |
| Monocyte % | 4.00 ± 0.45 | 3.80 ± 0.37 | 0.343 | 0.74 |
| Parameters | Control | Hilyses | t-Value | p-Value |
|---|---|---|---|---|
| ALT (U/L) | 5.44 ± 0.43 | 4.54 ± 0.28 | 1.776 | 0.11 |
| AST (U/L) | 42.24 ± 3.66 | 38.58 ± 3.45 | 0.730 | 0.49 |
| Total protein (g/dL) | 3.10 ± 0.09 | 2.97 ± 0.14 | 0.762 | 0.47 |
| Albumin (g/dL) | 1.89 ± 0.05 | 1.71 ± 0.07 | 2.020 | 0.08 |
| Globulin (g/dL) | 1.21 ± 0.08 | 1.26 ± 0.11 | −0.387 | 0.71 |
| Urea (mg/dL) | 2.82 ± 0.29 | 2.91 ± 0.22 | −0.259 | 0.80 |
| Creatinine (mg/dL) | 0.36 ± 0.01 | 0.36 ± 0.01 | −0.065 | 0.95 |
| Treatments | Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Hilyses% 1 | Broodstock on Hilyses | Final BW at 3 m, g | Total BW gain, g | Total Feed Intake/fish, g | Final FCR 3, g/g | Final PER 4, g/g | Final SGR 5 |
| C-C | 0.0 | − | 15.4 c | 15.3 c | 20.5 d | 1.3 a | 2.5 c | 5.4 c |
| C-H | 0.4 | − | 19.8 b | 19.7 b | 23.8 c | 1.2 b | 2.8 b | 5.7 b |
| H-C | 0.0 | + | 20.8 b | 20.7 b | 25.2 b | 1.2 b | 2.7 b | 5.7 b |
| H-H | 0.4 | + | 26.2 a | 26.0 a | 28.9 a | 1.1 c | 3.0 a | 5.9 a |
| - | - | SEM 2 | 0.372 | 0.372 | 0.115 | 0.012 | 0.012 | 0.012 |
| Main Effects | ||||||||
| Hilyses | 0.0% | 18.1 b | 17.9 b | 22.9 b | 1.3 a | 2.6 b | 5.5 b | |
| - | 0.4% | 22.9 a | 22.9 a | 26.4 a | 1.1 b | 2.9 a | 5.8 a | |
| - | SEM | 0.256 | 0.256 | 0.082 | 0.008 | 0.008 | 0.008 | |
| Broodstock on Hilyses | − | 18.3 b | 18.2 b | 22.7 b | 1.2 a | 2.6 b | 5.5 b | |
| - | + | 24.4 a | 24.3 a | 27.7 a | 1.1 b | 2.9 a | 5.9 a | |
| - | SEM | 0.259 | 0.259 | 0.082 | 0.008 | 0.008 | 0.008 | |
| p-Value | ||||||||
| Hilyses | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Broodstock | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Hilyses × broodstock on Hilyses | 0.235 | 0.235 | 0.137 | 0.253 | 0.563 | 0.213 | ||
| F-Value | ||||||||
| Hilyses | 179.203 | 179.203 | 906.941 | 111.447 | 557.261 | 524.053 | ||
| Broodstock | 261.810 | 261.810 | 1810.735 | 93.995 | 468.853 | 764.349 | ||
| Hilyses × broodstock on Hilyses | 1.437 | 1.437 | 2.728 | 1.514 | 0.365 | 1.827 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Elala, N.M.; Ali, T.E.-S.; Ragaa, N.M.; Ali, S.E.; Abd-Elsalam, R.M.; Younis, N.A.; Abdel-Moneam, D.A.; Hamdien, A.H.; Bonato, M.; Dawood, M.A.O. Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial. Animals 2021, 11, 815. https://doi.org/10.3390/ani11030815
Abu-Elala NM, Ali TE-S, Ragaa NM, Ali SE, Abd-Elsalam RM, Younis NA, Abdel-Moneam DA, Hamdien AH, Bonato M, Dawood MAO. Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial. Animals. 2021; 11(3):815. https://doi.org/10.3390/ani11030815
Chicago/Turabian StyleAbu-Elala, Nermeen M., Tamer El-Sayed Ali, Naela M. Ragaa, Sara E. Ali, Reham M. Abd-Elsalam, Nehal A. Younis, Dalia A. Abdel-Moneam, Aya H. Hamdien, Melina Bonato, and Mahmoud A.O. Dawood. 2021. "Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial" Animals 11, no. 3: 815. https://doi.org/10.3390/ani11030815
APA StyleAbu-Elala, N. M., Ali, T. E.-S., Ragaa, N. M., Ali, S. E., Abd-Elsalam, R. M., Younis, N. A., Abdel-Moneam, D. A., Hamdien, A. H., Bonato, M., & Dawood, M. A. O. (2021). Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial. Animals, 11(3), 815. https://doi.org/10.3390/ani11030815