A Systematic Review of Genomic Regions and Candidate Genes Underlying Behavioral Traits in Farmed Mammals and Their Link with Human Disorders
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Data Gathering and Editing
2.2. Identification of Candidate Genes
2.3. Common Genomic Regions
2.4. Functional Analyses
2.4.1. Functional Analyses within Species
2.4.2. Functional Analysis across Species
3. Results
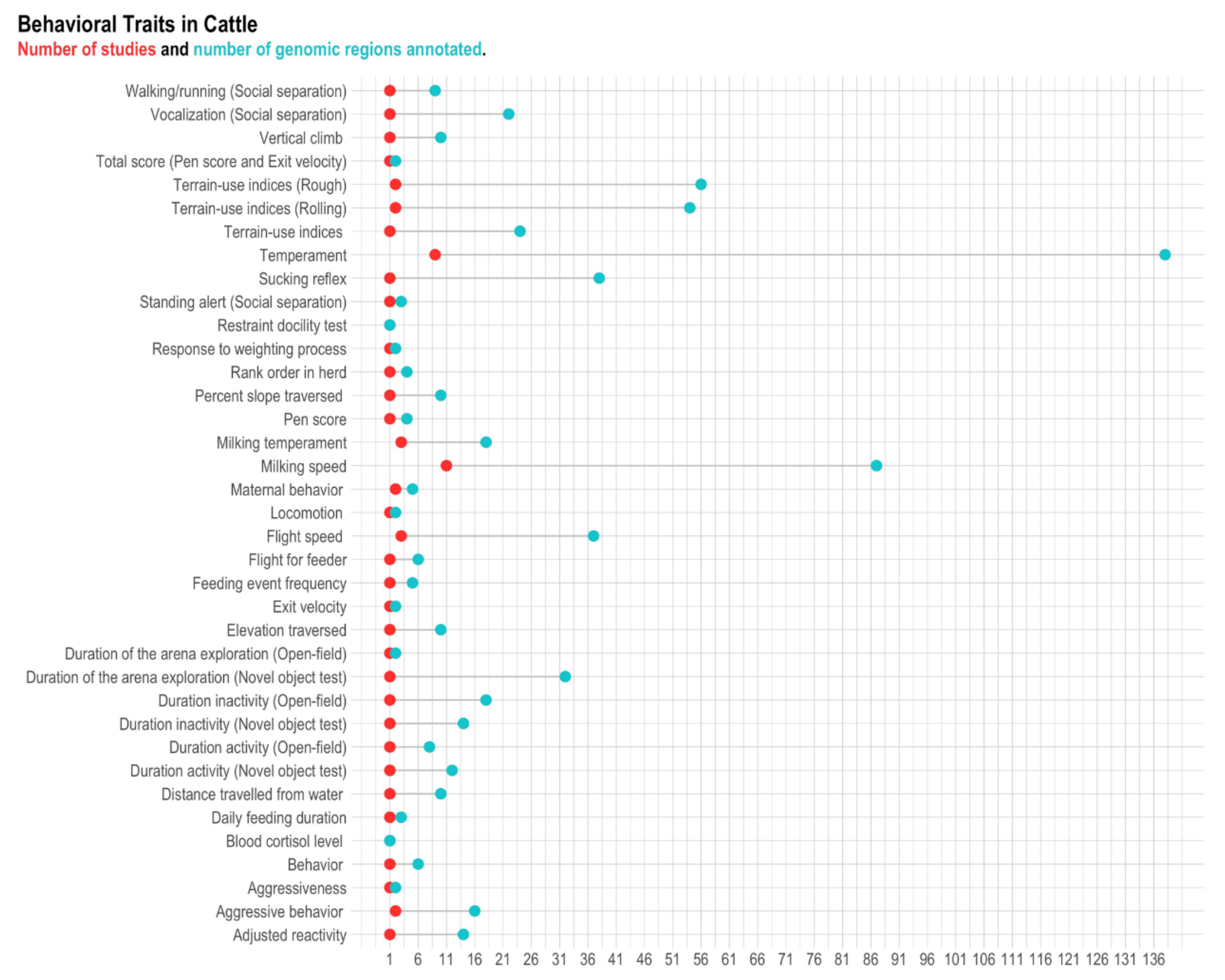
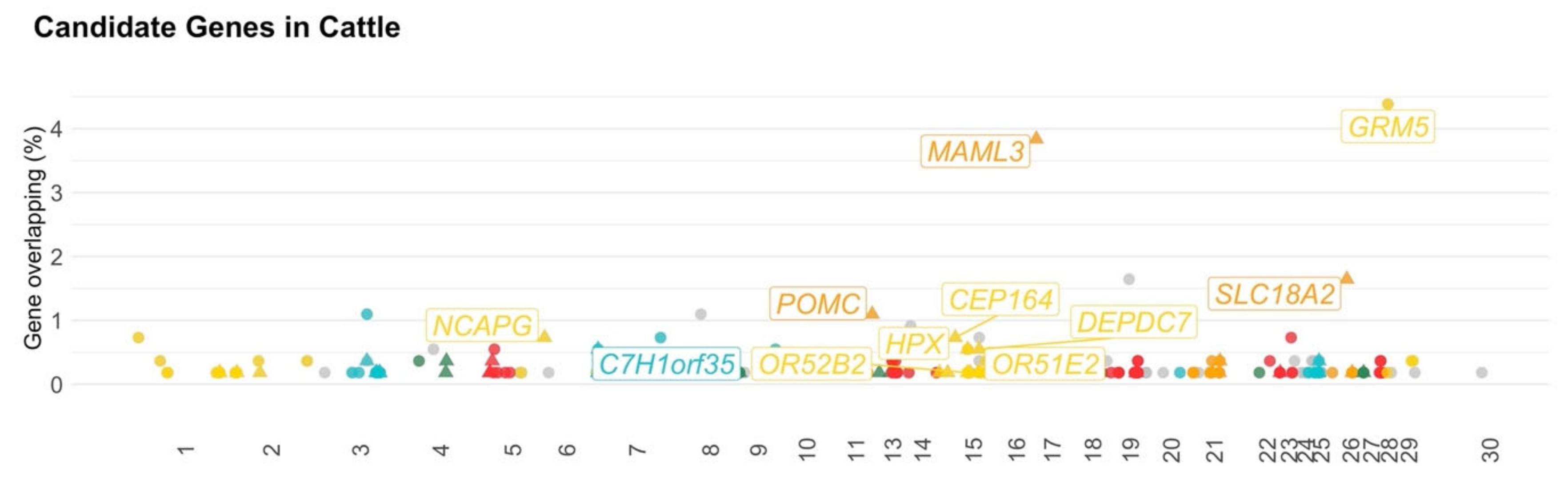
3.1. Beef and Dairy Cattle
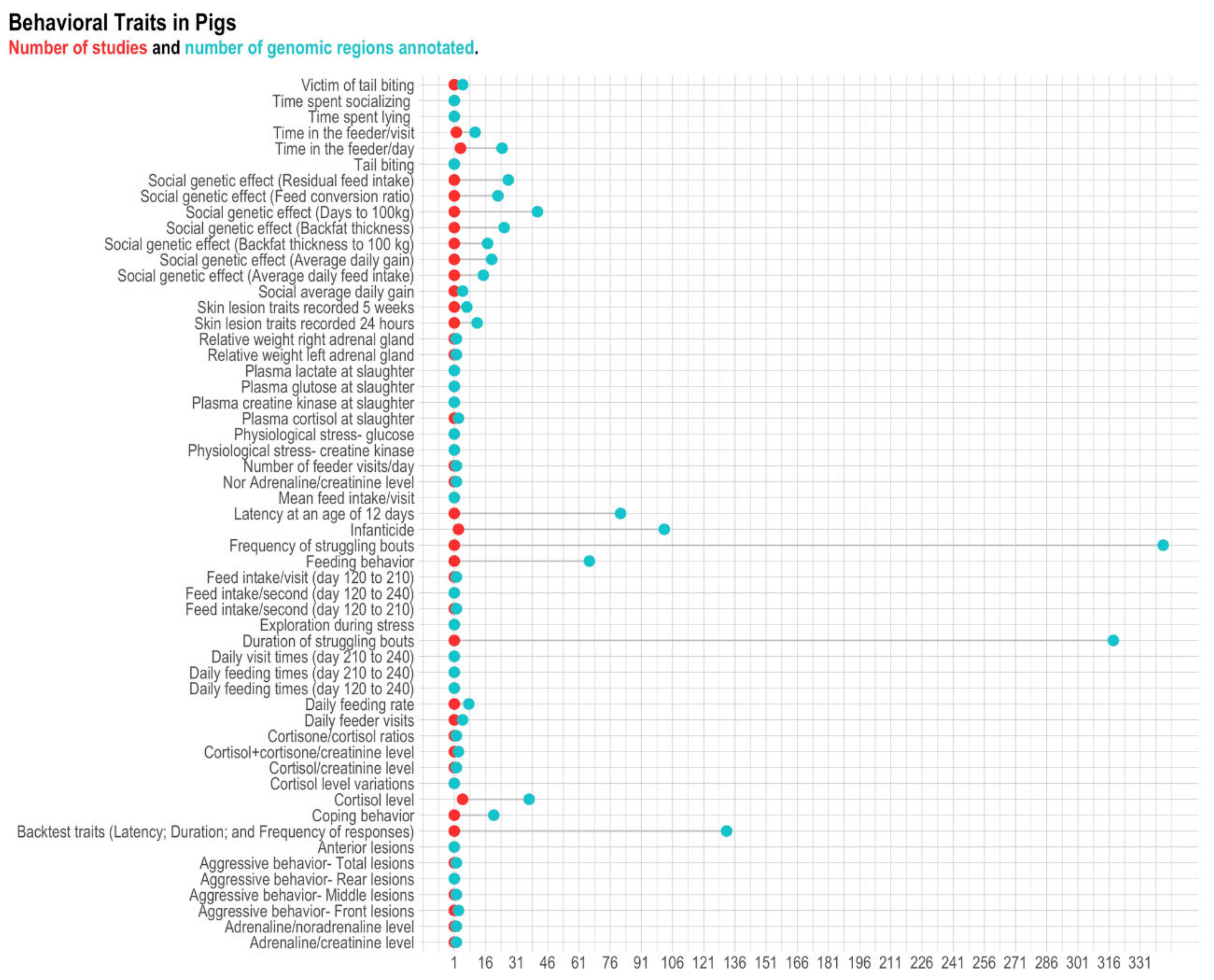
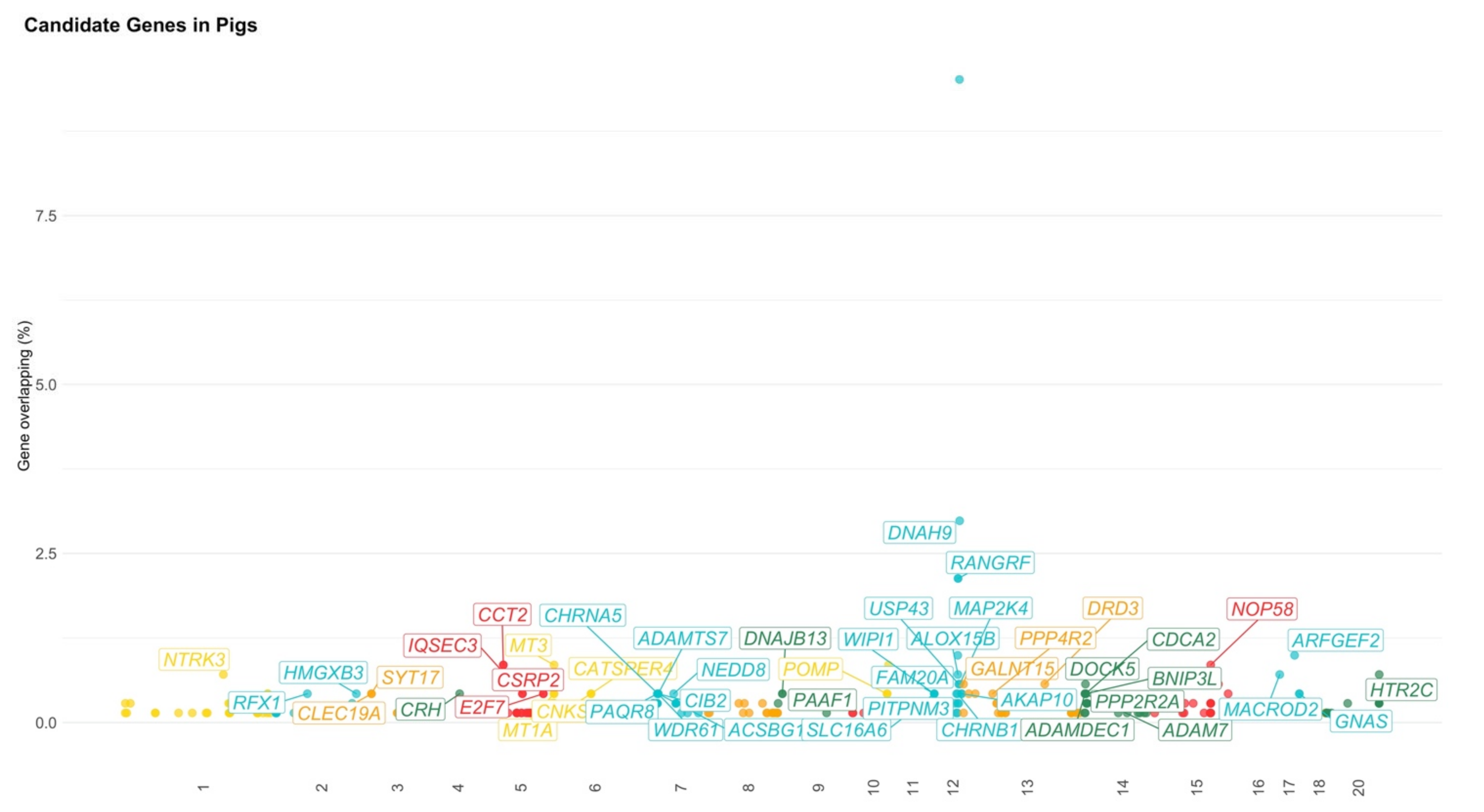
3.2. Pigs
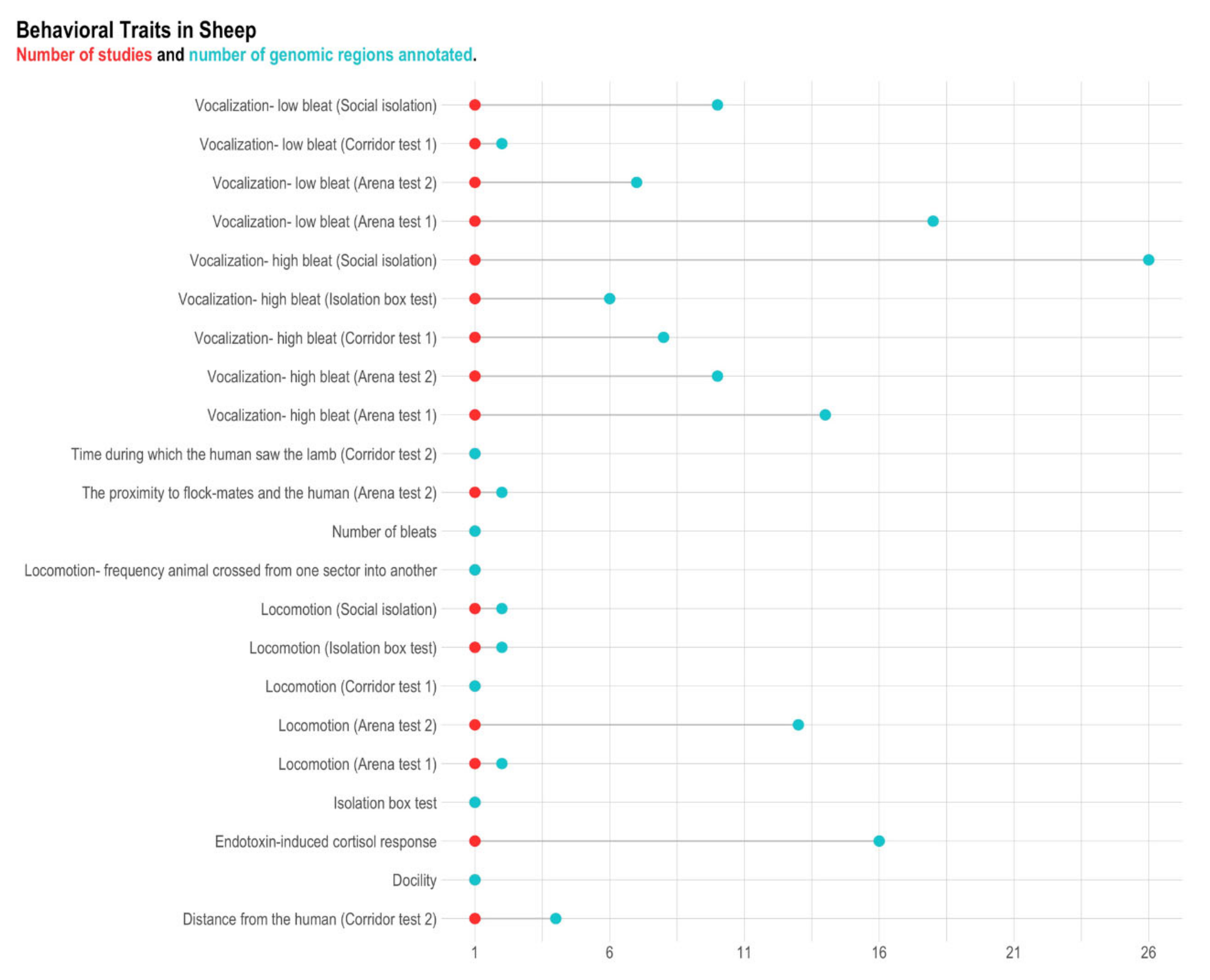
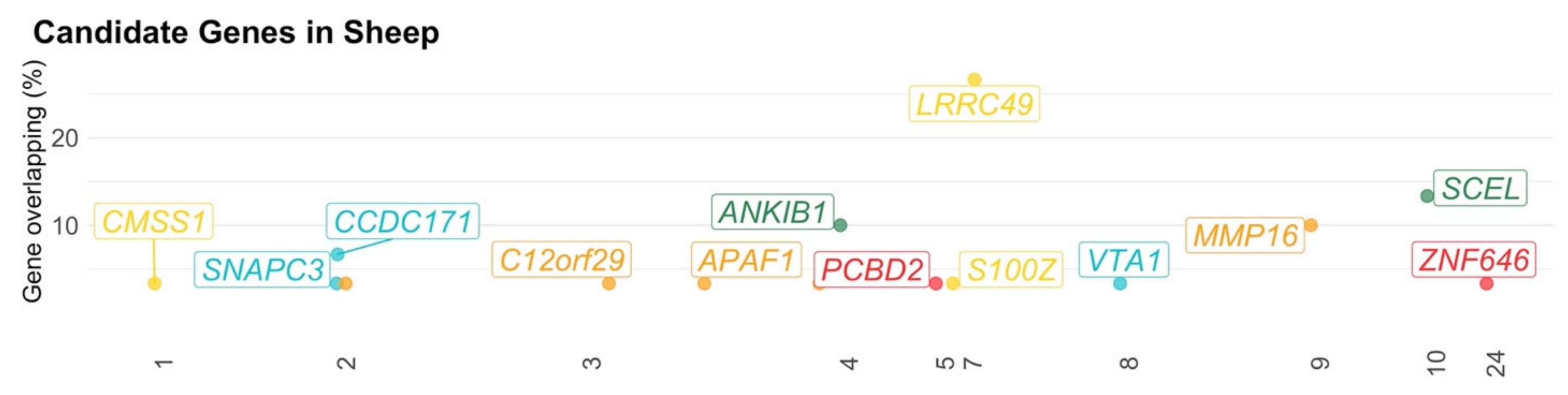
3.3. Sheep
3.4. Common Regions across Farmed Mammals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Variation in Animals and Plants under Domestication; Murray, J., Ed.; Cambridge University Press: London, UK, 1868; ISBN 978-1-108-01-423-6. [Google Scholar]
- Belyaev, D.K. Domestication of animals. Sci. J. 1969, 5, 47–52. [Google Scholar]
- Mignon-Grasteau, S.; Boissy, A.; Bouix, J.; Faure, J.M.; Fisher, A.D.; Hinch, G.N.; Jensen, P.; Le Neindre, P.; Mormède, P.; Prunet, P.; et al. Genetics of adaptation and domestication in livestock. Livest. Prod. Sci. 2005, 93, 3–14. [Google Scholar] [CrossRef]
- Wilkins, A.S.; Wrangham, R.W.; Tecumseh Fitch, W. The “domestication syndrome” in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics 2014, 197, 795–808. [Google Scholar] [CrossRef]
- Adamczyk, K.; Pokorska, J.; Makulska, J.; Earley, B.; Mazurek, M. Genetic analysis and evaluation of behavioural traits in cattle. Livest. Sci. 2013, 154, 1–12. [Google Scholar] [CrossRef]
- Stephansen, R.S.; Fogh, A.; Norberg, E. Genetic parameters for handling and milking temperament in Danish first-parity Holstein cows. J. Dairy Sci. 2018, 101, 11033–11039. [Google Scholar] [CrossRef]
- Coutinho, M.A.S.; Ramos, P.M.; Silva, S.L.; Martello, L.S.; Pereira, A.S.C.; Delgado, E.F. Divergent temperaments are associated with beef tenderness and the inhibitory activity of calpastatin. Meat Sci. 2017, 134, 61–67. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Schuehle Pfeiffer, C.E.; Randel, R.D.; Welsh, T.H.; Oliphint, R.A.; Baird, B.E.; Curley, K.O.; Vann, R.C.; Hale, D.S.; Savell, J.W. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.F.; Bohnert, D.W.; Meneghetti, M.; Losi, T.C.; Vasconcelos, J.L.M. Effects of temperament on pregnancy rates to fixed-timed AI in Bos indicus beef cows. Livest. Sci. 2011, 142, 108–113. [Google Scholar] [CrossRef]
- Phocas, F.; Boivin, X.; Sapa, J.; Trillat, G.; Boissy, A.; Le Neindre, P. Genetic correlations between temperament and breeding traits in Limousin heifers. Anim. Sci. 2006, 82, 805–811. [Google Scholar] [CrossRef]
- Hoppe, S.; Brandt, H.R.; König, S.; Erhardt, G.; Gauly, M. Temperament traits of beef calves measured under field conditions and their relationships to performance. J. Anim. Sci. 2010, 88, 1982–1989. [Google Scholar] [CrossRef]
- Sant’Anna, A.C.; Da Silva Valente, T.; Magalhaes, A.F.B.; Espigolan, R.; Ceballos, M.C.; De Albuquerque, L.G.; Da Costa, M.J.R.P. Relationships between temperament, meat quality, and carcass traits in Nellore cattle. J. Anim. Sci. 2019, 97, 4721–4731. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.S.; Sant’Anna, A.C.; Baldi, F.; Albuquerque, L.G.; da Costa, M.J.R.P. Genetic association between temperament and sexual precocity indicator traits in Nellore cattle. J. Appl. Genet. 2015, 56, 349–354. [Google Scholar] [CrossRef]
- Nkrumah, J.D.; Crews, D.H.; Basarab, J.A.; Price, M.A.; Okine, E.K.; Wang, Z.; Li, C.; Moore, S.S. Genetic and phenotypic relationships of feeding behavior and temperament with performance, feed efficiency, ultrasound, and carcass merit of beef cattle. J. Anim. Sci. 2007, 85, 2382–2390. [Google Scholar] [CrossRef]
- Ivemeyer, S.; Knierim, U.; Waiblinger, S. Effect of human-animal relationship and management on udder health in Swiss dairy herds. J. Dairy Sci. 2011, 94, 5890–5902. [Google Scholar] [CrossRef] [PubMed]
- Neja, W.; Sawa, A.; Jankowska, M.; Bogucki, M.; Krezel-Czopek, S. Effect of the temperament of dairy cows on lifetime production efficiency. Arch. Anim. Breed. 2015, 58, 193–197. [Google Scholar] [CrossRef]
- Tóth, G.; Póti, P.; Abayné, E.H.; Gulyás, L.; Bodnar, A.; Pajor, F. Effect of temperament on milk production, somatic cell count, chemical composition and physical properties in Lacaune dairy sheep breed. Mljekarstvo 2017, 67, 261–266. [Google Scholar] [CrossRef]
- Cziszter, L.T.; Gavojdian, D.; Neamt, R.; Neciu, F.; Kusza, S.; Ilie, D.E. Effects of temperament on production and reproductive performances in Simmental dual-purpose cows. J. Vet. Behav. 2016, 15, 50–55. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Turner, S.P.; Kurt, E.; Evans, G.; Thölking, L.; Looft, H.; Wimmers, K.; Murani, E.; Klont, R.; Foury, A.; et al. Pigs’ aggressive temperament affects pre-slaughter mixing aggression, stress and meat quality. Animal 2010, 4, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M. Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. J. Anim. Sci. 2008, 86, 246–258. [Google Scholar] [CrossRef] [PubMed]
- D’Eath, R.B.; Roehe, R.; Turner, S.P.; Ison, S.H.; Farish, M.; Jack, M.C.; Lawrence, A.B. Genetics of animal temperament: Aggressive behaviour at mixing is genetically associated with the response to handling in pigs. Animal 2009, 3, 1544–1554. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhang, H.F.; Johnston, L.J.; Martin, W.; Peterson, J.D.; Coetzee, J.F. Effects of tail docking and tail biting on performance and welfare of growing–finishing pigs in a confinement housing system. J. Anim. Sci. 2017, 95, 4835–4845. [Google Scholar] [CrossRef]
- Dogan, K.; Demirci, S. Livestock-Handling Related Injuries and Deaths. In Livestock Production; IntechOpen: London, UK, 2012. [Google Scholar]
- Knap, P.W. The scientific development that we need in the animal breeding industry. J. Anim. Breed. Genet. 2020, 137, 343–344. [Google Scholar] [CrossRef]
- Dawkins, M.S. Animal welfare and efficient farming: Is conflict inevitable? Anim. Prod. Sci. 2017, 57, 201–208. [Google Scholar] [CrossRef]
- Welfare Quality Network. Available online: www.welfarequality.net (accessed on 7 January 2020).
- Desautes, C.; Bidanel, J.P.; Milan, D.; Iannuccelli, N.; Amigues, Y.; Bourgeois, F.; Caritez, J.C.; Renard, C.; Chevalet, C.; Mormede, P. Genetic linkage mapping of quantitative trait loci for behavioral and neuroendocrine stress response traits in pigs. J. Anim. Sci. 2002, 80, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Geburt, K.; Piechotta, M.; König von Borstel, U.; Gauly, M. Influence of testosterone on the docility of German Simmental and Charolais heifers during behavior tests. Physiol. Behav. 2015, 141, 164–171. [Google Scholar] [CrossRef]
- Curley, K.O.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Michenet, A.; Saintilan, R.; Venot, E.; Phocas, F. Insights into the genetic variation of maternal behavior and suckling performance of continental beef cows. Genet. Sel. Evol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Quilter, C.R.; Blott, S.C.; Wilson, A.E.; Bagga, M.R.; Sargent, C.A.; Oliver, G.L.; Southwood, O.I.; Gilbert, C.L.; Mileham, A.; Affara, N.A. Porcine maternal infanticide as a model for puerperal psychosis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144, 862–868. [Google Scholar] [CrossRef]
- Quilter, C.R.; Sargent, C.A.; Bauer, J.; Bagga, M.R.; Reiter, C.P.; Hutchinson, E.L.; Southwood, O.I.; Evans, G.; Milaham, A.; Griffin, D.K.; et al. An association and haplotype analysis of porcine maternal infanticide: A model for human puerperal psychosis? Am. J. Med. Genet. Neuropsychiatr. Genet. 2012, 908–927. [Google Scholar] [CrossRef]
- Valente, T.S.; Baldi, F.; Sant’Anna, A.C.; Albuquerque, L.G.; Costa, M.J.R.P. Da Genome-wide association study between single nucleotide polymorphisms and flight speed in Nellore cattle. PLoS ONE 2016, 11, e0156956. [Google Scholar] [CrossRef]
- Bailey, D.W.; Lunt, S.; Lipka, A.; Thomas, M.G.; Medrano, J.F.; Cánovas, A.; Rincon, G.; Stephenson, M.B.; Jensen, D. Genetic influences on cattle grazing distribution: Association of genetic markers with terrain use in cattle. Rangel. Ecol. Manag. 2015, 68, 142–149. [Google Scholar] [CrossRef]
- Hazard, D.; Moreno, C.; Foulquié, D.; Delval, E.; Franҫois, D.; Bouix, J.; Sallé, G.; Boissy, A. Identification of QTLs for behavioral reactivity to social separation and humans in sheep using the OvineSNP50 BeadChip. BMC Genom. 2014, 15, 778. [Google Scholar] [CrossRef]
- Dreher, C.; Stratz, P.; Wellmann, R.; Hamann, H.; Bennewitz, J. Genome-wide association studies using BayesC and estimation of genetic parameters for perinatal sucking reflex in Brown Swiss calves. In Proceedings of the 11th World Congress on Genetics Applied Livestock Production, Auckland, New Zealand, 7–11 February 2018. [Google Scholar]
- Hiendleder, S.; Thomsen, H.; Reinsch, N.; Bennewitz, J.; Leyhe-Horn, B.; Looft, C.; Xu, N.; Medjugorac, I.; Russ, I.; Kühn, C.; et al. Mapping of QTL for Body Conformation and Behavior in Cattle. J. Hered. 2003, 94, 496–506. [Google Scholar] [CrossRef]
- Marete, A.; Sahana, G.; Fritz, S.; Lefebvre, R.; Barbat, A.; Lund, M.S.; Guldbrandtsen, B.; Boichard, D. Genome-wide association study for milking speed in French Holstein cows. J. Dairy Sci. 2018, 101, 6205–6219. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.C.; Peixoto, M.G.C.D.; De Souza Fonseca, P.A.; De Fátima Ávila Pires, M.; Ventura, R.V.; Da Cruz Rosse, I.; Bruneli, F.A.T.; Machado, M.A.; Carvalho, M.R.S. Identification of candidate genes for reactivity in guzerat (bos indicus) cattle: A genome-wide association study. PLoS ONE 2017, 12, e0169163. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, S.M. A QTL study of cattle behavioral traits in embryo transfer families. J. Hered. 2001, 92, 290–292. [Google Scholar] [CrossRef]
- Vallee, A.; Daures, J.; van Arendonk, J.A.M.; Bovenhuis, H. Genome-wide association study for behavior, type traits, and muscular development in Charolais beef cattle. J. Anim. Sci. 2016, 94, 2307–2316. [Google Scholar] [CrossRef]
- Ding, R.; Yang, M.; Wang, X.; Quan, J.; Zhuang, Z.; Zhou, S.; Li, S.; Xu, Z.; Zheng, E.; Cai, G.; et al. Genetic architecture of feeding behavior and feed efficiency in a Duroc pig population. Front. Genet. 2018, 9, 220. [Google Scholar] [CrossRef]
- Hong, J.K.; Jeong, Y.D.; Cho, E.S.; Choi, T.J.; Kim, Y.M.; Cho, K.H.; Lee, J.B.; Lim, H.T.; Lee, D.H. A genome-wide association study of social genetic effects in Landrace pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 784–790. [Google Scholar] [CrossRef]
- Desire, S.; Turner, S.P.; D’Eath, R.B.; Doeschl-Wilson, A.B.; Lewis, C.R.G.; Roehe, R. Genetic associations of short- and long-term aggressiveness identified by skin lesion with growth, feed efficiency, and carcass characteristics in growing pigs. J. Anim. Sci. 2015, 93, 3303–3312. [Google Scholar] [CrossRef]
- Wu, P.; Wang, K.; Yang, Q.; Zhou, J.; Chen, D.; Liu, Y.; Ma, J. association study for direct genetic effects and social genetic effects of six growth traits in Large White pigs. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ponsuksili, S.; Zebunke, M.; Murani, E.; Trakooljul, N.; Krieter, J.; Puppe, B.; Schwerin, M.; Wimmers, K. Integrated Genome-wide association and hypothalamus eQTL studies indicate a link between the circadian rhythm-related gene PER1 and coping behavior. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Keel, B.N.; Brown-Brandl, T.M.; Cassady, J.P.; Rohrer, G.A. Genome-wide association of changes in swine feeding behaviour due to heat stress. Genet. Sel. Evol. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Garza-Brenner, E.; Sifuentes-Rincón, A.M.; Randel, R.D.; Paredes-Sánchez, F.A.; Parra-Bracamonte, G.M.; Arellano Vera, W.; Rodríguez Almeida, F.A.; Segura Cabrera, A. Association of SNPs in dopamine and serotonin pathway genes and their interacting genes with temperament traits in Charolais cows. J. Appl. Genet. 2017, 58, 363–371. [Google Scholar] [CrossRef]
- Lindholm-Perry, A.K.; Kuehn, L.A.; Freetly, H.C.; Snelling, W.M. Genetic markers that influence feed efficiency phenotypes also affect cattle temperament as measured by flight speed. Anim. Genet. 2015, 46, 60–64. [Google Scholar] [CrossRef]
- Pierce, C.F. Identifying single nucleotide polymorphisms associated with beef cattle terrain-use in the western United States. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2019. [Google Scholar]
- Glenske, K.; Prinzenberg, E.M.; Brandt, H.; Gauly, M.; Erhardt, G. A chromosome-wide QTL study on BTA29 affecting temperament traits in German Angus beef cattle and mapping of DRD4. Animal 2011, 5, 195–197. [Google Scholar] [CrossRef]
- Chan, F.Y. Genome Wide Association Studies for Temperament in New Zealand Dairy Cattle. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2012; p. 106. [Google Scholar]
- Hulsman Hanna, L.L.; Garrick, D.J.; Gill, C.A.; Herring, A.D.; Riggs, P.K.; Miller, R.K.; Sanders, J.O.; Riley, D.G. Genome-wide association study of temperament and tenderness using different Bayesian approaches in a Nellore-Angus crossbred population. Livest. Sci. 2014, 161, 17–27. [Google Scholar] [CrossRef]
- Kolbehdari, D.; Wang, Z.; Grant, J.R.; Murdoch, B.; Prasad, A.; Xiu, Z.; Marques, E.; Stothard, P.; Moore, S.S. A Whole-Genome Scan to Map Quantitative Trait Loci for Conformation and Functional Traits in Canadian Holstein Bulls. J. Dairy Sci. 2008, 91, 2844–2856. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Erbe, M.; Seefried, F.R.; Gredler, B.; Bapst, B.; Bieber, A.; Simianer, H. Accuracy of direct genomic values for functional traits in Brown Swiss cattle. J. Dairy Sci. 2014, 97, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Macleod, I.M.; Bowman, P.J.; Chamberlain, A.J.; Vander Jagt, C.J.; Daetwyler, H.D.; Hayes, B.J.; Goddard, M.E. Genomic prediction and candidate gene discovery for dairy cattle temperament using sequence data and functional biology. In Proceedings of the 23rd Conference of the Association for the Advancement of Animal Breeding and Genetics (AAABG), Armidale, NSW, Australia, 27 October–1 November 2019; Volume 23, pp. 416–419. [Google Scholar]
- Riley, D.G.; Gill, C.A.; Boldt, C.R.; Funkhouser, R.R.; Herring, A.D.; Riggs, P.K.; Sawyer, J.E.; Lunt, D.K.; Sanders, J.O. Crossbred Bos indicus steer temperament as yearlings and whole genome association of steer temperament as yearlings and calf temperament post-weaning. J. Anim. Sci. 2016, 94, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ismail, M.K.; Miller, S.P.; Sargolzaei, M.; Grossi, D.A.; Nayeri, S.; Moore, S.S.; Plastow, G.; Stothard, P.; Schenkel, F. Genome Wide Association Analyses Identify New Loci for Milking Speed and Temperament in North American Holsteins. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Volume Genetics of Trait Complexes: Lactation, Vancouver, BC, Canada, 17–22 August 2014; pp. 4–6. [Google Scholar]
- Boichard, D.; Grohs, C.; Bourgeois, F.; Cerqueira, F.; Faugeras, R.; Neau, A.; Rupp, R.; Amigues, Y.; Boscher, M.Y.; Leveziel, H. Detection of genes influencing economic traits in three French dairy cattle breeds. Genet. Sel. Evol. 2003, 40, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jorjani, H.; Carlborg, Ö. A genome-wide association study using international breeding-evaluation data identifies major loci affecting production traits and stature in the Brown Swiss cattle breed. BMC Genet. 2012, 13, 82. [Google Scholar] [CrossRef]
- Jardim, J.G.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Association analysis for udder index and milking speed with imputed whole-genome sequence variants in Nordic Holstein cattle. J. Dairy Sci. 2017, 101, 2199–2212. [Google Scholar] [CrossRef]
- Sandor, C.; Farnir, F.; Hansoul, S.; Coppieters, W.; Meuwissen, T.; Georges, M. Linkage disequilibrium on the bovine X chromosome: Characterization and use in quantitative trait locus mapping. Genetics 2006, 173, 1777–1786. [Google Scholar] [CrossRef]
- Schrooten, C.; Bink, M.C.A.M.; Bovenhuis, H. Whole genome scan to detect chromosomal regions affecting multiple traits in dairy cattle. J. Dairy Sci. 2004, 87, 3550–3560. [Google Scholar] [CrossRef]
- Schrooten, C.; Bovenhuis, H.; Coppieters, W.; van Arendonk, J.A.M. Whole genome scan to detect quantitative trait loci for conformation and functional traits in dairy cattle. J. Dairy Sci. 2000, 83, 795–806. [Google Scholar] [CrossRef]
- Bauer, J. Investigation of the genetic components of maternal infanticide in Sus scrofa. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2018. [Google Scholar]
- Ma, Y.; Zhang, H.; Zhang, Q.; Ding, X. Identification of selection footprints on the X Chromosome in pig. PLoS ONE 2014, 9, e0094911. [Google Scholar] [CrossRef]
- Wilson, K.; Zanella, R.; Ventura, C.; Johansen, H.L.; Framstad, T.; Janczak, A.; Zanella, A.J.; Neibergs, H.L. Identification of chromosomal locations associated with tail biting and being a victim of tail-biting behaviour in the domestic pig (Sus scrofa domesticus). J. Appl. Genet. 2012, 53, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Reyer, H.; Shirali, M.; Ponsuksili, S.; Murani, E.; Varley, P.F.; Jensen, J.; Wimmers, K. Exploring the genetics of feed efficiency and feeding behaviour traits in a pig line highly selected for performance characteristics. Mol. Genet. Genom. 2017, 292, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Zhang, Z.Y.; Ma, J.W.; Ai, H.S.; Ren, J.; Huang, L.S. A genomewide association study of feed efficiency and feeding behaviors at two fattening stages in a white duroc × erhualian F2 population. J. Anim. Sci. 2015, 93, 1481–1489. [Google Scholar] [CrossRef]
- Qiu, X.; Ledger, J.; Zheng, C.; Martin, G.B.; Blache, D. Associations between temperament and gene polymorphisms in the brain dopaminergic system and the adrenal gland of sheep. Physiol. Behav. 2016, 153, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dreher, C.; Wellmann, R.; Stratz, P.; Schmid, M.; Preuß, S.; Hamann, H.; Bennewitz, J. Genomic analysis of perinatal sucking reflex in German Brown Swiss calves. J. Dairy Sci. 2019, 102, 6296–6305. [Google Scholar] [CrossRef]
- Yin, L.; Liu, C.; Hong, T.; Zhou, H.; Kae Hsiang, K. Design of system for monitoring dairy cattle’s behavioral features based on wireless sensor networks. Trans. Chin. Soc. Agric. Eng. 2010, 26, 203–208. [Google Scholar]
- Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens. Bio-Sens. Res. 2017, 12, 15–29. [Google Scholar] [CrossRef]
- Brito, L.F.; Oliveira, H.R.; McConn, B.; Schinckel, A.; Arrazola, A.; Marchant-Forde, J.; Johnson, J. Large-scale phenotyping of livestock welfare in commercial production systems: A new frontier in animal breeding. Front. Genet. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Velie, B.D.; Maltecca, C.; Cassady, J.P. Genetic relationships among pig behavior, growth, backfat, and loin muscle area. J. Anim. Sci. 2009, 87, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Archie, E.A.; Tung, J. Social behavior and the microbiome. Curr. Opin. Behav. Sci. 2015, 6, 28–34. [Google Scholar] [CrossRef]
- Silva, B.; Gonzalo, A.; Cañón, J. Genetic parameters of aggressiveness, ferocity and mobility in the fighting bull breed. Anim. Res. 2011, 55, 65–70. [Google Scholar] [CrossRef]
- Haskell, M.J.; Simm, G.; Simon, P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 368. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team, R. A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2019. [Google Scholar]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Warr, A.; Affara, N.; Aken, B.; Beiki, H.; Bickhart, D.M.; Billis, K.; Chow, W.; Eory, L.; Finlayson, H.A.; Flicek, P.; et al. An improved pig reference genome sequence to enable pig genetics and genomics research. Gigascience 2020, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Archibald, A.L.; Cockett, N.E.; Dalrymple, B.P.; Faraut, T.; Kijas, J.W.; Maddox, J.F.; McEwan, J.C.; Hutton Oddy, V.; Raadsma, H.W.; Wade, C.; et al. The sheep genome reference sequence: A work in progress. Anim. Genet. 2010, 41, 449–453. [Google Scholar] [CrossRef]
- Blake, J.A.; Dolan, M.; Drabkin, H.; Hill, D.P.; Ni, L.; Sitnikov, D.; Bridges, S.; Burgess, S.; Buza, T.; McCarthy, F.; et al. Gene ontology annotations and resources. Nucleic Acids Res. 2013, 41, 530–535. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4. [Google Scholar] [CrossRef]
- Chen, N.; Fu, W.; Zhao, J.; Shen, J.; Chen, Q.; Zheng, Z.; Chen, H.; Sonstegard, T.S.; Lei, C.; Jiang, Y. The Bovine Genome Variation Database (BGVD): Integrated Web-database for Bovine Sequencing Variations and Selective Signatures. bioRxiv 2019, 802223. [Google Scholar] [CrossRef]
- Jones, T.S. Measurement of Temperament in Beef Cattle and its Relationships to Animal Production Characteristics. Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2013. [Google Scholar]
- Smyth, G.; Hu, Y.; Dunn, P.; Phipson, B.; Chen, Y. Package ‘statmod’. 2020. [Google Scholar]
- Piñero, J.; Bravo, Á.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Genome Reference Consortium Human Build 38 Patch Release 13 (GRCh38.p13). Available online: www.ncbi.nlm.nih.gov/assembly/GCF_000001405.39 (accessed on 1 February 2020).
- Garza-Brenner, E. Analisis Genetico-Molecular del Temperament en Ganado Bovino: Busqueda y Associacion de Polimorfismos en Genes Candidatos. Available online: http://tesis.ipn.mx/handle/123456789/24604 (accessed on 1 September 2020).
- Esmailizadeh, A.K.; Morris, C.A.; Cullen, N.G.; Kruk, Z.A.; Lines, D.S.; Hickey, S.M.; Dobbie, P.M.; Bottema, C.D.K.; Pitchford, W.S. Genetic mapping of quantitative trait loci for meat quality and muscle metabolic traits in cattle. Anim. Genet. 2011, 42, 592–599. [Google Scholar] [CrossRef]
- Friedrich, J.; Brand, B.; Ponsuksili, S.; Graunke, K.L.; Langbein, J.; Knaust, J.; Kühn, C.; Schwerin, M. Detection of genetic variants affecting cattle behaviour and their impact on milk production: A genome-wide association study. Anim. Genet. 2016, 47, 12–18. [Google Scholar] [CrossRef]
- Glenske, K.; Brandt, H.; Prinzenberg, E.M.; Gauly, M.; Erhardt, G. Verification of a QTL on BTA1 for temperament in German Simmental and German Angus calves (Short Communication). Arch. Anim. Breed. 2010, 53, 388–392. [Google Scholar] [CrossRef][Green Version]
- Gutiérrez-Gil, B.; Ball, N.; Burton, D.; Haskell, M.; Williams, J.L.; Wiener, P. Identification of quantitative trait loci affecting cattle temperament. J. Hered. 2008, 99, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Spelman, R.J.; Huisman, A.E.; Singireddy, S.R.; Coppieters, W.; Arranz, J.; Georges, M.; Garrick, D.J. Short Communication: Quantitative Trait Loci Analysis on 17 Nonproduction Traits in the New Zealand Dairy Population. J. Dairy Sci. 1999, 82, 2514–2516. [Google Scholar] [CrossRef]
- Valente, T.; Abo-ismail, M.; Gentec, L.; Basarab, J.A.; Plastow, G.S. Genomic insights for feeding behavior traits in beef cattle. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Volume Biology—Feed Intake and Efficiency 1, 234, Auckland, New Zealand, 7–11 February 2018. [Google Scholar]
- Terenina, E.; Babigumira, B.M.; Le Mignon, G.; Bazovkina, D.; Rousseau, S.; Salin, F.; Bendixen, C.; Mormede, P. Association study of molecular polymorphisms in candidate genes related to stress responses with production and meat quality traits in pigs. Domest. Anim. Endocrinol. 2013, 44, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Desire, S. Genetic and Environmental Dissection of Short- and Long-Term Social Aggression in Pigs. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 2015. [Google Scholar]
- Murani, E.; Ponsuksili, S.; D’Eath, R.B.; Turner, S.P.; Kurt, E.; Evans, G.; Tholking, L.; Klont, R.; Foury, A.; Mormede, P.; et al. Association of HPA axis-related genetic variation with stress reactivity and aggressive behaviour in pigs. BMC Genet. 2010, 11, 74. [Google Scholar] [CrossRef]
- Murani, E.; Reyer, H.; Ponsuksili, S.; Fritschka, S.; Wimmers, K. A Substitution in the Ligand Binding Domain of the Porcine Glucocorticoid Receptor Affects Activity of the Adrenal Gland. PLoS ONE 2012, 7, e0045518. [Google Scholar] [CrossRef]
- Okamura, T.; Onodera, W.; Tayama, T.; Kadowaki, H.; Kojima-Shibata, C.; Suzuki, E.; Uemoto, Y.; Mikawa, S.; Hayashi, T.; Awata, T.; et al. A genome-wide scan for quantitative trait loci affecting respiratory disease and immune capacity in Landrace pigs. Anim. Genet. 2012, 43, 721–729. [Google Scholar] [CrossRef]
- Ding, R.; Quan, J.; Yang, M.; Wang, X.; Zheng, E.; Yang, H.; Fu, D.; Yang, Y.; Yang, L.; Li, Z.; et al. Genome-wide association analysis reveals genetic loci and candidate genes for feeding behavior and eating efficiency in Duroc boars. PLoS ONE 2017, 12, e0183244. [Google Scholar] [CrossRef]
- Do, D.N.; Strathe, A.B.; Ostersen, T.; Jensen, J.; Mark, T.; Kadarmideen, H.N. Genome-wide association study reveals genetic architecture of eating behavior in pigs and its implications for humans obesity by comparative mapping. PLoS ONE 2013, 8, e0071509. [Google Scholar] [CrossRef] [PubMed]
- Reiner, G.; Köhler, F.; Berge, T.; Fischer, R.; Hübner-Weitz, K.; Scholl, J.; Willems, H. Mapping of quantitative trait loci affecting behaviour in swine. Anim. Genet. 2009, 40, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Gley, K.; Murani, E.; Haack, F.; Trakooljul, N.; Zebunke, M.; Puppe, B.; Wimmers, K.; Ponsuksili, S. Haplotypes of coping behavior associated QTL regions reveal distinct transcript profiles in amygdala and hippocampus. Behav. Brain Res. 2019, 372, 112038. [Google Scholar] [CrossRef] [PubMed]
- Görres, A.; Ponsuksili, S.; Wimmers, K.; Muráni, E. Analysis of non-synonymous SNPs of the porcine SERPINA6 gene as potential causal variants for a QTL affecting plasma cortisol levels on SSC7. Anim. Genet. 2015, 46, 239–246. [Google Scholar] [CrossRef]
- Wurtz, K.E.; Siegford, J.M.; Ernst, C.W.; Raney, N.E.; Bates, R.O.; Steibel, J.P. Genome-wide association analyses of lesion counts in group-housed pigs. Anim. Genet. 2018, 49, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Houston, R.D.; Haley, C.S.; Archibald, A.L.; Rance, K.A. A QTL affecting daily feed intake maps to Chromosome 2 in pigs. Mamm. Genome 2005, 16, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.D.; You, Q.; Schenkel, L.C.; Voort, G.V.; Schenkel, F.S.; Wilton, J.; Cain, L.; Karrow, N.A. A genome-wide association study to identify chromosomal regions influencing ovine cortisol response. Livest. Sci. 2016, 187, 40–47. [Google Scholar] [CrossRef]
- Poissant, J.; Réale, D.; Martin, J.G.A.; Festa-Bianchet, M.; Coltman, D.W. A quantitative trait locus analysis of personality in wild bighorn sheep. Ecol. Evol. 2013, 3, 474–481. [Google Scholar] [CrossRef]
- Yu, H.; Morota, G.; Celestino, E.F.; Dahlen, C.R.; Wagner, S.A.; Riley, D.G.; Hulsman Hanna, L.L. Deciphering cattle temperament measures derived from a four-platform standing scale using genetic factor analytic modeling. Front. Genet. 2020. [Google Scholar] [CrossRef]
- Rohrer, G.A.; Brown-Brandl, T.; Rempel, L.A.; Schneider, J.F.; Holl, J. Genetic analysis of behavior traits in swine production. Livest. Sci. 2013, 157, 28–37. [Google Scholar] [CrossRef]
- Sant’Anna, A.C.; Baldi, F.; Valente, T.S.; Albuquerque, L.G.; Menezes, L.M.; Boligon, A.A.; Paranhos da Costa, M.J.R. Genetic associations between temperament and performance traits in Nellore beef cattle. J. Anim. Breed. Genet. 2015, 132, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, A.L.; Gipson, T.A.; Askar, A.R.; Puchala, R. Invited review: Feeding behavior of goats. J. Anim. Sci. 2010, 88, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.M. Individual differences in temperament of dairy goats and the inhibition of milk ejection. Appl. Anim. Behav. Sci. 1989, 22, 269–282. [Google Scholar] [CrossRef]
- Marino, L. Thinking chickens: A review of cognition, emotion, and behavior in the domestic chicken. Anim. Cogn. 2017, 20, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M.; Williams, M.J.; Jensen, P.; Wright, D. Genetical genomics of behavior: A novel chicken genomic model for anxiety behavior. Genetics 2016, 202, 327–340. [Google Scholar] [CrossRef]
- Niepoth, N.; Bendesky, A. How Natural Genetic Variation Shapes Behavior. Annu. Rev. Genomics Hum. Genet. 2020, 21, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Köhn, L.; Kadzhaev, K.; Burstedt, M.S.I.; Haraldsson, S.; Hallberg, B.; Sandgren, O.; Golovleva, I. Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. Eur. J. Hum. Genet. 2007, 15, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Nakabayashi, K.; Fujimoto, T.; Gu, N.; Baba, I.; Takashima, Y.; Doi, K.; Harada, H.; Kato, N.; Sasazuki, T.; et al. ZFAT expression in B and T lymphocytes and identification of ZFAT-regulated genes. Genomics 2008, 91, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Jenko, J.; McClure, M.C.; Matthews, D.; McClure, J.; Johnsson, M.; Gorjanc, G.; Hickey, J.M. Analysis of a large dataset reveals haplotypes carrying putatively recessive lethal and semi-lethal alleles with pleiotropic effects on economically important traits in beef cattle. Genet. Sel. Evol. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Schwarz, J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012, 33, 267–286. [Google Scholar] [CrossRef]
- Romio, L.; Fry, A.M.; Winyard, P.J.D.; Malcolm, S.; Woolf, A.S.; Feather, S.A. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J. Am. Soc. Nephrol. 2004, 15, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, G.; Alfieri, M.; Prattichizzo, C.; Zullo, A.; Cairo, S.; Franco, B. Functional Characterization of the OFD1 Protein Reveals a Nuclear Localization and Physical Interaction with Subunits of a Chromatin Remodeling Complex. Mol. Biol. Cell 2007, 18, 4397–4404. [Google Scholar] [CrossRef]
- Gerdes, J.M.; Davis, E.E.; Katsanis, N. The Vertebrate Primary Cilium in Development, Homeostasis, and Disease. Cell 2009, 137, 32–45. [Google Scholar] [CrossRef]
- Bisgrove, B.W.; Yost, H.J. The roles of cilia in developmental disorders and disease. Development 2006, 133, 4131–4143. [Google Scholar] [CrossRef]
- Cheng, H.W. Breeding of tomorrow’s chickens to improve well-being. Poult. Sci. 2010, 89, 805–813. [Google Scholar] [CrossRef]
- Grimsby, J.; Toth, M.; Chenl, K.; Kumazawa, T.; Klaidman, L.; Adams, J.D.; Karoum, F.; Gap, J.; Shih, J.C. Increased stress response and Beta-phenylethylamine in MAOB-deficient mice. Nature 1997, 17, 206–210. [Google Scholar]
- Chung, H.Y.; McClureb, M.C. Effects of SNPs from the differentially expressed swine odorant binding protein gene on average daily gain. J. Appl. Anim. Res. 2011, 39, 61–64. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Jones, R.B.; Schofield, C.P.; White, R.P.; Wathes, C.M. The use of olfactory and other cues for social recognition by juvenile pigs. Appl. Anim. Behav. Sci. 2001, 72, 321–333. [Google Scholar] [CrossRef]
- Ho-Shing, O.; Dulac, C. Influences of genomic imprinting on brain function and behavior. Curr. Opin. Behav. Sci. 2019, 25, 66–76. [Google Scholar] [CrossRef]
- Bando, Y.; Hirano, T.; Tagawa, Y. Dysfunction of KCNK potassium channels impairs neuronal migration in the developing mouse cerebral cortex. Cereb. Cortex 2014, 24, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, I.; Zanzouri, M.; Honoré, E.; Duprat, F.; Ehrengruber, M.U.; Lazdunski, M.; Patel, A.J. K+-dependent cerebellar granule neuron apoptosis. Role of TASK leak K+ channels. J. Biol. Chem. 2003, 278, 32068–32076. [Google Scholar] [CrossRef]
- Brenner, T.; O’Shaughnessy, K.M. Both TASK-3 and TREK-1 two-pore loop K channels are expressed in H295R cells and modulate their membrane potential and aldosterone secretion. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Barel, O.; Shalev, S.A.; Ofir, R.; Cohen, A.; Zlotogora, J.; Shorer, Z.; Mazor, G.; Finer, G.; Khateeb, S.; Zilberberg, N.; et al. Maternally Inherited Birk Barel Mental Retardation Dysmorphism Syndrome Caused by a Mutation in the Genomically Imprinted Potassium Channel KCNK9. Am. J. Hum. Genet. 2008, 83, 193–199. [Google Scholar] [CrossRef]
- Aykaç, A.; Şehirli, A.Ö. The Role of the SLC Transporters Protein in the Neurodegenerative Disorders. Clin. Psychopharmacol. Neurosci. 2020, 18, 174–187. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J. Agonistic Behavior in Food Animals: Review of Research and Techniques. J. Anim. Sci. 1986, 62, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Dorries, K.M.; Adkins-regan, E.; Halpern, B.P. Olfactory sensitivity to the pheromone, androstenone, is sexually dimorphic in the pig. Physiol. Behav. 1995, 57, 255–259. [Google Scholar] [CrossRef]
- Rekwot, P.I.; Ogwu, D.; Oyedipe, E.O.; Sekoni, V.O. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 2001, 65, 157–170. [Google Scholar] [CrossRef]
- Carter, C.S.; Grippo, A.J.; Pournajafi-Nazarloo, H.; Ruscio, M.G.; Porges, S.W. Oxytocin, vasopressin and sociality. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 170, pp. 331–336. ISBN 9780444532015. [Google Scholar]
- Johnson, Z.V.; Young, L.J. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef]
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J.L. Salivary oxytocin in pigs, cattle, and goats during positive human-animal interactions. Psychoneuroendocrinology 2020, 115, 104636. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Hall, J.B.; Estill, C.T.; Kastelic, J.P.; Joseph, C.; Abdel Aziz, R.L.; Nak, D. Flunixin meglumine improves pregnancy rate in embryo recipient beef cows with an excitable temperament. Theriogenology 2018, 107, 70–77. [Google Scholar] [CrossRef]
- Yang, F.L.; Anschutz, K.S.; Ball, J.J.; Hornsby, P.; Reynolds, J.L.; Pohlman, F.W. Evaluating the Relationship of Animal Temperament to Carcass Characteristics and Meat Quality. Meat Muscle Biol. 2019, 3, 70. [Google Scholar] [CrossRef]
- Gianola, D.; de los Campos, G.; Toro, M.A.; Naya, H.; Schön, C.C.; Sorensen, D. Do molecular markers inform about pleiotropy? Genetics 2015, 201, 23–29. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef]
- Calvo, J.H.; Iguácel, L.P.; Kirinus, J.K.; Serrano, M.; Ripoll, G.; Casasús, I.; Joy, M.; Pérez-Velasco, L.; Sarto, P.; Albertí, P.; et al. A new single nucleotide polymorphism in the calpastatin (CAST) gene associated with beef tenderness. Meat Sci. 2014, 96, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, C.R.; Plante, Y.; Schmutz, S.M. Comparison of Angus cattle populations using gene variants and microsatellites. Can. J. Anim. Sci. 2011, 91, 81–85. [Google Scholar] [CrossRef][Green Version]
- Curi, R.A.; Chardulo, L.A.L.; Mason, M.C.; Arrigoni, M.D.B.; Silveira, A.C.; De Oliveira, H.N. Effect of single nucleotide polymorphisms of CAPN1 and CAST genes on meat traits in Nellore beef cattle (Bos indicus) and in their crosses with Bos taurus. Anim. Genet. 2009, 40, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hu, V.W.; Addington, A.; Hyman, A. Novel autism subtype-dependent genetic variants are revealed by quantitative trait and subphenotype association analyses of Published GWAS Data. PLoS ONE 2011, 6, e0019067. [Google Scholar] [CrossRef]
- Yokoyama, M.; Suzuki, E.; Sato, T.; Maruta, S.; Watanabe, S.; Miyaoka, H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci. Lett. 2005, 379, 37–41. [Google Scholar] [CrossRef]
- Hull, E.M.; Muschamp, J.W.; Sato, S. Dopamine and serotonin: Influences on male sexual behavior. Physiol. Behav. 2004, 83, 291–307. [Google Scholar] [CrossRef]
- Pittenger, C.; Bloch, M.H.; Williams, K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol. Ther. 2011, 132, 314–332. [Google Scholar] [CrossRef] [PubMed]

| Species | Breed Background 1 |
|---|---|
| Cattle | Angus, Angus Moiled, Australian Red, Blonde d’aquitaine, Brown Swiss, Brangus, Brahman, Belgian Blue, Charolais, Finnish Ayrshire, Guzerat, Gelbvieh, Hereford, Holstein, Jersey, Limousin, Montbeliarde, Normande, Red Angus, Simmental, Tarentaise |
| Pigs | Landrace, Large White, Duroc, White Duroc, Erhualian, Yorkshire, Meishan, Pietran, Songliao |
| Sheep | Merino, Romanov, Berrichon du Cher, Ram Mountain, Rideu-Arcott |
| Reference | Behavioral Trait (Number of Genomic Regions) | Reference | Behavioral Trait (Number of Genomic Regions) |
|---|---|---|---|
| Abo-Ismail et al. [58] | Milking speed (13) 1, Milking temperament (15) | Kolbehdari et al. [54] | Milking speed (20), Temperament (16) |
| Bailey et al. [34] | Terrain-use indices (rolling; 40), Terrain-use indices (rough; 42) | Kramer et al. [55] | Aggressiveness (2), Milking speed (5), Milking temperament (1), Rank order in herd (4), Temperament (2) |
| Boichard et al. [59] | Milking speed (4) | Lindholm-Perry et al. [49] | Flight speed (24) |
| Chan [52] | Temperament (21) | MacLeod et al. [56] | Temperament (10) |
| Santos [39] | Adjusted reactivity (14) | Marete et al. [38] | Milking speed (7) |
| Dreher et al. [71] | Sucking reflex (38) | Michenet et al. [30] | Maternal behavior (1) |
| Esmailizadeh et al. [93] | Blood cortisol level (1) | Sandor et al. [62] | Milking speed (2) |
| Friedrich et al. [94] | Duration activity (novel object test— OT; 12), Duration activity (open-field; 8), Duration inactivity (OT; 14), Duration inactivity (open-field; 18), Duration of the arena exploration (OT; 32), Duration of the arena exploration (open-field; 2) | Pierce [50] | Distance travelled from water (10), Elevation traversed (10), Percent slope traversed (10), Terrain-use indices (24)Terrain-use indices (rolling; 14), Terrain-use indices (rough; 14), Vertical climb (10) |
| Garza-Brenner [92] | Exit velocity (2), Pen score (4), Total score (Pen score and Exit velocity; 2) | Riley et al. [57] | Aggressive behavior (2), Temperament (6) |
| Garza-Brenner et al. [48] | Flight speed (4), Temperament (12) | Schmutz [40] | Behavior (6) |
| Glenske et al. [95] | Response to weighting process (2), Restraint docility test (1), Temperament (2) | Schrooten et al. [64] | Milking speed (2) |
| Guo et al. [69] | Milking speed (4) | Schrooten et al. [63] | Milking speed (9) |
| Gutierrez-Gil et al. [96] | Flight for feeder (6), Standing alert (3), Vocalization (22), Walking/running (9) | Spelman et al. [97] | Milking temperament (2) |
| Hanna et al. [53] | Temperament (66) | Valente et al. [33] | Flight speed (9) |
| Hiendleder et al. [37] | Milking speed (3)Temperament (3) | Valente et al. [98] | Daily feeding duration (3)Feeding event frequency (5) |
| Jardim et al. [61] | Milking speed (18) | Valle et al. [41] | Aggressive behavior (14), Locomotion (2), Maternal behavior (4) |
| Reference | Behavioral Trait (Number of Genomic Regions) | Reference | Behavioral Trait (Number of Genomic Regions) |
|---|---|---|---|
| Bauer [65] | Infanticide (93) 1 | Murani et al. [101] | Aggressive behavior (front lesions; 3; middle lesions; 2; rear lesions; 1; total lesions; 2), Cortisol level (1), Physiological stress (creatine kinase; 1), Physiological stress (glucose; 1) |
| Cross et al. [47] | Feeding behavior (66) | Murani et al. [102] | Cortisol level (27) |
| Desautes et al. [27] | Cortisol level (2), Cortisol level variations (1), Exploration during stress (1) | Ponsuksili et al. [46] | Backtest traits (latency, duration; and frequency of response; 132), Duration of struggling bouts (318), Frequency of struggling bouts (342), Latency at an age of 12 days (81) |
| Desire et al. [44] | Skin lesion traits (19) | Okamura et al. [103] | Cortisol level (1) |
| Ding et al. [104] | Mean feed intake/visit (1), Number of feeder visits/day (2), Time in the feeder/visit (1), Time in the feeder/day (2) | Quilter et al. [32] | Infanticide (8) |
| Do et al. [105] | Time in the feeder/day (12), Time in the feeder/visit (10) | Reiner et al. [106] | Time spent lying (1), Time spent socializing (1) |
| Gley et al. [107] | Coping behavior (20) | Reyer et al. [68] | Daily feeder visits (5), Daily feeding rate (8), Time in the feeder/day (9) |
| Gorres et al. [108] | Cortisol level (6) | Wurtz et al. [109] | Anterior lesions (1) |
| Guo et al. [69] | Daily feeding times (day 120 to 240; 1), Daily feeding times (day 210 to 240; 1), Daily visit times (day 210 to 240; 1), Feed intake/second (day 120 to 240; 2), Feed intake/second (day 210 to 240; 1), Feed intake/visit (day 120 to 240; 2) | Terenina et al. [99] | Adrenaline/creatinine level (2), Adrenaline/noradrenaline level (2) Cortisol/creatinine level (2), Cortisol+cortisone/creatinine level (3), Cortisone/cortisol ratios (2), Nor Adrenaline/creatinine level (2) Plasma cortisol at slaughter (3), Plasma creatine kinase at slaughter (1), Plasma glucose measured at slaughter (1), Plasma lactate at slaughter (1), Relative weight left adrenal gland (2), Relative weight right adrenal gland (2) |
| Hong et al. [43] | Social average daily gain (5) | Wilson et al. [67] | Tail biting (1), Victim of tail biting (5) |
| Houston et al. [110] | Time in the feeder/day (1) | Wu et al. [45] | Social genetic effect (average daily feed intake; 15), Social genetic effect (average daily gain; 19), Social genetic effect (backfat thickness to 100 kg; 17), Social genetic effect (backfat thickness; 25), Social genetic effect (days to 100 kg; 41), Social genetic effect (feed conversion ratio; 22), Social genetic effect (residual feed intake; 27) |
| Ma et al. [66] | Infanticide (1) |
| Reference | Behavioral Trait (Number of Genomic Regions) |
|---|---|
| Hazard et al. [35] | Distance from the human (corridor test 2; 4) 1, Locomotion (arena test 1; 2), Locomotion (arena test 2; 13), Locomotion (corridor test 1; 1), Locomotion (isolation box test; 2), Locomotion (social isolation; 2), The proximity to flock-mates and the human (arena test 2; 2), Time during which the human saw the lamb (corridor test 2; 1), Vocalization—high bleat (arena test 1; 14), Vocalization—high bleat (arena test 2; 10), Vocalization—high bleat (corridor test 1; 8), Vocalization—high bleat (isolation box test; 6), Vocalization—high bleat (social isolation; 26), Vocalization—low bleat (arena test 1; 18), Vocalization—low bleat (arena test 2; 7), Vocalization—low bleat (corridor test 1; 2), Vocalization—low bleat (social isolation; 10) |
| Pant et al. [111] | Endotoxin-induced cortisol response (16) |
| Poissant et al. [112] | Docility (1) |
| Qiu et al. [70] | Locomotion—frequency the animal crossed from one sector into another (1), Number of bleats (1) |
| Human Gene | Gene Description | Human CHR | Cattle Gene | Cattle CHR | Pig Gene | Pig CHR | Sheep Gene | Sheep CHR |
|---|---|---|---|---|---|---|---|---|
| ADAM10 | ADAM metallopeptidase domain 10 | 15 | ADAM10 | 10 | ADAM10 | 1 | ENSOARG00000002399 | |
| ADAMTS2 | ADAM metallopeptidase with thrombospondin type 1 motif 2 | 5 | ADAMTS2 1 | 7 | ADAMTS2 | 2 | ADAMTS2 | 5 |
| CAST | Calpastatin | 5 | CAST | 7 | CAST | 2 | CAST | 5 |
| CDC6 | Cell division cycle 6 | 17 | CDC6 | 19 | CDC6 | 12 | CDC6 | 11 |
| CSE1L | Chromosome segregation 1 like | 20 | CSE1L | 13 | CSE1L | 17 | CSE1L | 13 |
| FAM135B | Family with sequence similarity 135 member B | 8 | FAM135B | 14 | FAM135B | 4 | FAM135B | 9 |
| FAM177A1 | Family with sequence similarity 177 member A1 | 14 | FAM177A1 | 21 | FAM177A1 | 7 | FAM177A1 | 18 |
| GRM5 | Glutamate metabotropic receptor 5 | 11 | GRM5 | 29 | GRM5 | 9 | ENSOARG00000014407 | 21 |
| HTR2C | 5-Hydroxytryptamine receptor 2C | X | HTR2C | X | HTR2C | X | HTR2C | X |
| KCNK9 | Potassium two pore domain channel subfamily K member 9 | 8 | KCNK9 | 14 | KCNK9 | 4 | ENSOARG00000004303 | 9 |
| MACROD2 | Mono-ADP ribosylhydrolase 2 | 20 | ENSBTAG00000048850 | 13 | MACROD2 | 17 | ENSOARG00000011613 | 13 |
| MAML3 | Mastermind like transcriptional coactivator 3 | 4 | MAML3 | 17 | MAML3 | 8 | MAML3 | 17 |
| MAOA | Monoamine oxidase A | X | MAOA | X | ENSSSCG00000012257 | X | MAOA | X |
| MAOB | Monoamine oxidase B | X | MAOB | X | MAOB | X | MAOB | X |
| MIR1912 | MicroRNA 1912 | X | MIR1912 | X | MIR1912 | X | ||
| MUSK | Muscle associated receptor tyrosine kinase | 9 | MUSK | 8 | MUSK | 1 | MUSK | 2 |
| NR3C2 | Nuclear receptor subfamily 3 group C member 2 | 4 | NR3C2 | 17 | NR3C2 | 8 | ENSOARG00000007116 | 17 |
| OFD1 | OFD1 centriole and centriolar satellite protein | X | OFD1 | X | ENSSSCG00000012125 | X | OFD1 | X |
| OR2B6 | Olfactory receptor family 2 subfamily B member 6 | 6 | OR2B6 | 23 | OR2B6 | 7 | ENSOARG00000009162 | 20 |
| OR51E2 | Olfactory receptor family 51 subfamily E member 2 | 11 | OR51E2 | 15 | OR51E2 | 9 | OR51E2 | 15 |
| OR52J3 | Olfactory receptor family 52 subfamily J member 3 | 11 | ENSBTAG00000038075 | 15 | OR52J3 | 9 | ENSOARG00000006229 | 15 |
| OR56A1 | Olfactory receptor family 56 subfamily A member 1 | 11 | OR56A1 | 15 | OR56A1 | 9 | OR56A1 | 15 |
| OR9Q2 | Olfactory receptor family 9 subfamily Q member 2 | 11 | OR9Q2 | 15 | OR9Q2 | 2 | OR9Q2 | 15 |
| PITPNM3 | PITPNM family member 3 | 17 | PITPNM3 | 19 | PITPNM3 | 12 | PITPNM3 | 11 |
| PLCB1 | Phospholipase C beta 1 | 20 | PLCB1 | 13 | PLCB1 | 17 | PLCB1 | 13 |
| POMC | Proopiomelanocortin | 2 | POMC | 11 | ENSSSCG00000033439 | POMC | 3 | |
| RERG | RAS like estrogen regulated growth inhibitor | 12 | RERG | 5 | RERG | 5 | RERG | 3 |
| SLC13A5 | Solute carrier family 13 member 5 | 17 | SLC13A5 | 19 | SLC13A5 | 12 | SLC13A5 | 11 |
| SLC16A11 | Solute carrier family 16 member 11 | 17 | SLC16A11 | 19 | SLC16A11 | 12 | SLC16A11 | 11 |
| SLC18A2 | Solute carrier family 18 member A2 | 10 | SLC18A2 | 26 | SLC18A2 | 14 | SLC18A2 | 22 |
| SLC25A4 | Solute carrier family 25 member 4 | 4 | SLC25A4 | 27 | SLC25A4 | 15 | SLC25A4 | 26 |
| SLC5A2 | Solute carrier family 5 member 2 | 16 | SLC5A2 | 25 | SLC5A2 | 3 | SLC5A2 | 24 |
| SLC6A2 | Solute carrier family 6 member 2 | 16 | SLC6A2 | 18 | SLC6A2 | 6 | SLC6A2 | 14 |
| SPNS3 | Sphingolipid transporter 3 (putative) | 17 | SPNS3 | 19 | SPNS3 | 12 | SPNS3 | 11 |
| ZFAT | Zinc finger and AT-hook domain containing | 8 | ZFAT | 14 | ZFAT | 4 | ZFAT | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarenga, A.B.; Oliveira, H.R.; Chen, S.-Y.; Miller, S.P.; Marchant-Forde, J.N.; Grigoletto, L.; Brito, L.F. A Systematic Review of Genomic Regions and Candidate Genes Underlying Behavioral Traits in Farmed Mammals and Their Link with Human Disorders. Animals 2021, 11, 715. https://doi.org/10.3390/ani11030715
Alvarenga AB, Oliveira HR, Chen S-Y, Miller SP, Marchant-Forde JN, Grigoletto L, Brito LF. A Systematic Review of Genomic Regions and Candidate Genes Underlying Behavioral Traits in Farmed Mammals and Their Link with Human Disorders. Animals. 2021; 11(3):715. https://doi.org/10.3390/ani11030715
Chicago/Turabian StyleAlvarenga, Amanda B., Hinayah R. Oliveira, Shi-Yi Chen, Stephen P. Miller, Jeremy N. Marchant-Forde, Lais Grigoletto, and Luiz F. Brito. 2021. "A Systematic Review of Genomic Regions and Candidate Genes Underlying Behavioral Traits in Farmed Mammals and Their Link with Human Disorders" Animals 11, no. 3: 715. https://doi.org/10.3390/ani11030715
APA StyleAlvarenga, A. B., Oliveira, H. R., Chen, S.-Y., Miller, S. P., Marchant-Forde, J. N., Grigoletto, L., & Brito, L. F. (2021). A Systematic Review of Genomic Regions and Candidate Genes Underlying Behavioral Traits in Farmed Mammals and Their Link with Human Disorders. Animals, 11(3), 715. https://doi.org/10.3390/ani11030715







