Impacts of in Utero Heat Stress on Carcass and Meat Quality Traits of Market Weight Gilts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Preparation
2.2. Carcass Evaluation
2.3. pH and Water-Holding Ability
2.4. Instrumental Display Color
2.5. Shear Force
2.6. Western Blotting
2.7. Transmission Value
2.8. Lipid Oxidation
2.9. Fatty Acid Profiling
2.10. Statistical Analysis
3. Results
3.1. Carcass Evaluation
3.2. Meat Quality
3.2.1. pH and Water-Holding Ability
3.2.2. Instrumental Display Color
3.2.3. Shear Force and Western Blotting
3.2.4. Transmission Value, Lipid Oxidation and Fatty Acid Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgard, L.H.; Rhoads, R.P.; Rhoads, M.L.; Gabler, N.K.; Ross, J.W.; Keating, A.F.; Boddicker, R.L.; Lenka, S.; Sejian, V. Impact of Climate Change on Livestock Production. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S.M.K., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 413–468. ISBN 978-3-642-29204-0. [Google Scholar]
- Ross, J.W.; Hale, B.J.; Gabler, N.K.; Rhoads, R.P.; Keating, A.F.; Baumgard, L.H. Physiological Consequences of Heat Stress in Pigs. Anim. Prod. Sci. 2015, 55, 1381. [Google Scholar] [CrossRef]
- Johnson, J.S.; Stewart, K.R.; Safranski, T.J.; Ross, J.W.; Baumgard, L.H. In Utero Heat Stress Alters Postnatal Phenotypes in Swine. Theriogenology 2020, 154, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Boddicker, R.L.; Seibert, J.T.; Johnson, J.S.; Pearce, S.C.; Selsby, J.T.; Gabler, N.K.; Lucy, M.C.; Safranski, T.J.; Rhoads, R.P.; Baumgard, L.H.; et al. Gestational Heat Stress Alters Postnatal Offspring Body Composition Indices and Metabolic Parameters in Pigs. PLoS ONE 2014, 9, e110859. [Google Scholar] [CrossRef]
- Serviento, A.M.; Merlot, E.; Prunier, A.; Quesnel, H.; Louveau, I.; Lebret, B.; Renaudeau, D. Heat stress in pregnant sows: Effects on growth performance and carcass composition of the offspring. In Proceedings of the Energy and Protein Metabolism and Nutrition, Vichy, France, 9–13 September 2007; Wageningen Academic Publishers: Belo Horizonte, Brazil, 2019; pp. 351–353. [Google Scholar]
- Johnson, J.S.; Sanz Fernandez, M.V.; Patience, J.F.; Ross, J.W.; Gabler, N.K.; Lucy, M.C.; Safranski, T.J.; Rhoads, R.P.; Baumgard, L.H. Effects of in Utero Heat Stress on Postnatal Body Composition in Pigs: II. Finishing Phase. J. Anim. Sci. 2015, 93, 82–92. [Google Scholar] [CrossRef]
- Johnson, J.S.; Sanz Fernandez, M.V.; Gutierrez, N.A.; Patience, J.F.; Ross, J.W.; Gabler, N.K.; Lucy, M.C.; Safranski, T.J.; Rhoads, R.P.; Baumgard, L.H. Effects of in Utero Heat Stress on Postnatal Body Composition in Pigs: I. Growing Phase. J. Anim. Sci. 2015, 93, 71–81. [Google Scholar] [CrossRef]
- Cruzen, S.M.; Boddicker, R.L.; Graves, K.L.; Johnson, T.P.; Arkfeld, E.K.; Baumgard, L.H.; Ross, J.W.; Safranski, T.J.; Lucy, M.C.; Lonergan, S.M. Carcass Composition of Market Weight Pigs Subjected to Heat Stress in Utero and during Finishing. J. Anim. Sci. 2015, 93, 2587–2596. [Google Scholar] [CrossRef]
- Byrd, C.; Anderson, N.; Lugar, D.; Safranski, T.; Lucy, M.; Johnson, J. Evaluating the Effects of In Utero Heat Stress on Piglet Physiology and Behavior Following Weaning and Transport. Animals 2019, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Maskal, J.M.; Duttlinger, A.W.; Kpodo, K.R.; McConn, B.R.; Byrd, C.J.; Richert, B.T.; Marchant-Forde, J.N.; Lay, D.C.; Perry, S.D.; Lucy, M.C.; et al. Evaluation and Mitigation of the Effects of in Utero Heat Stress on Piglet Growth Performance, Postabsorptive Metabolism, and Stress Response Following Weaning and Transport. J. Anim. Sci. 2020, 98, skaa265. [Google Scholar] [CrossRef] [PubMed]
- Chapel, N.M.; Byrd, C.J.; Lugar, D.W.; Morello, G.M.; Baumgard, L.H.; Ross, J.W.; Safranski, T.J.; Lucy, M.C.; Johnson, J.S. Determining the Effects of Early Gestation in Utero Heat Stress on Postnatal Fasting Heat Production and Circulating Biomarkers Associated with Metabolism in Growing Pigs. J. Anim. Sci. 2017, 95, 3914. [Google Scholar] [CrossRef]
- Machado-Neto, R.; Graves, C.N.; Curtis, S.E. Immunoglobulins in Piglets from Sows Heat-Stressed Prepartum. J. Anim. Sci. 1987, 65, 445–455. [Google Scholar] [CrossRef]
- Čobanović, N.; Stanković, S.D.; Dimitrijević, M.; Suvajdžić, B.; Grković, N.; Vasilev, D.; Karabasil, N. Identifying Physiological Stress Biomarkers for Prediction of Pork Quality Variation. Animals 2020, 10, 614. [Google Scholar] [CrossRef]
- Bee, G.; Anderson, A.L.; Lonergan, S.M.; Huff-Lonergan, E. Rate and Extent of PH Decline Affect Proteolysis of Cytoskeletal Proteins and Water-Holding Capacity in Pork. Meat Sci. 2007, 76, 359–365. [Google Scholar] [CrossRef]
- Bidner, B.S.; Ellis, M.; Brewer, M.S.; Campion, D.; Wilson, E.R.; McKeith, F.K. Effect of ultimate pH on the quality characteristics of pork. J. Muscle Foods 2004, 15, 139–154. [Google Scholar] [CrossRef]
- Lindahl, G.; Henckel, P.; Karlsson, A.H.; Andersen, H.J. Significance of Early Postmortem Temperature and PH Decline on Colour Characteristics of Pork Loin from Different Crossbreeds. Meat Sci. 2006, 72, 613–623. [Google Scholar] [CrossRef]
- Lee, Y.B.; Choi, Y.I. PSE (Pale, Soft, Exudative) Pork: The Causes and Solutions-Review. Asian Australas. J. Anim. Sci 1999, 12, 244–252. [Google Scholar] [CrossRef]
- Penny, I.F. Protein Denaturation and Water-Holding Capacity in Pork Muscle. Int. J. Food Sci. Technol. 1969, 4, 269–273. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Baas, T.J.; Malek, M.; Dekkers, J.C.M.; Prusa, K.; Rothschild, M.F. Correlations among Selected Pork Quality Traits. J. Anim. Sci. 2002, 80, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Federation of Animal Science Societies. Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Chapter 11; Federation of Animal Science Societies: Champaign, IL, USA, 2010; ISBN 978-1-884706-11-0. [Google Scholar]
- Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine, 11th ed.; National Research Council (U.S.), Ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Suárez-Belloch, J.; Latorre, M.A.; Guada, J.A. The Effect of Protein Restriction during the Growing Period on Carcass, Meat and Fat Quality of Heavy Barrows and Gilts. Meat Sci. 2016, 112, 16–23. [Google Scholar] [CrossRef]
- Burson, D. Procedures for Estimating Pork Carcass Composition; National Pork Producers Council: Des Moines, IA, USA, 2001. [Google Scholar]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Liesse, C.; Kemp, R.; Balan, P. Evaluation of Combined Effects of Ageing Period and Freezing Rate on Quality Attributes of Beef Loins. Meat Sci. 2015, 110, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.B.; Meyers, B.; Kim, H.-W.; Liceaga, A.M.; Lemenager, R.P. Effects of Stepwise Dry/Wet-Aging and Freezing on Meat Quality of Beef Loins. Meat Sci. 2017, 123, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kim, J.-H.; Seo, J.-K.; Setyabrata, D.; Kim, Y.H.B. Effects of Aging/Freezing Sequence and Freezing Rate on Meat Quality and Oxidative Stability of Pork Loins. Meat Sci. 2018, 139, 162–170. [Google Scholar] [CrossRef] [PubMed]
- AMSA. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; pp. 1–17. [Google Scholar]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-Oxygen Modified Atmosphere Packaging System Induces Lipid and Myoglobin Oxidation and Protein Polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Cahill, V.R. Water Extractability of Muscle Protein and Factors Which Affect This Procedure as a Method of Determining Pork Quality. J. Anim. Sci. 1968, 27, 31–38. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. [30] Microsomal Lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. ISBN 978-0-12-181952-1. [Google Scholar]
- Tuell, J.R.; Kim, H.-W.; Zhang, J.; Guedes, J.; Seo, J.-K.; Schoonmaker, J.P.; Kim, Y.H.B. Arginine Supplementation May Improve Color and Redox Stability of Beef Loins through Delayed Onset of Mitochondrial-Mediated Apoptotic Processes. Food Chem. 2021, 343, 128552. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Johnson, J.S.; Baumgard, L.H. PHYSIOLOGY SYMPOSIUM: Postnatal Consequences of in Utero Heat Stress in Pigs. J. Anim. Sci. 2019, 97, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.J. What Is Pork Quality? In Quality of Meat and Fat in Pigs as Affected by Genetics and Nutrition; Publication-European Association for Animal Production: Rome, Italy, 2000; Volume 100, pp. 15–26. ISBN 978-90-74134-74-3. [Google Scholar]
- Meisinger, D. A System for Assuring Pork Quality; National Pork Producers Council: Des Moines, IA, USA, 1999. [Google Scholar]
- Maltin, C.; Balcerzak, D.; Tilley, R.; Delday, M. Determinants of Meat Quality: Tenderness. Proc. Nutr. Soc. 2003, 62, 337–347. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Farouk, M.M.; Mustafa, N.M.; Wu, G.; Krsinic, G. The “Sponge Effect” Hypothesis: An Alternative Explanation of the Improvement in the Waterholding Capacity of Meat with Ageing. Meat Sci. 2012, 90, 670–677. [Google Scholar] [CrossRef]
- Ma, D.; Guedes, J.M.; Duttlinger, A.W.; Johnson, J.S.; Zuelly, S.M.; Lay, D.C.; Richert, B.T.; Kim, Y.H.B. Impact of L-Glutamine as Replacement of Dietary Antibiotics during Post Weaning and Transport Recovery on Carcass and Meat Quality Attributes in Pigs. Livest. Sci. 2020, 104350. [Google Scholar] [CrossRef]
- Mitacek, R.M.; Ke, Y.; Prenni, J.E.; Jadeja, R.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Mitochondrial Degeneration, Depletion of NADH, and Oxidative Stress Decrease Color Stability of Wet-Aged Beef Longissimus Steaks. J. Food Sci. 2019, 84, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lee, J.R.; Kim, M.K.; Jo, C.; Lee, K.H.; You, I.; Jung, S. Quality Improvement of Pork Loin by Dry Aging. Korean J. Food Sci. Anim. Resour. 2016, 36, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Seideman, S.C.; Cross, H.R.; Smith, G.C.; Durland, P.R. Factors associated with fresh meat color: A review. J. Food Qual. 1984, 6, 211–237. [Google Scholar] [CrossRef]
- Brewer, M.S.; Zhu, L.G.; Bidner, B.; Meisinger, D.J.; McKeith, F.K. Measuring Pork Color: Effects of Bloom Time, Muscle, pH and Relationship to Instrumental Parameters. Meat Sci. 2001, 57, 169–176. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A Structural Approach to Understanding the Interactions between Colour, Water-Holding Capacity and Tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current Research in Meat Color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and Lipid Oxidation Interactions: Mechanistic Bases and Control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.A.; Galvin, K.; Kerry, J.P.; Buckley, D.J. Lipid Stability in Meat and Meat Products. Meat Sci. 1998, 49, S73–S86. [Google Scholar] [CrossRef]
- Li, S.; Ma, R.; Pan, J.; Lin, X.; Dong, X.; Yu, C. Combined Effects of Aging and Low Temperature, Long Time Heating on Pork Toughness. Meat Sci. 2019, 150, 33–39. [Google Scholar] [CrossRef]
- Anderson, M.J.; Lonergan, S.M.; Fedler, C.A.; Prusa, K.J.; Binning, J.M.; Huff-Lonergan, E. Profile of Biochemical Traits Influencing Tenderness of Muscles from the Beef Round. Meat Sci. 2012, 91, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C.; Safranski, T.J. Heat Stress in Pregnant Sows: Thermal Responses and Subsequent Performance of Sows and Their Offspring. Mol. Reprod. Dev. 2017, 84, 946–956. [Google Scholar] [CrossRef]
- Johnson, J.S.; Abuajamieh, M.; Fernandez, M.V.S.; Seibert, J.T.; Stoakes, S.K.; Keating, A.F.; Ross, J.W.; Selsby, J.T.; Rhoads, R.P.; Baumgard, L.H. The Impact of in Utero Heat Stress and Nutrient Restriction on Progeny Body Composition. J. Therm. Biol. 2015, 53, 143–150. [Google Scholar] [CrossRef]
- Harris, A.J.; Duxson, M.J.; Fitzsimons, R.B.; Rieger, F. Myonuclear Birthdates Distinguish the Origins of Primary and Secondary Myotubes in Embryonic Mammalian Skeletal Muscles. Development 1989, 107, 771–784. [Google Scholar]
- Reik, W. Stability and Flexibility of Epigenetic Gene Regulation in Mammalian Development. Nature 2007, 447, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Lefaucheur, L.; Juin, H.; Louveau, I.; Lebret, B. Low Birth Weight Is Associated with Enlarged Muscle Fiber Area and Impaired Meat Tenderness of the Longissimus Muscle in Pigs. J. Anim. Sci. 2006, 84, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.M.; Oksbjerg, N. Birth Weight and Postnatal Dietary Protein Level Affect Performance, Muscle Metabolism and Meat Quality in Pigs. Animal 2011, 5, 1382–1389. [Google Scholar] [CrossRef]
- Muroya, S.; Ertbjerg, P.; Pomponio, L.; Christensen, M. Desmin and Troponin T Are Degraded Faster in Type IIb Muscle Fibers than in Type I Fibers during Postmortem Aging of Porcine Muscle. Meat Sci. 2010, 86, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, D.; Kim, Y.H.B. Mitochondrial Apoptosis and Proteolytic Changes of Myofibrillar Proteins in Two Different Pork Muscles during Aging. Food Chem. 2020, 319, 126571. [Google Scholar] [CrossRef] [PubMed]

| Trait | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| IUTN | IUHS | |||
| BW 1, kg | 118.1 | 116.4 | 1.7 | 0.475 |
| HCW 2, kg | 90.8 | 88.8 | 1.4 | 0.314 |
| CCW 3 (left), kg | 43.6 | 42.6 | 0.7 | 0.343 |
| Dressing percentage | 76.9 | 76.3 | 0.4 | 0.311 |
| Head, kg | 5.42 a | 5.09 b | 0.09 | 0.021 |
| Liver + Gallbladder, kg | 1.49 | 1.54 | 0.05 | 0.553 |
| Heart, kg | 0.41 a | 0.37 b | 0.02 | 0.046 |
| Kidney, kg | 0.31 | 0.32 | 0.01 | 0.995 |
| Spleen, kg | 0.17 | 0.17 | 0.01 | 0.563 |
| Trait | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| IUTN | IUHS | |||
| Muscle score (1–3) | 2.6 | 2.4 | 0.1 | 0.258 |
| Lean firmness score (1–3) | 1.9 | 1.9 | 0.2 | 0.921 |
| Lean wetness score (1–3) | 1.9 | 2.0 | 0.1 | 0.382 |
| Lean color score (1–6) | 3.0 | 2.7 | 0.2 | 0.328 |
| Marbling score (1–10) | 1.4 | 1.3 | 0.1 | 0.517 |
| Carcass length, cm | 84.1 | 84.2 | 0.7 | 0.890 |
| Last rib fat, cm | 2.6 | 2.6 | 0.2 | 0.718 |
| Fat depth, cm | 1.8 | 1.8 | 0.1 | 0.838 |
| Loin muscle area, cm2 | 52.9 a | 47.4 b | 1.1 | 0.002 |
| Standardized fat free lean, kg | 50.2 | 48.0 | 1.0 | 0.154 |
| Percent lean | 55.1 | 54.0 | 0.6 | 0.254 |
| Aging Period (Days) | pH | Water-Holding Ability (%) | |||||
|---|---|---|---|---|---|---|---|
| Purge Loss | Drip Loss | Display Weight Loss | Freezing/Thawing Loss | Cooking Loss | |||
| Treatment effect (T) | |||||||
| IUTN | - | 5.57 | 6.0 | 6.0 | 6.1 | 5.3 | 23.0 |
| IUHS | - | 5.57 | 5.8 | 6.1 | 6.4 | 5.2 | 24.2 |
| SEM | - | 0.01 | 0.8 | 0.5 | 0.6 | 0.5 | 1.4 |
| Aging period effect (A) | |||||||
| 1 d | - | 5.55 y | - | 7.6 x | - | 6.1 x | 25.2 x |
| 7 d | - | 5.59 x | - | 4.5 y | - | 4.3 y | 22.0 y |
| SEM | - | 0.01 | - | 0.5 | - | 0.4 | 1.1 |
| Two-way interaction (T × D) | |||||||
| IUTN | 1 d | 5.54 | - | 7.6 | - | 6.1 | 24.3 |
| 7 d | 5.59 | - | 4.5 | - | 4.4 | 21.7 | |
| IUHS | 1 d | 5.55 | - | 7.6 | - | 6.2 | 26.1 |
| 7 d | 5.59 | - | 4.6 | - | 4.2 | 22.3 | |
| SEM | 0.01 | - | 0.6 | - | 0.6 | 1.6 | |
| Significance of p-Value | |||||||
| Treatment effect (T) | 0.754 | 0.905 | 0.850 | 0.743 | 0.938 | 0.549 | |
| Aging period effect (A) | <0.001 | - | <0.001 | - | <0.001 | 0.012 | |
| Two-way interaction (T × A) | 0.688 | - | 0.942 | - | 0.714 | 0.596 | |
| Display Period (Days) | CIE L* (Lightness) | CIE a* (Redness) | CIE b* (Yellowness) | Chroma (Color Intensity) | Hue Angle (Discoloration) | |
|---|---|---|---|---|---|---|
| Treatment effect (T) | ||||||
| IUTN | - | 53.9 | 5.8 | 4.7 | 7.3 | 36.3 |
| IUHS | - | 54.8 | 6.2 | 4.3 | 7.8 | 36.6 |
| SEM | - | 0.8 | 0.3 | 0.4 | 0.5 | 1.3 |
| Display period effect (D) | ||||||
| 0 d | - | 53.7 c | 5.6 d | 4.2 bc | 7.0 c | 36.3 b |
| 1 d | - | 54.4 b | 6.3 a | 4.8 a | 7.9 a | 36.8 b |
| 2 d | - | 54.1 b | 6.3 a | 4.3 b | 7.7 b | 34.1 c |
| 3 d | - | 54.4 b | 6.0 b | 4.8 a | 7.7 ab | 38.4 a |
| 4 d | - | 54.8 a | 5.9 bc | 4.8 a | 7.6 b | 38.5 a |
| 5 d | - | 54.7 a | 5.8 cd | 4.1 c | 7.1 c | 34.8 c |
| SEM | - | 0.6 | 0.2 | 0.3 | 0.3 | 1.0 |
| Two-way interaction (T × D) | ||||||
| IUTN | 0 d | 53.2 | 5.5 | 4.0 | 6.8 | 36.1 |
| 1 d | 54.1 | 6.2 | 4.7 | 7.8 | 37.0 | |
| 2 d | 53.8 | 6.1 | 4.2 | 7.5 | 34.6 | |
| 3 d | 53.8 | 5.8 | 4.5 | 7.4 | 38.0 | |
| 4 d | 54.1 | 5.7 | 4.5 | 7.3 | 38.0 | |
| 5 d | 54.2 | 5.5 | 3.8 | 6.7 | 34.3 | |
| IUHS | 0 d | 54.3 | 5.8 | 4.4 | 7.3 | 36.4 |
| 1 d | 54.6 | 6.4 | 4.8 | 8.1 | 36.7 | |
| 2 d | 54.5 | 6.5 | 4.4 | 7.9 | 33.7 | |
| 3 d | 54.9 | 6.2 | 5.0 | 8.0 | 38.7 | |
| 4 d | 55.5 | 6.2 | 5.0 | 8.0 | 39.0 | |
| 5 d | 55.2 | 6.0 | 4.3 | 7.5 | 35.2 | |
| SEM | 0.8 | 0.3 | 0.4 | 0.5 | 1.5 | |
| Significance of p-Value | ||||||
| Treatment effect (T) | 0.415 | 0.413 | 0.488 | 0.422 | 0.881 | |
| Display period effect (D) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Two-way interaction (T × D) | 0.232 | 0.791 | 0.177 | 0.299 | 0.727 |
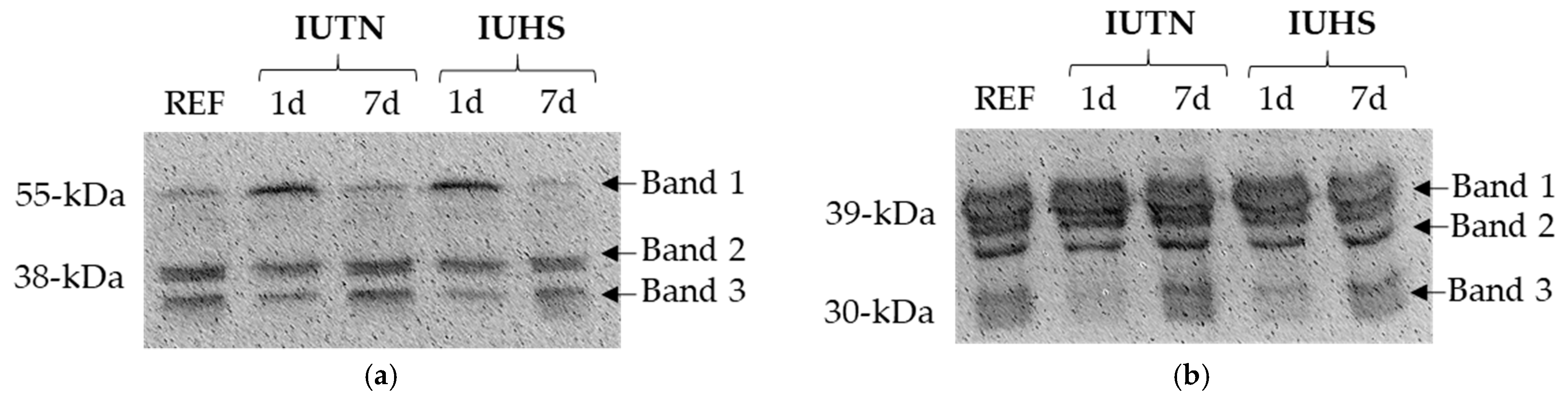
| Aging Period (Days) | Desmin Band 1 | Desmin Band 2 | Desmin Band 3 | Troponin T Band 1 | Troponin T Band 2 | Troponin T Band 3 | |
|---|---|---|---|---|---|---|---|
| Treatment effect (T) | |||||||
| IUTN | - | 1.23 | 0.85 | 0.71 | 1.01 | 0.86 | 0.92 |
| IUHS | - | 1.10 | 0.88 | 0.84 | 0.98 | 0.88 | 0.85 |
| SEM | - | 0.05 | 0.04 | 0.05 | 0.03 | 0.02 | 0.03 |
| Aging period effect (A) | |||||||
| 1 d | - | 1.33 x | 0.73 y | 0.61 y | 0.98 | 0.77 y | 0.76 y |
| 7 d | - | 1.00 y | 0.99 x | 0.94 x | 1.01 | 0.97 x | 1.00 x |
| SEM | - | 0.04 | 0.03 | 0.04 | 0.03 | 0.02 | 0.02 |
| Two-way interaction (T × A) | |||||||
| IUTN | 1 d | 1.39 | 0.72 | 0.58 | 0.98 | 0.75 | 0.73 |
| 7 d | 1.06 | 0.97 | 0.84 | 1.03 | 0.96 | 0.96 | |
| IUHS | 1 d | 1.27 | 0.74 | 0.64 | 0.98 | 0.79 | 0.79 |
| 7 d | 0.93 | 1.02 | 1.04 | 0.99 | 0.98 | 1.05 | |
| SEM | 0.05 | 0.05 | 0.06 | 0.04 | 0.03 | 0.04 | |
| Significance of p-Value | |||||||
| Treatment effect (T) | 0.073 | 0.581 | 0.067 | 0.655 | 0.412 | 0.111 | |
| Aging period effect (A) | <0.001 | <0.001 | <0.001 | 0.132 | <0.001 | <0.001 | |
| Two-way interaction (T × A) | 0.901 | 0.655 | 0.130 | 0.347 | 0.740 | 0.427 |
| Display Period (Days) | TBARS Value 1 | Transmission Value (%) | |
|---|---|---|---|
| Treatment effect (T) | |||
| IUTN | - | 0.64 | 46.0 |
| IUHS | - | 0.64 | 46.1 |
| SEM | - | 0.03 | 3.4 |
| Display period effect (D) | |||
| 0 d | - | 0.51 b | - |
| 5 d | - | 0.76 a | - |
| SEM | - | 0.02 | - |
| Two-way interaction (T × A) | |||
| IUTN | 0 d | 0.53 | - |
| 5 d | 0.75 | - | |
| IUHS | 0 d | 0.50 | - |
| 5 d | 0.77 | - | |
| SEM | 0.03 | - | |
| Significance of p-Value | |||
| Treatment effect (T) | 0.957 | 0.982 | |
| Display period effect (D) | <0.001 | - | |
| Two-way interaction (T × D) | 0.070 | - |
| Treatment | ||||
|---|---|---|---|---|
| Fatty Acid 1 | IUTN | IUHS | SEM | p-Value |
| C4:0 | N.D. 2 | N.D. | - | - |
| C6:0 | N.D. | N.D. | - | - |
| C8:0 | N.D. | N.D. | - | - |
| C10:0 | 0.13 | 0.12 | 0.01 | 0.195 |
| C12:0 | 0.03 | 0.03 | <0.01 | 0.494 |
| C14:0 | 1.30 | 1.27 | 0.04 | 0.673 |
| C14:1 | 0.23 | 0.19 | 0.05 | 0.619 |
| C16:0 | 24.01 | 24.08 | 0.24 | 0.825 |
| C16:1 | 3.48 | 3.26 | 0.12 | 0.192 |
| C18:0 | 10.66 | 11.05 | 0.22 | 0.233 |
| C18:1n-9c | 37.21 | 37.27 | 0.61 | 0.953 |
| C18:1n-9t | 0.32 | 0.33 | 0.01 | 0.347 |
| C18:2n-6c | 11.66 | 11.44 | 0.46 | 0.742 |
| C18:2n-6t | 0.07 | 0.04 | 0.02 | 0.361 |
| C18:3n-3 | 0.27 | 0.24 | 0.02 | 0.172 |
| C18:3n-6 | 0.07 | 0.05 | 0.01 | 0.128 |
| C20:0 | 0.15 | 0.14 | 0.01 | 0.748 |
| C20:1n-9 | 0.46 | 0.47 | 0.02 | 0.689 |
| C20:2 | 0.27 | 0.28 | 0.01 | 0.513 |
| C20:3n-3 | 0.01 | 0.03 | 0.01 | 0.249 |
| C20:3n-6 | 0.26 | 0.27 | 0.02 | 0.746 |
| C20:4n-6 | 2.17 | 2.14 | 0.15 | 0.900 |
| C20:5n-3 | 0.01 | 0.01 | <0.01 | 0.161 |
| C22:0 | 0.04 | 0.04 | 0.01 | 0.627 |
| C22:1n-9 | N.D. | N.D. | - | - |
| C22:6n-3 | 0.11 | 0.01 | <0.01 | 0.230 |
| C24:1n-9 | N.D. | N.D. | - | - |
| Total SFA 3 | 36.55 | 37.05 | 0.38 | 0.367 |
| Total MUFA 4 | 41.88 | 41.71 | 0.63 | 0.848 |
| Total PUFA 5 | 14.83 | 14.51 | 0.66 | 0.736 |
| Total UFA 6 | 56.73 | 56.23 | 0.27 | 0.204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuell, J.R.; Nondorf, M.J.; Maskal, J.M.; Johnson, J.S.; Kim, Y.H.B. Impacts of in Utero Heat Stress on Carcass and Meat Quality Traits of Market Weight Gilts. Animals 2021, 11, 717. https://doi.org/10.3390/ani11030717
Tuell JR, Nondorf MJ, Maskal JM, Johnson JS, Kim YHB. Impacts of in Utero Heat Stress on Carcass and Meat Quality Traits of Market Weight Gilts. Animals. 2021; 11(3):717. https://doi.org/10.3390/ani11030717
Chicago/Turabian StyleTuell, Jacob R., Mariah J. Nondorf, Jacob M. Maskal, Jay S. Johnson, and Yuan H. Brad Kim. 2021. "Impacts of in Utero Heat Stress on Carcass and Meat Quality Traits of Market Weight Gilts" Animals 11, no. 3: 717. https://doi.org/10.3390/ani11030717
APA StyleTuell, J. R., Nondorf, M. J., Maskal, J. M., Johnson, J. S., & Kim, Y. H. B. (2021). Impacts of in Utero Heat Stress on Carcass and Meat Quality Traits of Market Weight Gilts. Animals, 11(3), 717. https://doi.org/10.3390/ani11030717








