Simple Summary
The aquaculture sector provides for nearly half of the world’s seafood consumption, thanks to its large expansion over the last 30 years. Despite this intense growth, clear guidelines for responsible practices and animal wellbeing are lacking. Gene expression studies are a fundamental tool for understanding welfare, but stress-markers in aquaculture fish species are poorly studied. In addition, the biostatistical analysis of gene expression data is not trivial, and this study applies different statistical methods in order to evaluate potential differences in the gene expression levels between control fish and fish acutely stressed by exposure to air.
Abstract
In this study, a stress trial was conducted with common carp, one of the most important species in aquaculture worldwide, to identify relevant gene regulation pathways in different areas of the brain. Acute distress due to exposure to air significantly activated the expression of the immediate early gene c-fos in the telencephalon. In addition, evidence for regulation of the two corticotropin-releasing factor (crf) genes in relation to their binding protein (corticotropin-releasing hormone-binding protein, crh-bp) is presented in this preliminary study. Inferences on the effects of due to exposure to air were obtained by using point estimation, which allows the prediction of a single value. This constitutes the best description to date of the previously generally unknown effects of stress in different brain regions in carp. Furthermore, principal component analyses were performed to reveal possible regulation patterns in the different regions of the fish brain. In conclusion, these preliminary studies on gene regulation in the carp brain that has been influenced by exposure to a stressor reveal that a number of genes may be successfully used as markers for exposure to unfavourable conditions.
1. Introduction
Distress is defined as a condition that interferes with the wellbeing of an animal if the adaptation processes of the animal fail to return the organism to physiological and/or psychological homeostasis [1,2]. Fish in aquaculture are often subjected to distress for short periods (acute stress), but can also be exposed to stressors for longer periods (chronic stress), meaning that the body’s biological functions are sufficiently altered and its coping mechanisms overwhelmed [1].
Understanding the immediate effects of stressors, such as handling and crowding, on gene regulation in fish, has been a focus of aquaculture research [3,4,5], in the interests of improving survival, growth, reproduction, and fillet quality. The brain, however, despite its role as first actor in the stress-cascade, has received comparatively little attention. Recently, a study on European seabass (Dicentrarchus labrax L.) and gilthead seabream (Sparus aurata L.) not only revealed relevant differences between species, but the importance of studying the different fish brain regions separately [6]. In addition, certain brain areas may even exhibit significant differences in gene expression [7]. The main purpose of this research is to identify stress-related biomarkers in different brain regions of common carp (Cyprinus carpio) to allow a precise evaluation of their rearing conditions.
The diversity of primary functions between the regions of a fish brain has been already studied in the past. The telencephalon, for instance, has long been regarded as solely fulfilling olfactory functions [8]. However, more recent research has confirmed its important role in the expression of emotional and motivational behaviour, as well as in fear conditioning in teleosts, including a pivotal role in these behaviours that are attributed to the amygdala [9,10,11]. The amygdala is known to play an essential role in mediating negative and positive emotions in mammals, which also involves the appraisal of incoming signals [12,13,14,15]. The optic tectum, which directly connects the efferent neurons with incoming retinal fibres in teleosts, was already recognized at an early stage as being essential for visually mediated behaviours already in early times [16,17]. However, the optic tectum is not solely required for the perception of motions, but has more recently been proven essential for the correct pacing of saccades during optokinetic responses in zebrafish (Danio rerio) [18]. In addition, the hypothalamus plays an important role in energy homeostasis and appetite regulation. The activated or suppressed neurons then lead to adjustments in behaviour and metabolism. Proopiomelanocortin (pomc) neurons appear to drive satiety in the hypothalamic arcuate nucleus of mice [19]. The cerebellum of fish is responsible for the coordination of body movements [20], and has been linked to spatial navigation [21].
The effects of stressors on the brain can be assessed by analysing the activities of different gene sets. Firstly, brain activity can be assessed, for instance, by measuring the increased expression of stress-related immediate early genes (IEGs). One example of an IEG is c-fos, which, due to its very rapid and robust expression, is widely used as a functional marker of neuronal activity after a diversity of stimuli in vertebrates [22]. In fish, different stimuli have been shown to induce varying levels of c-fos expression. Light avoidance, as an innate choice behaviour, involves rapid changes in the expression of c-fos in the medial zone of the dorsal telencephalon in adult zebrafish [11]. In addition, the administration of D-amphetamine, known to activate the reward system, resulted in increased expression of c-fos in the same brain region 30 min after the injection of the substance [9]. Moreover, the sleep and wake behaviour of zebrafish leads to typical differences in c-fos patterns in zebrafish [23]. Even caffeine has been proven to act as a stimulator of c-fos in the zebrafish brain [24]. In addition, exposure to neurotoxins for 60 min resulted in rapid changes of the c-fos protein in different regions of the brain in killifish (Fundulus heteroclitus) [25].
Another gene belonging to the IEG group is egr-1 (encodes the early growth response protein 1), which has already been shown to undergo change during the breeding cycle of sticklebacks [26]. Furthermore, the immunohistochemical detection of phosphorylated extracellular signal–regulated kinase (erk) has also been used as a read-out for neural activity in fish at the whole-brain level [27]. The protein palladin (palld) is essential for the organization of the actin cytoskeleton and a deficiency can lead to a failure of neurite outgrowth in rats [28]. Interestingly, palld has also been described as an immune-related gene in mirror carp (Cyprinus carpio) exposed to koi herpes virus [29]. The importance of this protein in cytoskeleton organization and kidney function has been confirmed in zebrafish [30,31]. Since it may also play an important role in the organization of the fish brain, this gene was included in this study. In addition, one metabolic gene (glyceraldehyde-3-phosphate dehydrogenase, gadph) was included in this study as its activity in tissues implicates higher energy demands and, therefore, increases in metabolic gene expression.
Secondly, the response to stressors also commonly involves activation of the hypothalamus-pituitary-interrenal (HPI) axis genes [32] and a number of these genes have therefore also been included in study. The corticotropin-releasing factor (crf) and its receptors play an important role in stress signalling via the HPI axis [33]. The abundance of the corticotropin-releasing hormone-binding protein (crh-bp) determines the availability of crh to its receptors, although other biological functions have been proposed for crh-bp [34]. Finally, the release of stress hormones, such as cortisol and 11-deoxycorticosterone, leads to the activation of glucocorticoid receptor (gr)- and mineralocorticoid receptor (mr)-mediated signalling pathways in teleosts [35]. In carp, gr2 is the most sensitive corticoid receptor, followed by mr, and gr1a and gr1b [36].
Thirdly, other brain networks are also known to be involved and/or affected by stress responses in other fish species. One example involves the serotonergic pathways being affected by stress in rainbow trout, Oncorhynchus mykiss [37]. Serotonin also plays a role in the habituation to startling acoustic stimuli in zebrafish [38]. While serotonin agonists have anxiolytic effects in humans [39], chemicals including ethanol have shown that the acute anxiolytic effects caused by these substances are likely to be mediated by γ-aminobutyric acid receptors A (gabaa, [40]). The same psychoactive compounds have also been used to influence normal behaviour in zebrafish [41]. In addition, together with vasotocin, isotocin is a neurotransmitter and neuromodulator that is produced in distinct neurosecretory neurons in the hypothalamic nuclei [42]. Both influence the outcomes of various behaviours, the establishment of social status, but are also involved in osmoregulation and stress responses in fish [42]. Osmoregulation is also regulated by prolactin in fish [43]. In addition, early development, behaviour, growth, and immunoregulation depend on prolactin and prolactin receptor expression [30,44]. In neuronal tissue, prolactin is also involved in the activation of neurons that evoke action potentials and/or calcium influx in neurons [45]. These reactions culminate in the release of neurotransmitters, e.g., dopamine in the hypothalamus of rats, which exert a negative feedback on the release of prolactin [46,47,48,49]. Interestingly, GABA-mediated inhibition of prolactin release from the pituitary of trout, probably by both, GABA receptors A and B, has been described by Prunet et al. [50]. However, the influence of stressors on these brain regulation pathways in other fish species is mostly unknown, which is the reason why a wider range of genes was included in this study.
This study was conducted to obtain preliminary data on the differential gene expression patterns of genes belonging (I) to the early immediate genes, (II) to the HPI axis, as well as (III) neurotransmitter-related pathways in the carp brain of stressed fish compared with non-stressed animals, used as controls, and to apply different biostatistical methods that allow the identification of a set of potential genes suitable as biomarkers of distress in fish.
2. Materials and Methods
2.1. Rearing Conditions and Sampling
The fish were reared at 23 °C–24 °C in a 290 L aquarium equipped with a settler and a moving-bed biofilter. The juvenile carp (Cyprinus carpio), approximately 2-months old, were kept for two months and fed 4 times daily at a feeding rate of 2 to 3% body weight per day. During the experiment, the mean weight of the eight fish was 28.3 ± 9.8 g and the mean standard length was 8.9 ± 1.1 cm. For stress treatment, four fish were exposed to the air for 1 min in a net, returned to the tank and anaesthetized 30 min afterwards. Following the acclimatization period, the four control fish were taken directly from the rearing tank. Anaesthesia was performed with an overdose of tricaine methanesulfonate (MS-222, Sigma-Aldrich, Buchs, Switzerland). The brains were sampled and stored in RNAlater® (Sigma-Aldrich, Buchs, Switzerland) for at least 24 h and then divided into the 4 brain regions (tel = telencephalon, hyp = hypothalamus, opt = optic tectum, rho = rhombencephalon, comprising the corpus cerebelli and the medulla oblongata). All experimental procedures were approved under permission number ZH-062-17 by the Cantonal veterinary authorities of Zurich (Switzerland).
2.2. PCR Conditions
Gene expression studies were performed by means of quantitative real-time polymerase chain reactions (qPCR) on a LC480 Light Cycler II (Roche, Basel, Switzerland). The total RNA from each of the four regions of each brain, tel, hyp, opt, and rho, was extracted using RNeasy Micro Kits (Qiagen AG, Hombrechtikon, Switzerland). The RNA content was confirmed using a spectrophotometer Q5000 (Quawell, San Jose, CA, USA). Subsequently, 20 μL of total RNA were reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, distributed by Thermo Fisher Scientific, Basel, Switzerland) according to the manufacturer’s instructions. Thereafter, the cDNA content was adjusted to 50 ng per μL using nuclease-free water (Ambion®, distributed by Thermo Fisher Scientific, Basel, Switzerland) and used for real-time PCR with the LightCycler® SYBR® Green I Master mix (Roche, Switzerland) using three replicates for each sample with the following cycling conditions: initial denaturation at 95 °C for 15 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. A melt curve step (95 °C for 5 s, 60 °C for 1 min, and 5 acquisitions per °C until 97 °C was included using a ramp rate of 0.06 °C per s) was added at the end of all runs to ensure that the individual PCR reactions yielded a single melting peak. All primer pairs used here are given in Table S1. Initially, the PCR products were mixed with the tracking dye orange G ((Thermo Fisher Scientific, Allschwil, Switzerland) and loaded onto 2% agarose (Bio-Rad Laboratories AG, Cressier, Switzerland) gels containing GelRed® (Chemie Brunschwig AG, Basel, Switzerland) and after horizontal gel electrophoresis in TAE buffer (242 g TRIS-HCl, 37.2 g sodium EDTA, 57.1 mL glacial acetic acid in 1 L distilled water, pH 7.5) for 35 min at 80 mV PCR products were visualized using UV illumination. In addition, for primer validation, the respective PCR products (with sizes ranging between 90 and 150 bp) confirmed by Sanger sequencing and the optimal reference genes were extracted from a set of 8 possible reference genes (Table S2). This was accomplished using the three genes with the best value for the expression stability M (i.e., eIF4E, bactin, and ef, for more details see Table S2), extracted with the geNorm function in the qbase+ software, version 3.0 (Biogazelle, Zwijnaarde, Belgium—www.qbaseplus.com accessed on 5 March 2020) established by Vandesompele et al. [51]. The target genes included early immediate genes (c-fos, egr-1, erk-1, erk-2, and palld), as well as the metabolic gene gapdh, in order to indicate active brain regions. The following genes related to the HPI axis were included: crf1, crf2, crfr1, crfr2, crhbp, pomc1, pomc2, gr1, gr2, and mr. In addition, serotonergic pathway genes (5-ht-r, serotr) as well as gabaa, iso-pre and prolr were investigated.
2.3. Calculations and Statistics
From the potential reference genes 18S RNA, b2m, egr-1, palld, gapdh, bactin, eIF4E, and ef, the reference genes that were used for gene normalization were bactin, eIF4E, and ef since these genes showed the lowest M value as calculated by the qbase+ software (Biogazelle, Zwijnaarde, Belgium, Table S2). The calculation of the normalized fold change in expression of each target gene was calculated according to Taylor et al. [52]. First, the mean quantitative cycle (mean ct) of the three technical replicates was calculated for each sample. The average ct of all control samples was calculated for each target gene and the relative difference (Δct) between the average ct for the control group and the mean ct for each sample was assessed within each target. Relative quantities are then calculated from the Δct values. For each biological group (i.e., control group versus air-exposed group), a normalization factor was derived from the geometric mean of the relative quantities of each reference gene. The relative quantity of each target gene was then divided by the normalization factor, followed by log transformation. The values hereby obtained were then used to calculate the geometric means for each treatment group. The standard deviation (SD) and the standard error of the mean (SEM) were calculated from the log transformed normalized expression data. The figures show the average relative normalized expression for each target gene ± SEM. Non-parametric tests (Mann–Whitney U tests) were run in IBM SPSS Statistics (version 26, IBM Schweiz, Zurich, Switzerland) to investigate the differences between the means for gene expression in each treatment group, since it has previously been shown that non-parametric methods may be better at controlling for false discoveries of significant differences between expression levels, for example, after RNA sequencing [53]. Differences between treatment groups were considered statistically significant when p < 0.05. Confidence intervals (95%) for the values of control and stressed fish have been derived by resampling 2000 times from the original sample based on the adjusted bootstrap percentile (BCa) method using the resample and the broom package in R studio. Assuming the independence of tests, multiple testing leads to an inflated probability of false positive results. To address this problem, the following mixed models with a fully Bayesian approach (as a part of the brms package [54] in R studio, Version 1.2.1335, RStudio Team 2018) and assuming a Gaussian distribution of the data were used to investigate potential differences between the two treatment groups:
yij~N(μij, s2)
μij, ~αj + βjxi + yi
αj~N(0, σ α2), 1, …, ngen
βj~N(0, σ β2)
yj~N(0, σ y2), i = 1, …, nanimal
The models include gene specific random effects for the constants (α), gene-specific random effects for the group differences (β) and animal-specific random effects for the constants (γ). The model fit was assessed through a comparison of graphical plots (QQ plots) showing the distribution of y and yrep. To improve the handling of possible outliers, posterior predictive checks based on the Markov Chain Monte Carlo (MCMC) approximation method were applied, which yielded simulated replicated data under the fitted model that were then compared with the observed data. The point estimators, their SEMs, credibility intervals and posterior predictive p values are reported.
A principal component analysis (PCA), as a data reduction method, was performed on the log transformed normalized expression data in R studio (Version 1.2.1335, RStudio Team 2018) for an initial description of the genes accounting for the main contribution towards the common variance within the gene expression patterns in the different brain regions. The representation of the variables for the principle components is calculated as a cos2 value. For a given variable, the sum of the cos2 on all the principal components is equal to one.
3. Results
3.1. Immediate Early Genes (c-fos, egr-1, erk-1 and erk-2, palld) and gapdh
A significant difference was observed between control fish and distressed fish for the IEG c-fos in the telencephalon (p < 0.05), but not in the other brain regions that were investigated (Figure 1). In addition, an increased probability for a reduction in the expression of this gene was observed in the optic tectum relative to other genes that were included in this study (Table 1 and Figure S4). However, the other IEGs were not significantly influenced by the stress treatment.
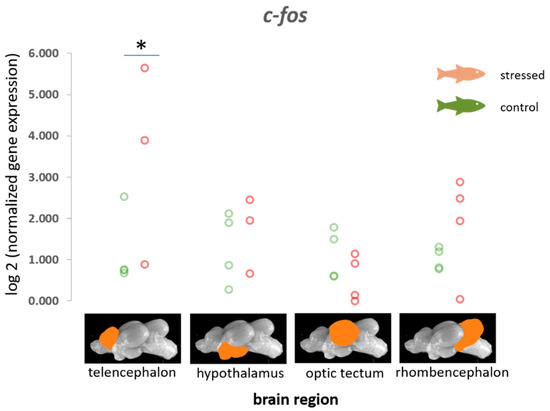
Figure 1.
Gene expression profile of the immediate early gene c-fos in each of the four brain parts in the control fish and fish 30 min after the air exposure with 95% confidence intervals of 0.72 to 2.1 for the control and 2.1 to 18.5 for the stressed fish for the values for the telencephalon derived from the adjusted bootstrap percentile (BCa) method; n = 4 per treatment, * = significance according to the Man–Whitney U tests, p < 0.05.

Table 1.
Probabilities for potential group differences between the control animals and the distressed fish (n = 4 each) in the different brain parts, the table shows for each of the genes the point estimator displayed in bold, the SEM in brackets, and the credibility interval and the posteriori p value in the second row.
For the IEGs, the first two components in the PCA for each brain region explained 77.1% of the variance in the IEG data in the telencephalon, 83.8% in the hypothalamus, 82.8% in the optic tectum, and 81.1% of the variance in the rhombencephalon (Figure S1).
However, the genes that indicated good representation on the principal component, i.e., displayed by a high cos2 value in Figure S1, differed for each brain region.
3.2. HPI Axis-Related Genes
For stress responses, it is essential to consider how the mRNA expression values of hormones, their binding proteins and receptors change relative to each other. A significant decrease in the ratio of crf1:crh-bp in the telencephalon and an increase in the hypothalamus were observed in stressed fish compared with the control fish (p = 0.021, Figure 2). Furthermore, the ratio of crf2:crh-bp was decreased in the telencephalon though the application of stress (p = 0.029, Figure 2).
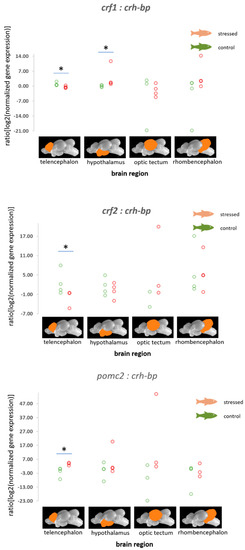
Figure 2.
Ratios of the normalized fold expression of crf1, crf2, and pomc2 relative to crh-bp in each of the 4 brain parts in the control fish and fish 30 min after the air exposure with 95% confidence intervals of 0.51 to 1.83 for the control and −0.77 to 0.17 for the stressed fish for the ratio of crf1:crh-bp in the telencephalon, −0.44 to 0.53 for the control and 1.16 to 9.20 for the stressed fish for the ratio of crf1:crh-bp in the hypothalamus; 0.00 to 6.48 for the control and −8.07 to −0.61 for the stressed fish for the ratio of crf2:crh-bp in the telencephalon, and −7.16 to −0.13 for the control and 2.62 to 4.42 for the stressed fish for the ratio of pomc2:crh-bp in the telencephalon derived from the adjusted bootstrap percentile (BCa) method; n = 4 per treatment, * = significance according to the Mann–Whitney U tests, p < 0.05.
In addition, the ratios of the normalized fold change in expression of pomc2 relative to crh-bp were found to be increased in the telencephalon of stressed fish compared with the control fish (p = 0.034, Figure 2). The ratio of the normalized fold change in expression of pomc1 to the crf receptor 2 was found to be significantly decreased (p = 0.043, Figure 3).
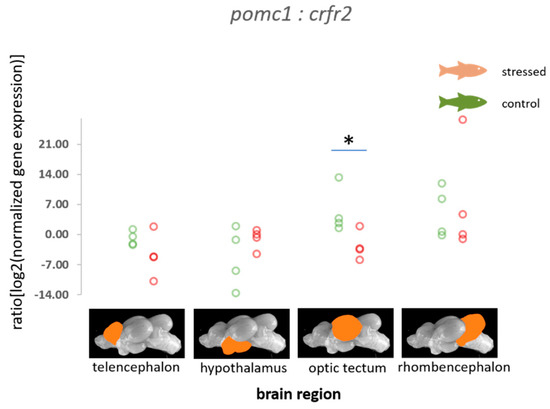
Figure 3.
Ratios of the normalized fold expression of pomc1 compared with the expression of crfr2 in each of the four brain parts in the control fish and fish 30 min after the air exposure with 95% confidence intervals of 2.14 to 10.98 for the control and −5.17 to 0.63 for the stressed fish for values for the optic tectum derived from the adjusted bootstrap percentile (BCa) method; n = 4 per treatment, * = significance according to the Mann–Whitney U tests, p < 0.05.
The PCA revealed that the first two components of the PCA represented 74.5% of the total variance in the HPI genes in the telencephalon, 74.2% of the variance in the same genes in the hypothalamus, and 78.8% and 60.6% of the variance in these genes in the optic tectum and the rhombencephalon (Figure S2). A high cos2 value was observed for crf-r2 in all four brain regions, which indicates good representation of this variable on the principal component. In these cases, the variables are positioned close to the circumference of the correlation circle in Figure S2. In contrast, a low cos2 indicates that the variable is not perfectly represented by the principal components. In this case, the variable is close to the centre of the circle, which, for example, can be seen for pomc2 in the telencephalon and rhombencephalon (Figure S2).
3.3. The Gene Expression Patterns of the Serotonergic Genes, gabaa, Isotocin Precursor, and the Prolactin Receptor
The ratios of normalized fold change in expression of gabaa relative to prolr were found to be increased in the optic tectum of stressed fish compared with the control fish (p = 0.021, Figure 4). Furthermore, calculations revealed an increased probability of a reduction in gabaa expression in the optic tectum relative to other genes in this study (Table 1 and Figure S4). In addition, the ratio of normalized fold change in expression of serotr relative to gabaa was found to be increased in the rhombencephalon of stressed fish compared with the control fish (p = 0.021, Figure 4), as well as an increased probability of a reduction in serotr in the telencephalon relative to other genes that were included in this study (Table 1 and Figure S4).
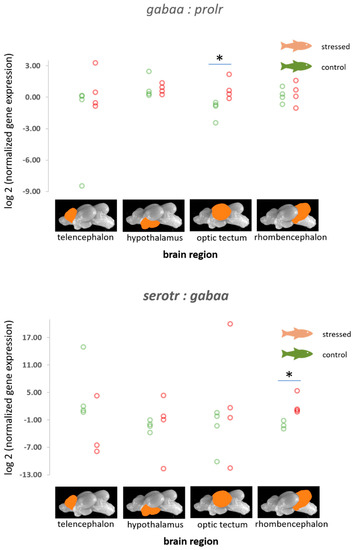
Figure 4.
Ratios of the normalized fold expression of gabaa to prolr and the normalized fold expression of serotr to the expression of the gabaa gene in each of the four brain parts in the control fish and fish 30 min after the air exposure with 95% confidence intervals of −2.43 to −0.63 for the control and 0.105 to 1.813 for the stressed fish for the gabaa:prolr ratio in the optic tectum, and −2.92 to −1.49 for the control and 0.95 to 4.39 for the stressed fish for the serotr:gabaa ratio in the rhombencephalon derived from the adjusted bootstrap percentile (BCa) method; n = 4 per treatment, * = significance according to the Mann–Whitney U tests, p < 0.05.
The PCA for the mRNA levels of the genes 5-ht-r, serotr, gabaa, isopre, and prolr in the telencephalon explained 72.2% of the variance in the data set, whereas the same calculations in the hypothalamus revealed that 85.9% of the variance is related to the selected genes (Figure S3). Similarly, 83.6% and 73.9% of the variance was attributed to the selected genes in the optic tectum and in the rhombencephalon, respectively (Figure S3). Consequently, an optimal set of genes was desired for a final PCA, for which gabaa, crfr1, crfr2, mr, egr-1, 5-ht-r, and c-fos were selected for each of the individual brain regions (Figure 5). The PCA for the telencephalon revealed that 74.0% of the variance in the data set was explained when these genes were selected as variables. Compared to this, the PCA for the hypothalamus showed that 89.5% of the variance was covered by the selected genes. Similarly, the respective variance levels were as high as 93.5% in the optic tectum and 86.1% in the rhombencephalon.

Figure 5.
Results for the first two components (Dim1 and Dim2) of the principal component analysis (PCA), including confidence ellipses for the selected genes gabaa, crfr1, crfr2, erg-1, mr, erg-1, 5-ht-r and c-fos in each in the four brain parts of control fish and fish 30 min after the air exposure (the numbers in the brackets indicate the percentage of the variance in the data sets that is explained by each components, mean ± SEM; n = 4 per treatment.
4. Discussion
Fish reared in aquaculture systems are continuously exposed to different stimuli, some of which are capable of inducing a distress situation once they overcome the physiological limits of the animals. With the continuous growth of the fish farming industry, ways to define and quantify welfare are becoming vital to producing recommendations for best practice and adaptations to legislation. In this study, we aimed to identify the diversity of gene expression profiles between the four brain regions (telencephalon, optic tectum, hypothalamus, and rhombencephalon) in distressed common carp.
Reference genes are fundamental to the exact determination of changes in gene expression [52,55]. These are usually chosen based on previous studies, but only a limited number of these have selected reference genes according to their stable expression pattern in distinct tissues [56,57]. Typical reference genes are related to maintenance of cell structures and metabolism. No such detailed investigation of suitable reference genes has been performed before for the fish brain. The reason for this is that even acute stress, for example in trout, can alter the gene expression of a number of genes involved in intracellular signalling and cytoskeletal changes [58]. Thus, the selection of housekeeping genes that typically have these functions in cells might be inadequate. Other genes, such as gapdh, may exhibit a high variability, which makes them unsuitable as references genes [59,60]. Here, only three genes were shown to be suitable as reference genes. Given the limited number of animals per treatment group, and in order to confirm the hypothesis that region-specific reference genes are required for the fish brain, an additional study using a higher sample size is recommended to confirm the suitability of the reference genes analysed in brain regulation studies on fish.
4.1. Immediate Early Genes (IEGs)
Different IEGs were investigated in this study to identify brain activity in the various brain regions. The increased expression of c-fos in the telencephalon of distressed carp and the increased probability of a reduction in the expression of this gene in the optic tectum relative to other genes (cf. Figure S4) confirms that c-fos is not only a suitable indicator of brain activity in higher vertebrates and fish species, such as zebrafish and goldfish [11,48,61], but also in carp. In situ hybridization has already shown that light avoidance leads to changes in the expression of c-fos in the medial zone of the dorsal telencephalon in adult zebrafish 30 min after the induction of neuronal activity [11]. Together with the current results from carp, it becomes clear that c-fos is capable of indicating changes in brain activity after exposure to acute stressors, in this case exposure to air.
4.2. HPI Axis-Related Genes
The fact that acute stress involves the crf system, e.g., the pre-optic area in the forebrain in fish, has already been reviewed in the past [61,62]. The investigations of acute and chronic stress responses and the crf system also included farmed fish species, such as rainbow trout, sea bass and gilthead seabream, but not carp [6,33,63]. The wide distribution of crf and crh-bp in the fish brain supports the assumption that the crf system fulfils important functions, even outside the cerebral system [64]. For example, changes in the crf system caused by stressors, including hypoxia, have also been observed in the caudal neurosecretory system and the heart of zebrafish [65,66]. Crh-bp is known to inhibit the crf-mediated activation of the crf receptors in a receptor subtype-specific fashion [67]. For this reason, the ratios of the normalized fold change in expression of the crf genes in this study were compared to the level of crh-bp, and the application of an acute stressor appears to influence this ratio, proving the assumption that the crh-bp actions are receptor-specific. Previous investigations have indicated that crh-bp is a more potent inhibitor of crfr2 activation than of crfr1 in fish [67]. Similarly, the crf2 receptors in humans have been described as being coupled to the cAMP–PKA signalling pathways similar to crfr2, but they mediate effects opposite to those of crfr1 receptors in arthritis [68]. This reflects the fact that crf1 and crf2, as well as the two crf receptors have different functions, which is probably also the case in fish. The differential effects of distress on the ratio of the normalized fold change in expression of the crf receptors compared with the fold change in expression of crh-bp in this study support the assumption that the two receptors have different functions in carp as well.
In this study, the ratio of the normalized fold change in expression of pomc1 to crfr2 is also reduced in the optic tectum. The hypothalamic circuit includes two populations of neurons: one co-expressing orexigenic neuropeptides, such as neuropeptide Y, and the other expressing pro-opiomelanocortin (pomc) and anorexigenic neuropeptides, thus regulating feed intake in fish [69]. The functions of pomc neurons in the optic tectum are less well described. Nevertheless, the results of this study support the hypothesis that the optic tectum is more than a predominantly retinorecipient structure.
4.3. Genes of the Serotonergic and the GABAergic Pathway
A link between prolactin release and GABA receptor signalling has previously been demonstrated in the hypothalamus of rodents, as well as in the pituitary in rodents and rainbow trout [47,49,50]. Surprisingly, the change in the ratio of the normalized fold change in expression of the GABA A receptor (gabaa) relative to the expression of prolr was observed in the optic tectum, but the role of prolr in this region of the brain remains unclear to date. The levels of GABA receptor mRNA expression, together with the expression of other important receptors, such as membrane receptors for serotonin and dopamine, affect memory loss in rats [70].
Serotonin transporter expression and activity is required for returning serotonin to the presynaptic neuron where it can be degraded or retained for later re-use. In higher vertebrates, selective serotonin reuptake inhibitors may lead to increased GABA concentrations [71], confirming an interaction between serotonin pathways and GABA levels. The current study on carp also indicated that the ratio of serotr to gabaa is influenced by acute distress. In trout, acute stress resulted in downregulation of a serotonin receptor subtype and mr in the telencephalon 4 h post-stress when compared with the levels 1 h post-stress, which indicates that a negative feedback exists for these receptors, that aims to downregulate the HPI axis after activation by stress [72]. More sampling time points would have been required in this study to show the dynamics of the activation of similar feedback mechanisms in carp.
In rodents, maternal care increases the 5-ht turnover at the serotonin receptor, elevating the activity of this receptor, which leads to the activation of expression of factors, such as egr-1 further downstream [73,74]. The PCA in the present study indicated that egr-1 is a suitable gene for indicating changes in brain regulation in carp as a result of exposure to stress.
5. Conclusions
Acute distress by air exposure regulates gene expression differently in the brain regions of common carp. This study, therefore, performed an analysis of gene expression stability of potential reference genes before calculation the changes of mRNA expression changes of target genes. The PCA showed that a relatively low number of variables (i.e., genes for which their mRNA expression was investigated) is sufficient to explain a high amount of variance within the data set. In the optic tectum, this amount of variance was found to be higher than 90%. This is a promising result to better identify optimal genes as markers for stress in fish and confirms that, besides the known HPI axis gene, genes belonging to the group of immediate early genes and to neurotransmitter pathways should also be included in future biomarker development studies. However, the basic concept of a PCA is that the components contribute to the common variance, which is certainly not an optimal assumption for gene expression data in the brain. The regulation of genes in the brain, especially as a result of a (dis-)stressor, probably leads to a common regulation pattern, as well as a unique regulation of certain genes. Therefore, exploratory factor analysis is currently assumed to yield better models for the description of gene regulations in the fish brain. Given the limited number of samples analysed in the present study, the calculations are preliminary ones. In the future, a broader study with a higher number of animals per treatment group and additional stressors will be conducted and will highlight more detailed insights in the gene regulation in carp. Furthermore, studies considering the genetical background of farmed fish would be needed to show the relevance of stress biomarkers under farm condition. In addition, future research on brain activity will have to include a number of physiological and behavioural parameters allowing the more precise assessment of stress responses in fish.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/11/2/585/s1, Figure S1: “Variable correlation plot of the PCA for the IEG-related genes in each in the 4 brain parts”, Figure S2: “Variable correlation plot of the PCA for the HPI axis-related genes in each in the 4 brain parts”, Figure S3: “Variable correlation plot of the PCA for the serotonin- and gabaa-related genes in each in the 4 brain parts”, Figure S4: “Posteriori probability plots of all genes in each in the 4 brain parts of control fish and fish 30 min after the air exposure”, Table S1: “Primer pairs selected for the gene expression studies”, Table S2: “Average expression stability (M value) of the potential reference genes”.
Author Contributions
Conceptualization, methodology, investigation, funding acquisition, project administration, writing—original draft preparation, software, formal analysis, and data curation, visualization, writing—review and editing: A.B. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Bridge programme (Project No. 40B2-0_180864) supported by the Swiss National Science Fonds (SNSF) and the Innosuisse—Swiss Innovation Agency.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Cantonal Veterinary Authorities of the Canton Zurich, Switzerland under the license number ZH-062-17 on the 25 July 2017.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Moberg, G.P. Problems in defining stress and distress in animals. J. Am. Veter. Med. Assoc. 1987, 191, 1207–1211. [Google Scholar]
- Carstens, E.; Moberg, G.P. Recognizing pain and distress in laboratory animals. ILAR J. 2000, 41, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Gornati, R.; Papis, E.; Rimoldi, S.; Terova, G.; Saroglia, M.; Bernardini, G. Rearing density influences the expression of stress-related genes in sea bass (Dicentrarchus labrax, L.). Gene 2004, 341, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, G.; Palti, Y.; Cleveland, B.M.; Weber, G.M.; Rexroad, C.E. RNA-seq analysis of early hepatic response to handling and confinement stress in rainbow trout. PLoS ONE 2014, 9, e88492. [Google Scholar] [CrossRef]
- Sun, P.; Yin, F.; Tang, B. Effects of acute handling stress on expression of growth-related genes in Pampus argenteus. J. World Aquac. Soc. 2016, 48, 166–179. [Google Scholar] [CrossRef]
- Samaras, A.; Santo, C.E.; Papandroulakis, N.; Mitrizakis, N.; Pavlidis, M.; Höglund, E.; Pelgrim, T.N.M.; Zethof, J.; Spanings, F.A.T.; Vindas, M.A.; et al. Allostatic load and stress physiology in European seabass (Dicentrarchus labrax L.) and gilthead seabream (Sparus aurata L.). Front. Endocrinol. 2018, 9, 451. [Google Scholar] [CrossRef]
- Vindas, M.A.; Gorissen, M.; Höglund, E.; Flik, G.; Tronci, V.; Damsgård, B.; Thörnqvist, P.O.; Nilsen, T.O.; Winberg, S.; Øverli, Ø.; et al. How do individuals cope with stress? Behavioural, physiological and neuronal differences between proactive and reactive coping styles in fish. J. Exp. Biol. 2017, 220, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Aronson, L.R. Forebrain function in teleost fishes. Trans. N. Y. Acad. Sci. 1967, 29, 390–396. [Google Scholar] [CrossRef]
- Von Trotha, J.W.; Vernier, P.; Bally-Cuif, L. Emotions and motivated behavior converge on an amygdala-like structure in the zebrafish. Eur. J. Neurosci. 2014, 40, 3302–3315. [Google Scholar] [CrossRef]
- Lal, P.; Tanabe, H.; Suster, M.L.; Ailani, D.; Kotani, Y.; Muto, A.; Itoh, M.; Iwasaki, M.; Wada, H.; Yaksi, E.; et al. Identification of a neuronal population in the telencephalon essential for fear conditioning in zebrafish. BMC Biol. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Lau, B.Y.B.; Mathur, P.; Gould, G.G.; Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 2581–2586. [Google Scholar] [CrossRef]
- Paton, J.J.; Belova, M.A.; Morrison, S.E.; Salzman, C.D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nat. Cell Biol. 2006, 439, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.A. The amygdala, reward and emotion. Trends Cogn. Sci. 2007, 11, 489–497. [Google Scholar] [CrossRef]
- Morrison, S.E.; Salzman, C.D. Re-valuing the amygdala. Curr. Opin. Neurobiol. 2010, 20, 221–230. [Google Scholar] [CrossRef]
- Johansen, J.P.; Cain, C.K.; Ostroff, L.E.; LeDoux, J.E. Molecular mechanisms of fear learning and memory. Cell 2011, 147, 509–524. [Google Scholar] [CrossRef]
- Ingle, D.J.; Irwin, L.N. Optic Tectum. In Comparative Neuroscience and Neurobiology. Readings from the Encyclopedia of Neuroscience; Birkhäuser: Boston, MA, USA, 1988; pp. 100–101. [Google Scholar]
- Springer, A.D.; Easter, S.S.; Agranoff, B.W. The role of the optic tectum in various visually mediated behaviors of goldfish. Brain Res. 1977, 128, 393–404. [Google Scholar] [CrossRef]
- Roeser, T.; Baier, H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J. Neurosci. 2003, 23, 3726–3734. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.W.; Elmquist, J.K. From neuroanatomy to behavior: Central integration of peripheral signals regulating feeding behavior. Nat. Neurosci. 2012, 15, 1350–1355. [Google Scholar] [CrossRef]
- Moens, C.B.; Prince, V.E. Constructing the hindbrain: Insights from the zebrafish. Dev. Dyn. 2002, 224, 1–17. [Google Scholar] [CrossRef]
- Duran, E.; Ocaña, F.M.; Martín-Monzón, I.; Rodríguez, F.; Salas, C. Cerebellum and spatial cognition in goldfish. Behav. Brain Res. 2014, 259, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Adams, D.H.; Simen, B.B. Transcription factors as modulators of stress responsivity. In Handbook of Molecular-Genetic Techniques for Brain and Behavior Research; Elsevier Science: Amsterdam, The Netherlands, 2005; Volume 15, pp. 679–698. [Google Scholar]
- Ashlin, T.G.; Blunsom, N.J.; Ghosh, M.; Cockcroft, S.; Rihel, J. Pitpnc1a regulates zebrafish sleep and wake behavior through modulation of insulin-like growth factor signaling. Cell Rep. 2018, 24, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Tran, S.; Shams, S.; Gerlai, R. A simple method for immunohistochemical staining of zebrafish brain sections for c-fos protein expression. Zebrafish 2015, 12, 414–420. [Google Scholar] [CrossRef]
- Salierno, J.; Snyder, N.; Murphy, A.; Poli, M.; Hall, S.; Baden, D.; Kane, A. Harmful algal bloom toxins alter c-Fos protein expression in the brain of killifish, Fundulus heteroclitus. Aquat. Toxicol. 2006, 78, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.; Bell, A.M. Changes in behavior and brain immediate early gene expression in male threespined sticklebacks as they become fathers. Horm. Behav. 2018, 97, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Randlett, O.; Wee, C.L.; Naumann, E.A.; Nnaemeka, O.; Schoppik, D.; Fitzgerald, J.E.; Portugues, R.; Lacoste, A.M.; Riegler, C.; Engert, F.; et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat. Methods 2015, 12, 1039–1046. [Google Scholar] [CrossRef]
- Boukhelifa, M.; Parast, M.M.; Valtschanoff, J.G.; LaMantia, A.S.; Meeker, R.B.; Otey, C.A. A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol. Biol. Cell. 2001, 12, 2721–2729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, Z.; Chen, L.; Ge, Y.; Li, S.; Peng, W.; Li, C.; Zhang, Y.; Hu, X.; Zhou, Z.; Shi, L.; et al. Genetic mapping of Koi herpesvirus resistance (KHVR) in mirror carp (Cyprinus carpio) revealed genes and molecular mechanisms of disease resistance. Aquaculture 2020, 519, 734850. [Google Scholar] [CrossRef]
- Artelt, N.; Ludwig, T.A.; Rogge, H.; Kavvadas, P.; Siegerist, F.; Blumenthal, A.; Brandt, J.V.D.; Otey, C.A.; Bang, M.-L.; Amann, K.; et al. The role of palladin in podocytes. J. Am. Soc. Nephrol. 2018, 29, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Flik, G.; Klaren, P.H.; Burg, E.H.V.D.; Metz, J.R.; Huising, M.O. CRF and stress in fish. Gen. Comp. Endocrinol. 2006, 146, 36–44. [Google Scholar] [CrossRef]
- Conde-Sieira, M.; Chivite, M.; Míguez, J.M.; Soengas, J.L. Stress effects on the mechanisms regulating appetite in teleost fish. Front. Endocrinol. 2018, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Doyon, C.; Trudeau, V.; Moon, T.W. Stress elevates corticotropin-releasing factor (CRF) and CRF-binding protein mRNA levels in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2005, 186, 123–130. [Google Scholar] [CrossRef]
- Huising, M.; Van Schooten, C.; Taverne-Thiele, A.J.; Hermsen, T.; Kemenade, B.M.L.V.-V.; Flik, G.; Metz, J.R. Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J. Mol. Endocrinol. 2004, 32, 627–648. [Google Scholar] [CrossRef]
- Seasholtz, A.F.; Valverde, R.A.; Denver, R.J. Corticotropin-releasing hormone-binding protein: Biochemistry and function from fishes to mammals. J. Endocrinol. 2002, 175, 89–97. [Google Scholar] [CrossRef]
- Sturm, A.; Bury, N.; Dengreville, L.; Fagart, J.; Flouriot, G.; Rafestin-Oblin, M.E.; Prunet, P. 11-deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 2005, 146, 47–55. [Google Scholar] [CrossRef]
- Stolte, E.H.; De Mazon, A.F.; Leon-Koosterziel, K.M.; Jesiak, M.; Bury, N.R.; Sturm, A.; Savelkoul, H.F.J.; Van Kemenade, B.M.L.V.; Flik, G. Corticosteroid receptors involved in stress regulation in common carp, Cyprinus carpio. J. Endocrinol. 2008, 198, 403–417. [Google Scholar] [CrossRef]
- Gesto, M.; López-Patiño, M.A.; Hernández, J.; Soengas, J.L.; Míguez, J.M. Gradation of the stress response in rainbow trout exposed to stressors of different severity: The role of brain serotonergic and dopaminergic systems. J. Neuroendocr. 2014, 27, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, C.; Hoagland, A.; Carroll, E.; Schoppik, D.; Isacoff, E.Y. Measuring behavioral individuality in the acoustic startle behavior in zebrafish. Bio-Protocol 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ohlsen, R.I.; Pilowsky, L.S. The place of partial agonism in psychiatry: Recent developments. J. Psychopharmacol. 2005, 19, 408–413. [Google Scholar] [CrossRef]
- Kumar, S.; Porcu, P.; Werner, D.F.; Matthews, D.B.; Diaz-Granados, J.L.; Helfand, R.S.; Morrow, A.L. The role of GABAA receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology 2009, 205, 529–564. [Google Scholar] [CrossRef]
- Abreu, M.S.; Giacomini, A.C.V.; Gusso, D.; Rosa, J.G.; Koakoski, G.; Kalichak, F.; Idalêncio, R.; Oliveira, T.A.; Barcellos, H.H.; Bonan, C.D.; et al. Acute exposure to waterborne psychoactive drugs attract zebrafish. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2016, 179, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, E.; Gozdowska, M.; Kulczykowska, E. Nonapeptides arginine, vasotocin and isotocin in fishes: Advantage of bioactive molecules measurement. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Manzon, L.A. The role of prolactin in fish osmoregulation: A review. Gen. Comp. Endocrinol. 2002, 125, 291–310. [Google Scholar] [CrossRef]
- Power, D. Developmental ontogeny of prolactin and its receptor in fish. Gen. Comp. Endocrinol. 2005, 142, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Henry, M.A.; Akopian, A.N. Prolactin receptor in regulation of neuronal excitability and channels. Channels 2014, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Grattan, D.R. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef]
- Fujikawa, Y.; Kozono, K.; Esaka, M.; Iijima, N.; Nagamatsu, Y.; Yoshida, M.; Uematsu, K. Molecular cloning and effect of c-fos mRNA on pharmacological stimuli in the goldfish brain. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2006, 1, 253–259. [Google Scholar] [CrossRef][Green Version]
- Sarkar, D.K. Evidence for prolactin feedback actions on hypothalamic oxytocin, vasoactive intestinal peptide and dopamine secretion. Neuroendocrinology 1989, 49, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Prunet, P.; Gonnard, J.-F.; Paboeuf, G. GABA-ergic control of prolactin release in rainbow trout (Oncorhynchus mykiss) pituitaries in vitro. Fish. Physiol. Biochem. 1993, 11, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The ultimate qPCR experiment: Producing publication quality, reproducible data the first time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Zhu, A.; Srivastava, A.; Ibrahim, J.G.; Patro, R.; Love, M.I. Nonparametric expression analysis using inferential replicate counts. Nucleic Acids Res. 2019, 47, e105. [Google Scholar] [CrossRef] [PubMed]
- Bürkner, P.-C. Advanced Bayesian multilevel modeling with the R package brms. R J. 2018, 10, 395–411. [Google Scholar] [CrossRef]
- Remans, T.; Keunen, E.; Bex, G.J.; Smeets, K.; Vangronsveld, J.; Cuypers, A. Reliable gene expression analysis by reverse transcription-quantitative PCR: Reporting and minimizing the uncertainty in data accuracy. Plant. Cell 2014, 26, 3829–3837. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Hernandez, N.; Véliz, D.; Vega-Retter, C. Selection of suitable reference genes for gene expression analysis in gills and liver of fish under field pollution conditions. Sci. Rep. 2019, 9, 3459. [Google Scholar] [CrossRef] [PubMed]
- Øvergård, A.-C.; Nerland, A.H.; Patel, S. Evaluation of potential reference genes for real time RT-PCR studies in Atlantic halibut (Hippoglossus hippoglossus, L.); during development, in tissues of healthy and NNV-injected fish, and in anterior kidney leucocytes. BMC Mol. Biol. 2010, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, A.; Koskinen, H.; Pehkonen, P.; Rexroad, C.E., III; Afanasyev, S.; Mölsä, H. Gene expression in the brain and kidney of rainbow trout in response to handling stress. BMC Genom. 2005, 6, 3. [Google Scholar] [CrossRef]
- Kocmarek, A.L.; Ferguson, M.M.; Danzmann, R.G. Differential gene expression in small and large rainbow trout derived from two seasonal spawning groups. BMC Genom. 2014, 15, 57. [Google Scholar] [CrossRef]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef]
- Pavlidis, M.; Theodoridi, A.; Tsalafouta, A. Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 60, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Bernier, N.J. The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen. Comp. Endocrinol. 2006, 146, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Karakatsouli, N.; Katsakoulis, P.; Leondaritis, G.; Kalogiannis, D.; Papoutsoglou, S.E.; Chadio, S.; Sakellaridis, N. Acute stress response of European sea bass Dicentrarchus labrax under blue and white light. Aquaculture 2012, 48–52. [Google Scholar] [CrossRef]
- Alderman, S.L.; Bernier, N.J. Localization of corticotropin-releasing factor, urotensin I, and CRF-binding protein gene expression in the brain of the zebrafish, Danio rerio. J. Comp. Neurol. 2007, 502, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Bernier, N.J.; Flik, G.; Klaren, P.H.M. Chapter 6: Regulation and contribution of the corticotropic, melanotropic and thyrotropic axes to the stress response in fishes. In Fish Physiology: Fish Neuroendocrinology; Academic Press: Cambridge, MA, USA, 2009; pp. 235–311. ISSN 1546-5098. [Google Scholar]
- Williams, T.A.; Bergstrome, J.C.; Scott, J.; Bernier, N.J. CRF and urocortin 3 protect the heart from hypoxia/reoxygenation-induced apoptosis in zebrafish. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R91–R100. [Google Scholar] [CrossRef]
- Manuel, R.; Metz, J.R.; Flik, G.; Vale, W.W.; Huising, M.O. Corticotropin-releasing factor-binding protein (CRF-BP) inhibits CRF- and urotensin-I-mediated activation of CRF receptor-1 and -2 in common carp. Gen. Comp. Endocrinol. 2014, 202, 69–75. [Google Scholar] [CrossRef]
- Fu, Y.; Neugebauer, V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci. 2008, 28, 3861–3876. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.J.; Cerdá-Reverter, J.M.; Soengas, J.L. Hypothalamic integration of metabolic, endocrine, and circadian signals in fish: Involvement in the control of food intake. Front. Neurosci. 2017, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Tellez, R.; Gómez-Viquez, L.; Liy-Salmeron, G.; Meneses, A. GABA, glutamate, dopamine and serotonin transporters expression on forgetting. Neurobiol. Learn. Mem. 2012, 98, 66–77. [Google Scholar] [CrossRef]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Krystal, J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 2002, 159, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Moltesen, M.; Laursen, D.C.; Thörnqvist, P.-O.; Andersson, M.; Winberg, S.; Höglund, E. Effects of acute and chronic stress on telencephalic neurochemistry and gene expression in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2016, 219, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J.; Szyf, M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005, 28, 456–463. [Google Scholar] [CrossRef]
- Meaney, M.J.; Diorio, J.; Francis, D.; Weaver, S.; Yau, J.; Chapman, K.; Seckl, J.R. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin. J. Neurosci. 2000, 20, 3926–3935. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).