Effect of Tank Size on Zebrafish Behavior and Physiology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Activity Pattern
2.3. Behavior Test
2.3.1. Shelter Leaving Test
2.3.2. Shelter Seeking Test
2.3.3. Shoaling Test
2.3.4. Stamina Test
2.4. Physiology Test
2.5. Statistical Analysis
3. Results
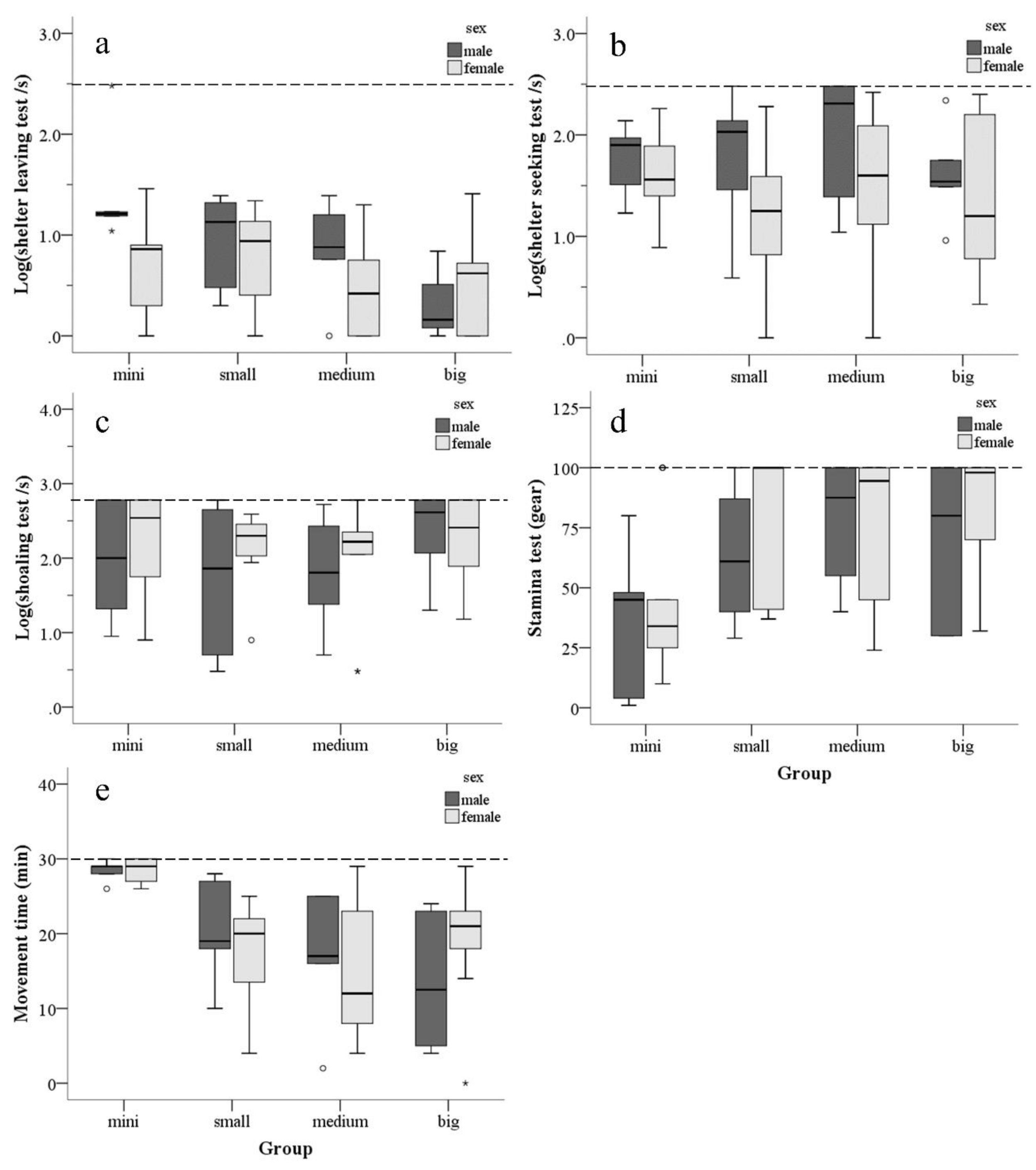
3.1. Effect of Tank Size on Behavior
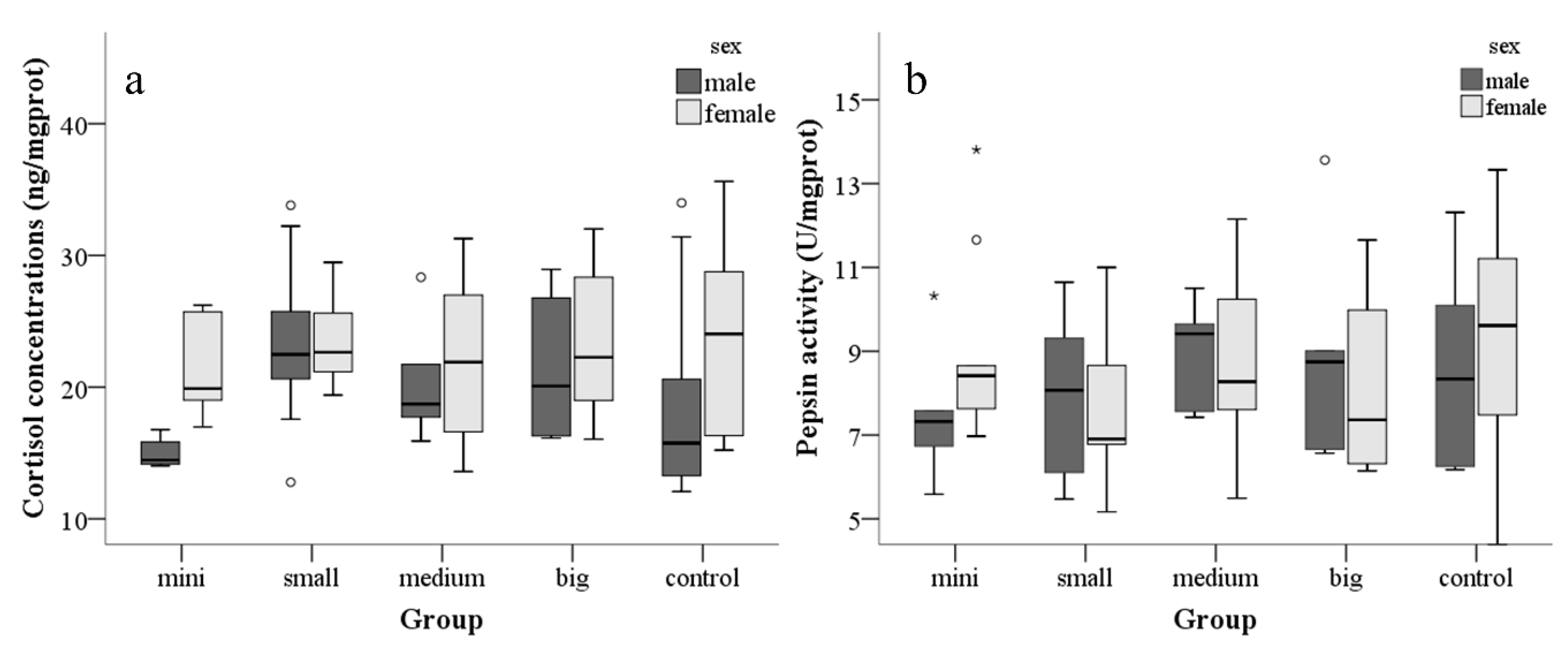
3.2. Effect of Tank Size on Physiology Test
3.3. Influence of Sex
4. Discussion
4.1. Effect of Tank Size on Zebrafish Behavior
4.2. Effect of Tank Size on Zebrafish Physiology
4.3. Sex Differences
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Test | Tank | Mean Time of Pass the Test (Before Dividing) | Mean Time of Pass the Test (After Feeding) | t-Value | d.f. | p-Value |
|---|---|---|---|---|---|---|
| Shelter leaving | mini | 1.270 | 0.950 | 1.477 | 13 | 0.164 |
| small | 1.338 | 0.841 | 2.620 | 15 | 0.019 * | |
| medium | 0.932 | 0.607 | 2.053 | 15 | 0.058 | |
| big | 1.340 | 0.398 | 4.647 | 14 | 0.001 * | |
| Shelter seeking | mini | 1.936 | 1.665 | 3.034 | 13 | 0.010 * |
| small | 1.906 | 1.531 | 1.622 | 15 | 0.126 | |
| medium | 1.805 | 1.690 | 0.550 | 15 | 0.591 | |
| big | 1.757 | 1.455 | 1.572 | 14 | 0.138 | |
| Shoaling | mini | 1.935 | 2.116 | −0.521 | 13 | 0.611 |
| small | 2.560 | 1.890 | 3.864 | 15 | 0.002 * | |
| medium | 2.391 | 1.896 | 2.076 | 15 | 0.056 | |
| big | 2.095 | 2.300 | −1.441 | 14 | 0.171 |
| Test | Group | Coef | HR | Z | p-Value | |
|---|---|---|---|---|---|---|
| Shelter leaving | sex | female | 0 | |||
| male | −0.982 | 0.374 | −1.171 | 0.242 | ||
| male | mini | 0 | ||||
| small | 0.544 | 1.723 | 0.547 | 0.585 | ||
| medium | 0.384 | 1.469 | 0.387 | 0.699 | ||
| big | 1.786 | 5.969 | 1.637 | 0.102 | ||
| female | mini | 0 | ||||
| small | 0.548 | 1.730 | 0.899 | 0.369 | ||
| medium | 1.074 | 2.929 | 1.839 | 0.066 | ||
| big | 0.490 | 1.632 | 0.748 | 0.454 | ||
| Shelter seeking | sex | female | 0 | |||
| male | −0.084 | 0.919 | −0.115 | 0.908 | ||
| male | mini | 0 | ||||
| small | −1.130 | 0.322 | −1.232 | 0.218 | ||
| medium | −0.804 | 0.447 | −0.867 | 0.386 | ||
| big | 0.184 | 1.202 | 0.185 | 0.853 | ||
| female | mini | 0 | ||||
| small | 0.929 | 2.533 | 1.579 | 0.114 | ||
| medium | 0.348 | 1.416 | 0.620 | 0.535 | ||
| big | −0.019 | 0.980 | −0.030 | 0.976 | ||
| Shoaling | sex | female | 0 | |||
| male | 0.286 | 1.332 | 0.392 | 0.695 | ||
| male | mini | 0 | ||||
| small | −0.402 | 0.668 | −0.443 | 0.658 | ||
| medium | −0.056 | 0.945 | −0.063 | 0.950 | ||
| big | −0.220 | 0.802 | −0.222 | 0.824 | ||
| female | mini | 0 | ||||
| small | 0.834 | 2.302 | 1.410 | 0.159 | ||
| medium | 0.830 | 2.295 | 1.475 | 0.140 | ||
| big | 0.012 | 1.012 | 0.019 | 0.985 | ||
| Stamina | sex | female | 0 | |||
| male | 0.278 | 1.321 | 0.470 | 0.638 | ||
| male | mini | 0 | ||||
| small | 0.481 | 1.618 | 0.531 | 0.595 | ||
| medium | −0.036 | 0.963 | −0.041 | 0.967 | ||
| big | −0.169 | 0.844 | −0.180 | 0.857 | ||
| female | mini | 0 | ||||
| small | −1.288 | 0.275 | −1.862 | 0.062 | ||
| medium | −1.153 | 0.315 | −1.959 | 0.050 | ||
| big | −1.162 | 0.312 | −1.973 | 0.048 * | ||
| Activity | sex | female | 0 | |||
| male | 0.267 | 1.306 | 0.414 | 0.679 | ||
| male | mini | 0 | ||||
| small | −0.940 | 0.390 | −1.130 | 0.258 | ||
| medium | −0.053 | 0.947 | 0.836 | 0.948 | ||
| big | 0.841 | 2.319 | 0.989 | 0.322 | ||
| female | mini | 0 | ||||
| small | 2.262 | 9.606 | 3.703 | <0.001 * | ||
| medium | 2.071 | 7.939 | 3.892 | <0.001 * | ||
| big | 1.494 | 4.457 | 2.781 | 0.005 * | ||
| Test | Group | Estimate | Std. Error | t-Value | p-Value | |
|---|---|---|---|---|---|---|
| Cortisol | Intercept | 24.912 | 3.292 | 7.567 | <0.001 * | |
| sex | female | |||||
| male | −5.987 | 2.863 | −2.091 | 0.041 * | ||
| male | mini | 0 | ||||
| small | 5.126 | 3.902 | 1.314 | 0.194 | ||
| medium | 3.575 | 3.986 | 0.897 | 0.373 | ||
| big | 3.385 | 3.999 | 0.847 | 0.401 | ||
| female | mini | 0 | ||||
| small | 3.011 | 2.634 | 1.143 | 0.258 | ||
| medium | 1.746 | 2.552 | 0.684 | 0.497 | ||
| big | 3.782 | 2.774 | 1.363 | 0.178 | ||
| Pepsin | Intercept | 12.618 | 1.161 | 10.862 | <0.001 * | |
| sex | female | 0 | ||||
| male | −1.290 | 1.010 | −1.277 | 0.207 | ||
| male | mini | 0 | ||||
| small | 0.902 | 1.376 | 0.656 | 0.514 | ||
| medium | 0.536 | 1.406 | 0.382 | 0.704 | ||
| big | 1.450 | 1.411 | 1.028 | 0.308 | ||
| female | mini | 0 | ||||
| small | 0.902 | 0.929 | −0.680 | 0.499 | ||
| medium | 0.536 | 0.900 | 1.253 | 0.215 | ||
| big | 1.450 | 0.978 | 0.760 | 0.450 | ||
References
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. Plos. Biol. 2010, 8, 1–5. [Google Scholar] [CrossRef]
- Simpson, J.; Kelly, J.P. The impact of environmental enrichment in laboratory rats-Behavioural and neurochemical aspects. Behav. Brain Res. 2011, 222, 246–264. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Baune, B.T. Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front. Cell Neurosci. 2014, 8, 97. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Brockmark, S.; Naeslund, J. Environmental effects on behavioural development consequences for fitness of captive-reared fishes in the wild. J. Fish. Biol. 2014, 85, 1946–1971. [Google Scholar] [CrossRef]
- Naslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish. Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Williams, T.D.; Readman, G.D.; Owen, S.F. Key issues concerning environmental enrichment for laboratory-held fish species. Lab. Anim. 2009, 43, 107–120. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Paulk, H.H.; Dienske, H.; Ribbens, L.G. Abnormal-Behavior in Relation to Cage Size in Rhesus-Monkeys. J. Abnorm. Psychol. 1977, 86, 87–92. [Google Scholar] [CrossRef]
- Manosevitz, M.; Pryor, J.B. Cage Size as a Factor in Environmental Enrichment. J. Comp. Physiol. Psych. 1975, 89, 648–654. [Google Scholar] [CrossRef]
- Polverino, G.; Manciocco, A.; Vitale, A.; Alleva, E. Stereotypic behaviours in Melopsittacus undulatus: Behavioural consequences of social and spatial limitations. Appl. Anim. Behav. Sci. 2015, 165, 143–155. [Google Scholar] [CrossRef]
- Espmark, A.M.; Kolarevic, J.; Asgard, T.; Terjesen, B.F. Tank size and fish management history matters in experimental design. Aquac. Res. 2017, 48, 2876–2894. [Google Scholar] [CrossRef]
- Tang, M.; Boisclair, D. Influence of the Size of Enclosures on the Swimming Characteristics of Juvenile Brook Trout (Salvelinus-Fontinalis). Can. J. Fish. Aquat. Sci. 1993, 50, 1786–1793. [Google Scholar] [CrossRef]
- Jha, P.; Barat, S.; Nayak, C.R. A comparison of growth, survival rate and number of marketable koi carp produced under different management regimes in earthen ponds and concrete tanks. Aquac. Int. 2006, 14, 615–626. [Google Scholar]
- Santaca, M.; Caja, T.; Miletto Petrazzini, M.E.; Agrillo, C.; Bisazza, A. Size discrimination in adult zebrafish (Danio rerio): Normative data and individual variation. Sci. Rep. 2020, 10, 1164. [Google Scholar] [CrossRef]
- Fishman, M.C. Genomics. Zebrafish--the canonical vertebrate. Science 2001, 294, 1290–1291. [Google Scholar] [CrossRef]
- Williams, F.E.; White, D.; Messer, W.S. A simple spatial alternation task for assessing memory function in zebrafish. Behav. Process. 2002, 58, 125–132. [Google Scholar] [CrossRef]
- Higgs, D.M.; Rollo, A.K.; Souza, M.J.; Popper, A.N. Development of form and function in peripheral auditory structures of the zebrafish (Danio rerio). J. Acoust. Soc. Am. 2003, 113, 1145–1154. [Google Scholar] [CrossRef]
- Gerlai, R. High-Throughput Behavioral Screens: The First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules 2010, 15, 2609–2622. [Google Scholar] [CrossRef]
- Lienart, G.D.H.; Mitchell, M.D.; Ferrari, M.C.O.; McCormick, M.I. Temperature and food availability affect risk assessment in an ectotherm. Anim. Behav. 2014, 89, 199–204. [Google Scholar] [CrossRef]
- Alestrom, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar]
- Piato, A.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.G.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog. Neuro-Psychopharmacol. 2011, 35, 561–567. [Google Scholar]
- Manuel, R.; Gorissen, M.; Stokkermans, M.; Zethof, J.; Ebbesson, L.O.E.; van de Vis, H.; Flik, G.; van den Bos, R. The Effects of Environmental Enrichment and Age-Related Differences on Inhibitory Avoidance in Zebrafish (Danio rerio Hamilton). Zebrafish 2015, 12, 152–165. [Google Scholar] [CrossRef]
- Wilkes, L.; Owen, S.F.; Readman, G.D.; Sloman, K.A.; Wilson, R.W. Does structural enrichment for toxicology studies improve zebrafish welfare? Appl. Anim. Behav. Sci. 2012, 139, 143–150. [Google Scholar] [CrossRef]
- Carter, A.J.; Feeney, W.E.; Marshall, H.H.; Cowlishaw, G.; Heinsohn, R. Animal personality: What are behavioural ecologists measuring? Biol Rev. 2013, 88, 465–475. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgard, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish. Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Kadri, S. Defining, assessing and promoting the welfare of farmed fish. Rev. Sci. Tech. Oie 2014, 33, 233–244. [Google Scholar] [CrossRef]
- Polverino, G.; Ruberto, T.; Staaks, G.; Mehner, T. Tank size alters mean behaviours and individual rank orders in personality traits of fish depending on their life stage. Anim. Behav. 2016, 115, 127–135. [Google Scholar] [CrossRef]
- Stewart, A.M.; Gaikwad, S.; Kyzar, E.; Kalueff, A.V. Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain. Res. 2012, 1451, 44–52. [Google Scholar] [CrossRef]
- Sneddon, L.U. The bold and the shy: Individual differences in rainbow trout. J. Fish. Biol. 2003, 62, 971–975. [Google Scholar] [CrossRef]
- Graham, C.; von Keyserlingk, M.A.G.; Franks, B. Zebrafish welfare: Natural history, social motivation and behaviour. Appl. Anim. Behav. Sci. 2018, 200, 13–22. [Google Scholar] [CrossRef]
- Wenk, C. Environmental effects on nutrient and energy metabolism in pigs. Arch. Anim. Nutr. 1998, 51, 211–224. [Google Scholar] [CrossRef]
- Braithwaite, V.A.; Ebbesson, L.O.E. Pain and stress responses in farmed fish. Rev. Sci. Tech. Oie 2014, 33, 245–253. [Google Scholar] [CrossRef]
- Shams, S.; Chatterjee, D.; Gerlai, R. Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav. Brain Res. 2015, 292, 283–287. [Google Scholar] [CrossRef]
- Wong, S.C.; Dykstra, M.; Campbell, J.M.; Earley, R.L. Measuring water-borne cortisol in convict cichlids (Amatitlania nigrofasciata): Is the procedure a stressor? Behaviour 2008, 145, 1283–1305. [Google Scholar]
- Brydges, N.M.; Braithwaite, V.A. Does environmental enrichment affect the behaviour of fish commonly used in laboratory work? Appl. Anim. Behav. Sci. 2009, 118, 137–143. [Google Scholar] [CrossRef]
- Ullah, I.; Zuberi, A.; Khan, K.U.; Ahmad, S.; Thornqvist, P.O.; Winberg, S. Effects of enrichment on the development of behaviour in an endangered fish mahseer (Tor putitora). Appl. Anim. Behav. Sci. 2017, 186, 93–100. [Google Scholar] [CrossRef]
- Bergendahl, I.A.; Salvanes, A.G.V.; Braithwaite, V.A. Determining the effects of duration and recency of exposure to environmental enrichment. Appl. Anim. Behav. Sci. 2016, 176, 163–169. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish. Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Wohr, A.C.; Erhard, M.H. Travel with dogs—Aspects of animal welfare. Tierarztl Prax. K H 2004, 32, 148–157. [Google Scholar] [CrossRef]
- Feist, G.; Schreck, C.B. Ontogeny of the stress response in chinook salmon, Oncorhynchus tshawytscha. Fish. Physiol. Biochem. 2001, 25, 31–40. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Feist, G.W.; Varga, Z.M.; Westerfield, M.; Kent, M.L.; Schreck, C.B. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture 2006, 258, 565–574. [Google Scholar] [CrossRef]
- Blessing, J.J.; Marshall, J.C.; Balcombe, S.R. Humane killing of fishes for scientific research: A comparison of two methods. J. Fish. Biol. 2010, 76, 2571–2577. [Google Scholar] [CrossRef]
- Roy, T.; Bhat, A. Population, sex and body size: Determinants of behavioural variations and behavioural correlations among wild zebrafish Danio rerio. Roy. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef]
- Maszczyk, P.; Gliwicz, Z.M. Selectivity by planktivorous fish at different prey densities, heterogeneities, and spatial scales. Limnol. Oceanogr. 2014, 59, 68–78. [Google Scholar] [CrossRef]
- Naslund, J.; Bererhi, B.; Johnsson, J.I. Design of Emergence Test Arenas Can Affect the Results of Boldness Assays. Ethology 2015, 121, 556–565. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Dong, J.Y.; Zhang, X.M.; Zhang, X.M. Effects of environmental enrichment on the distribution of Sebastes schlegelli in early developmental stages. Acta. Ecoligica. Sinica. 2018, 38, 1–10. [Google Scholar] [CrossRef]
- Harris, S.; Ramnarine, I.W.; Smith, H.G.; Pettersson, L.B. Picking personalities apart: Estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos 2010, 119, 1711–1718. [Google Scholar] [CrossRef]
- Ingley, S.J.; Rehm, J.; Johnson, J.B. Size doesn’t matter, sex does: A test for boldness in sister species of Brachyrhaphis fishes. Ecol. Evol. 2014, 4, 4361–4369. [Google Scholar]
- Moscicki, M.K.; Hurd, P.L. Sex, boldness and stress experience affect convict cichlid, Amatitlania nigrofasciata, open field behaviour. Anim. Behav. 2015, 107, 105–114. [Google Scholar]
- Roy, T.; Shukla, R.; Bhat, A. Risk-Taking during Feeding: Between- and Within-Population Variation and Repeatability across Contexts Among Wild Zebrafish. Zebrafish 2017, 14, 393–403. [Google Scholar]
- Weissman, M.M.; Bland, R.; Joyce, P.R.; Newman, S.; Wells, J.E.; Wittchen, H.U. Sex-Differences in Rates of Depression—Cross-National Perspectives. J. Affect. Disord. 1993, 29, 77–84. [Google Scholar] [CrossRef]
- Tian, D.; Xiao-Hong, X.U.; Hong, X.; Chen, L.; Xie, L.D.; Tao, L.I. Effects of Adulthood Exposure to Bisphenol-A on Behaviors in Mice. Acta. Psychologica Sinica. 2011, 43, 534–543. [Google Scholar] [CrossRef]
- Langenau, D.M.; Traver, D.; Ferrando, A.A.; Kutok, J.L.; Aster, J.C.; Kanki, J.P.; Lin, S.; Prochownik, E.; Trede, N.S.; Zon, L.I.; et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 2003, 299, 887–890. [Google Scholar] [CrossRef]
- Yang, P.; Kajiwara, R.; Tonoki, A.; Itoh, M. Successive and discrete spaced conditioning in active avoidance learning in young and aged zebrafish. Neurosci. Res. 2018, 130, 1–7. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; Metcalfe, N.B.; McKenzie, D.J.; Domenici, P. Environmental stressors alter relationships between physiology and behaviour. Trends Ecol. Evol 2013, 28, 651–658. [Google Scholar] [CrossRef]
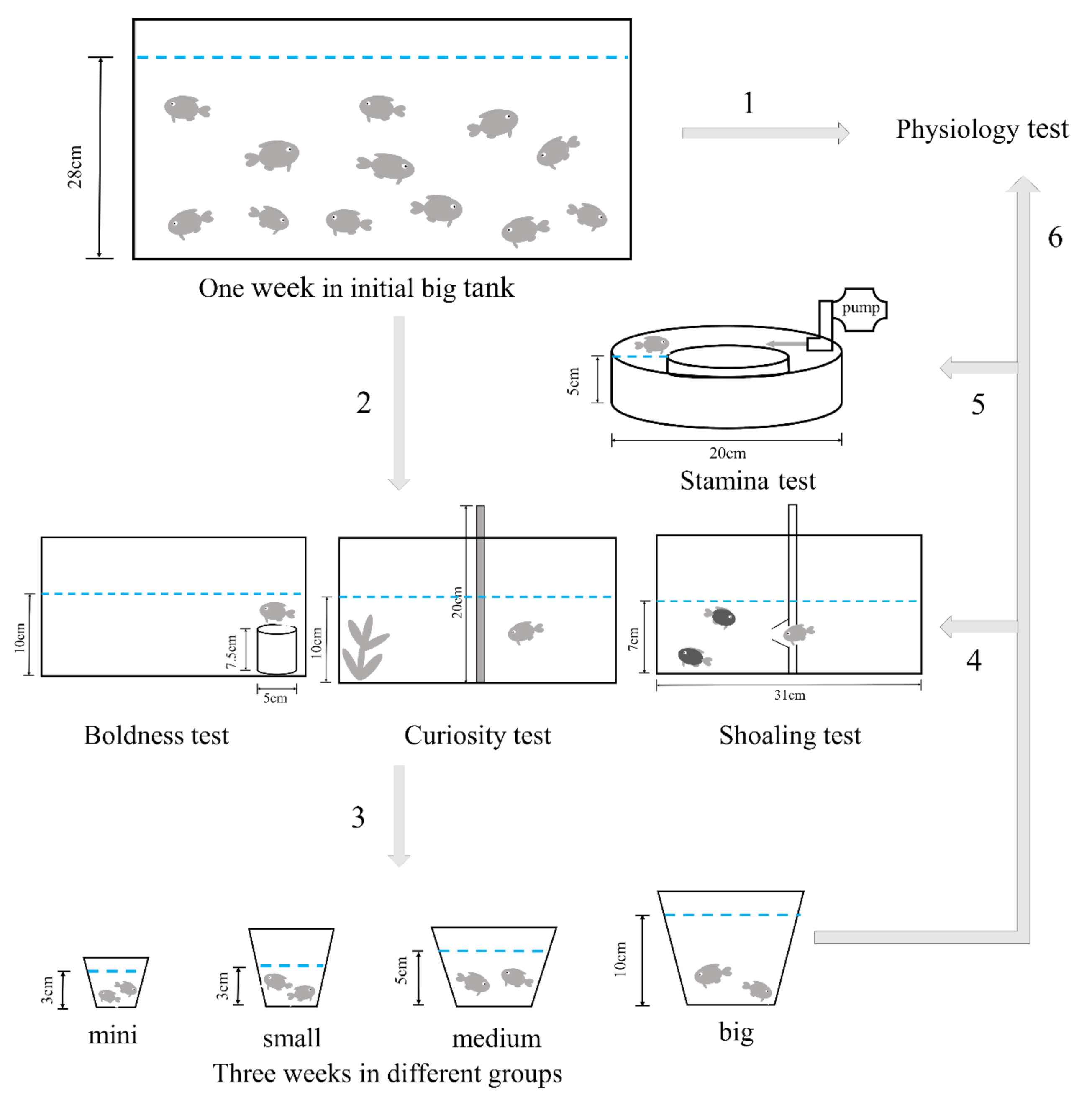
| Items | Length/cm | Width/cm | Height/cm | Depth of Water/cm | Volume of Water/cm3 |
|---|---|---|---|---|---|
| Mini tank A* | 3.6 | 4.8 | 3.5 | 3 | 40 |
| Small tank B * | 5 | 6.5 | 7.5 | 3 | 80 |
| Medium tank C * | 8 | 11.8 | 7.5 | 5 | 400 |
| Large tank D * | 12.5 | 17.5 | 13.5 | 10 | 1500 |
| Initial big tank | 50 | 25 | 31 | 28 | 3500 |
| Test tank | 31 | 23 | 16 | ||
| Opaque plate | 21 | 20 | |||
| Transparent plate ** | 21 | 20 | |||
| Water pump | 75 | 50 | 78 | ||
| Annular flume | Inner diameter: 15 | External diameter: 20 | 5 | 5 | |
| Shelter | External diameter:5 | 7.5 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maierdiyali, A.·.; Wang, L.; Luo, Y.; Li, Z. Effect of Tank Size on Zebrafish Behavior and Physiology. Animals 2020, 10, 2353. https://doi.org/10.3390/ani10122353
Maierdiyali A·, Wang L, Luo Y, Li Z. Effect of Tank Size on Zebrafish Behavior and Physiology. Animals. 2020; 10(12):2353. https://doi.org/10.3390/ani10122353
Chicago/Turabian StyleMaierdiyali, Abudusaimaiti ·, Lin Wang, Yunchao Luo, and Zhongqiu Li. 2020. "Effect of Tank Size on Zebrafish Behavior and Physiology" Animals 10, no. 12: 2353. https://doi.org/10.3390/ani10122353
APA StyleMaierdiyali, A. ·., Wang, L., Luo, Y., & Li, Z. (2020). Effect of Tank Size on Zebrafish Behavior and Physiology. Animals, 10(12), 2353. https://doi.org/10.3390/ani10122353








