Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sampling
2.2. Gene Expression Analysis
2.3. Analysis of Blood Biochemical/Immunological Variables
2.4. Next-Generation Sequencing (NGS) of Bacterial Community Profiles
2.5. Mathematical and Statistical Analyses
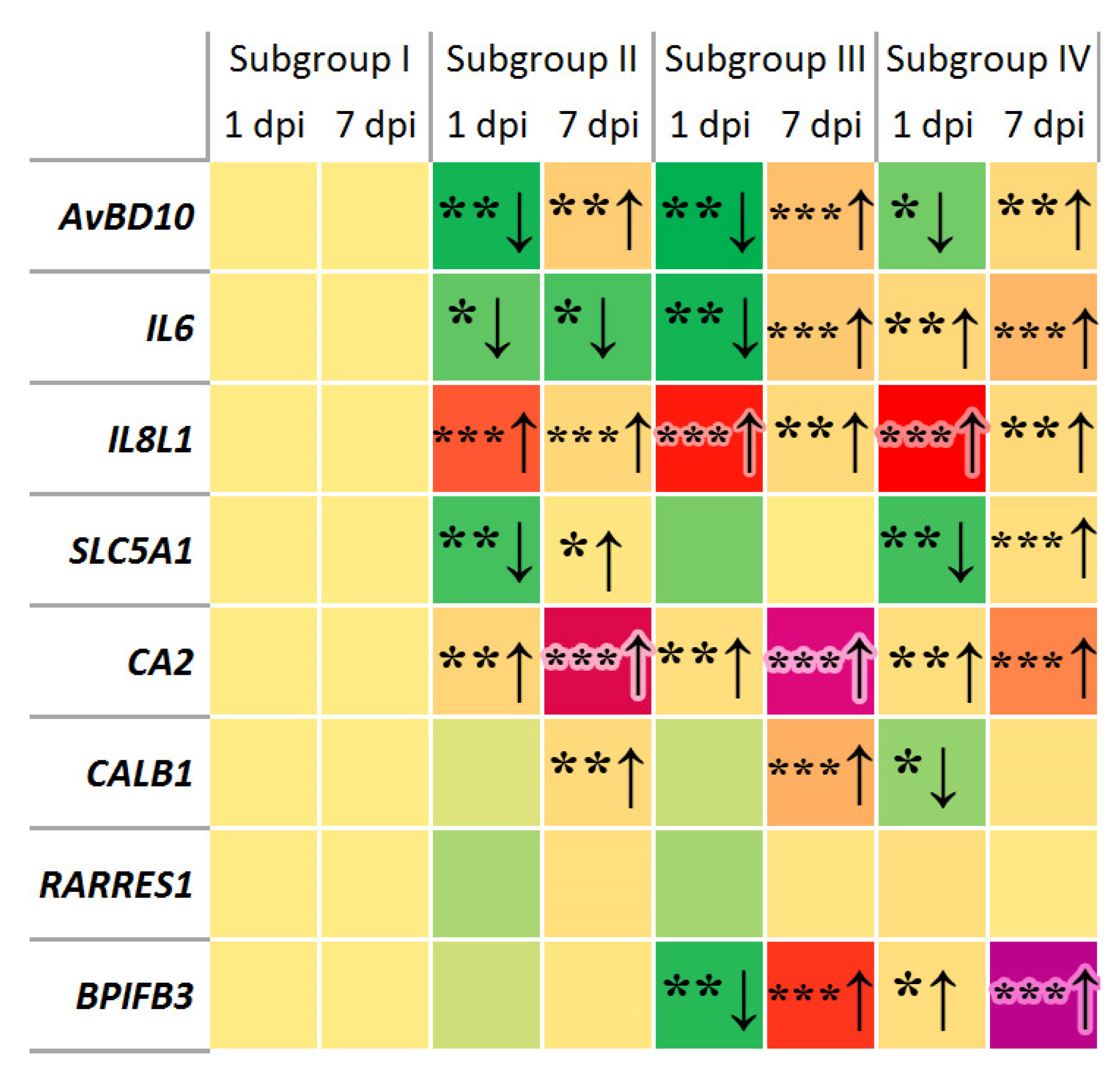
3. Results
3.1. Changes in Gene Expression
3.2. Dynamics of Blood Biochemical/Immunological Variables
3.3. Cecal Microbiome Diversity Characterization
3.4. Effects of SE and Intebio® on Egg Productivity
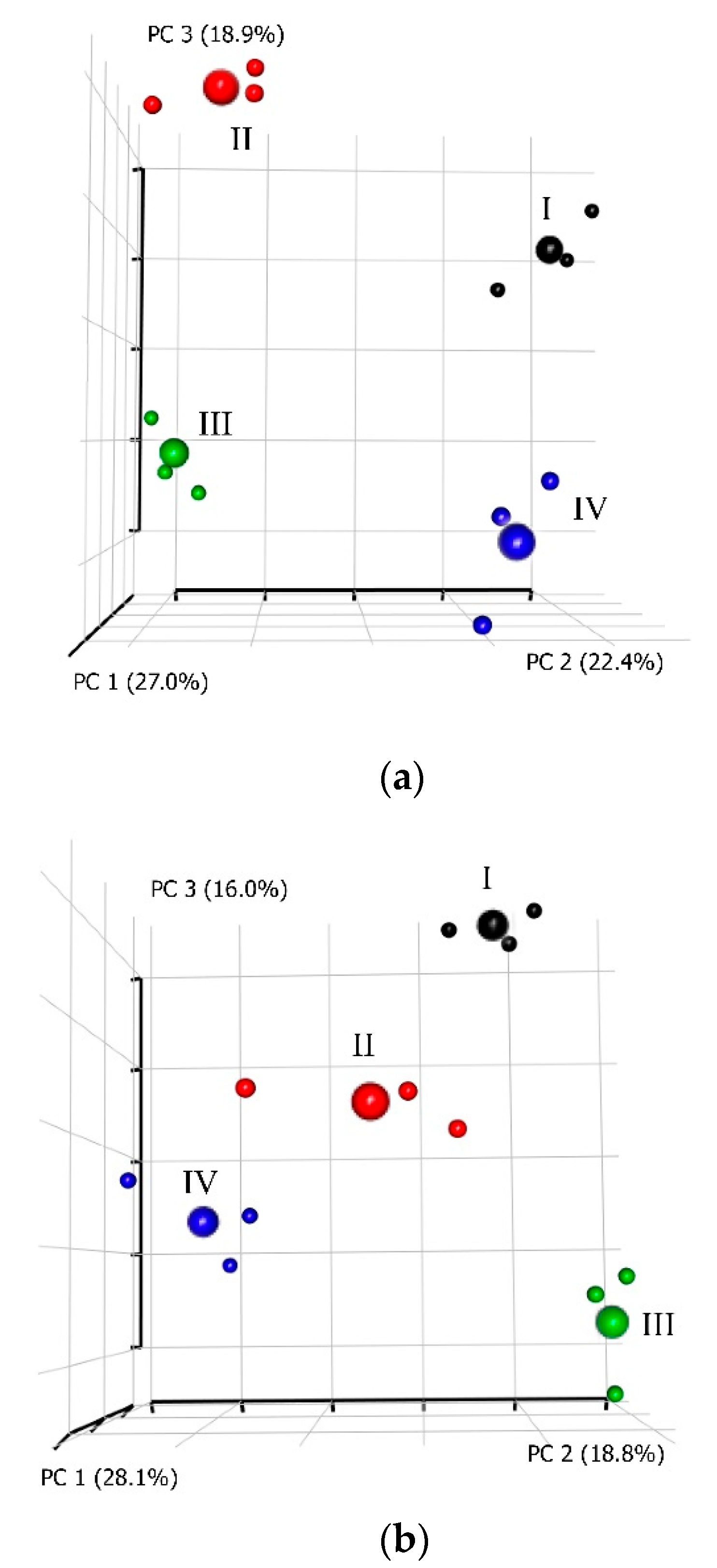
3.5. Mathematical Analyses Combining All Studied Characters
4. Discussion
4.1. Gene Expression Changes in Response to SE and Phytobiotic
4.2. Blood Biochemical/Immunological Variables as Affected by SE and Intebio®
4.3. Effects of SE and Intebio® on Cecal Microbiome Profiles
4.4. Egg Production Traits and Effects of SE and Intebio®
4.5. Interplay between Treatments and Studied Characters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Department of Agriculture. Food Safety and Inspection Service, 1998. Progress Report on Salmonella Testing of Raw Meat and Poultry Products, 1998–2003. Available online: https://www.fsis.usda.gov/Oa/background/salmtest4.htm (accessed on 3 January 2021).
- Galan, J.E.; Curtiss, R. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 1989, 86, 6383–6387. [Google Scholar] [CrossRef] [PubMed]
- Le Minor, L.E. The genus Salmonella. In The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community, Electronic Release 3.14, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Eds.; Springer: New York, NY, USA, 2003; p. 314. ISBN 0-387-14254-1. [Google Scholar]
- Mughini-Gras, L.; Enserink, R.; Friesema, I.; Heck, M.; van Duynhoven, Y.; van Pelt, W. Risk factors for human salmonellosis originating from pigs, cattle, broiler chickens and egg laying hens: A combined case-control and source attribution analysis. PLoS ONE 2014, 9, e87933. [Google Scholar] [CrossRef] [PubMed]
- Spiridonov, A.N.; Petrova, O.N.; Irza, V.N.; Karaulov, A.K.; Nikiforov, V.V. Epizootic situation on infectious avian diseases based on analysis of data from veterinary reports. Vet. Segodnia 2015, 4, 18–28. [Google Scholar]
- Withanage, G.S.K.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Hoffmann, C.; Hill, D.A.; Minkah, N.; Kirn, T.; Troy, A.; Artis, D.; Bushman, F.D. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect. Immun. 2009, 77, 4668–4678. [Google Scholar] [CrossRef]
- Hill, D.A.; Hoffmann, C.; Abt, M.C.; Du, Y.; Kobuley, D.; Kirn, T.J.; Bushman, F.; Artis, D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2009, 3, 148–158. [Google Scholar] [CrossRef]
- Sekirov, I.; Tam, N.M.; Jogova, M.; Robertson, M.L.; Li, Y.; Lupp, C.; Finlay, B.B. Antibiotic-induced perturbations of the in-testinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 2008, 76, 4726–4736. [Google Scholar] [CrossRef]
- Croswell, A.; Amir, E.; Teggatz, P.; Barman, M.; Salzman, N.H. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect. Immun. 2009, 77, 2741–2753. [Google Scholar] [CrossRef]
- Broeck, W.V.D.; Cox, E.; Goddeeris, B.M. Receptor-dependent immune responses in pigs after oral immunization with F4 fimbriae. Infect. Immun. 1999, 67, 520–526. [Google Scholar] [CrossRef]
- Baylis, M.; Goldmann, W. The genetics of scrapie in sheep and goats. Curr. Mol. Med. 2004, 4, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry1. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.S.; Glebova, I.V. Antibacterial effect of essential oils of medicinal plants (review). Probl. Biol. Produktivn. Zhivotn. 2017, 3, 5–25. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.C.; Hsu, C.L.; Yen, G.C. Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus het-erophyllus. J. Agric. Food Chem. 2008, 56, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Siani, A.C.; Souza, M.C.; Henriques, M.G.; Ramos, M.F. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013, 51, 881–887. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Johny, A.K.; Darre, M.J.; Donoghue, A.M.; Donoghue, D.J.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, thymol and carvacrol against Salmonella Enteritidis and Campylobacter jejuni in vitro. J. Appl. Poult. Res. 2010, 19, 237–244. [Google Scholar] [CrossRef]
- Piesova, E.; Makova, Z.; Levkut, M.; Faixova, Z.; Pistl, J.; Marcin, A.; Levkut, M. The effects of sage extract feed supplemen-tation on biochemical parameters, weight of internal organs and Salmonella counts in chickens. Res. Vet. Sci. 2012, 93, 1307–1308. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fascina, V.B.; Sartori, J.R.; Gonzales, E.; Carvalho, F.B.; Souza, I.M.G.P.; Polycarpo, G.V.; Stradiotti, A.C.; Pelícia, V.C. Phyto-genic additives and organic acids in broiler chicken diets. Rev. Bras. Zootec. 2012, 41, 2189–2197. [Google Scholar] [CrossRef]
- U.S. Food and Drugs Administration. Code of Federal Regulations. Title 21—Food and Drugs. Chapter I—Food and Drug Administration. Subchapter E—Animal Drugs, Feeds, and Related Products. Part 582—Substances Generally Recognized as Safe. Subpart A—Synthetic Flavoring Substances and adjuvants; U.S. Food and Drugs Administration: Silver Spring, MD, USA, 2011.
- Aldridge, P.D.; Gray, M.A.; Hirst, B.H.; Khan, C.M. Who’s talking to whom? Epithelial-bacterial pathogen interactions. Mol. Microbiol. 2005, 55, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Jenner, R.G.; Young, R.A. Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Genet. 2005, 3, 281–294. [Google Scholar] [CrossRef]
- Norkina, O.; Burnett, T.G.; de Lisle, R.C. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect. Immun. 2004, 72, 6040–6049. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Laubitz, D.; Hill, F.M.; Shehab, K.W.; Lipinski, L.; Midura-Kiela, M.T.; McFadden, R.-M.T.; Ramalingam, R.; Hassan, K.A.; Gołębiewski, M.; et al. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am. J. Physiol. Liver Physiol. 2013, 305, G667–G677. [Google Scholar] [CrossRef]
- Berkes, J.; Viswanathan, V.K.; Savkovic, S.D.; Hecht, G. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut 2003, 52, 439–451. [Google Scholar] [CrossRef]
- Temperley, N.D.; Berlin, S.; Paton, I.R.; Griffin, D.; Burt, D.W. Evolution of the chicken Toll-like receptor gene family: A story of gene gain and gene loss. BMC Genom. 2008, 9, 62. [Google Scholar] [CrossRef]
- Barrow, P.; Huggins, M.; Lovell, M.; Simpson, J. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res. Vet. Sci. 1987, 42, 194–199. [Google Scholar] [CrossRef]
- Kogut, M.H.; Tellez-Isaias, G.; McGruder, E.D.; Hargis, B.M.; Williams, J.D.; Corrier, D.E.; Deloach, J.R. Heterophils are decisive components in the early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 1994, 16, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Wolf, M.; Walz, A.; Loetscher, P. Chemokines: Multiple levels of leukocyte migration control. Trends Immunol. 2004, 25, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.R.; Haghighi, H.R.; Chambers, J.R.; Brisbin, J.; Read, L.R.; Sharif, S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar Typhimurium. Clin. Vaccine Immunol. 2008, 15, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P. Salmonella enterica in the chicken: How it has helped our understanding of immunology in a non-biomedical model species. Front. Immunol. 2014, 5, 482. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Biragyn, A.; Hoover, D.M.; Lubkowski, J.; Oppenheim, J.J. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004, 22, 181–215. [Google Scholar] [CrossRef] [PubMed]
- Menendez, A.; Finlay, B.B. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 2007, 19, 385–391. [Google Scholar] [CrossRef]
- Matulova, M.; Rajova, J.; Vlasatikova, L.; Volf, J.; Stepanova, H.; Havlickova, H.; Sisak, F.; Rychlik, I. Characterization of chicken spleen transcriptome after infection with Salmonella enterica serovar Enteritidis. PLoS ONE 2012, 7, e48101. [Google Scholar] [CrossRef]
- Klotman, M.E.; Chang, T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006, 6, 447–456. [Google Scholar] [CrossRef]
- Lynn, D.J.; Higgs, R.; Lloyd, A.T.; O’Farrelly, C.; Hervé-Grépinet, V.; Nys, Y.; Brinkman, F.S.; Yu, P.-L.; Soulier, A.; Kaiser, P.; et al. Avian beta-defensin nomenclature: A community proposed update. Immunol. Lett. 2007, 110, 86–89. [Google Scholar] [CrossRef]
- Yacoub, H.A.; Elazzazy, A.M.; Abuzinadah, O.A.H.; Al-Hejin, A.M.; Mahmoud, M.M.; Harakeh, S.M. Antimicrobial activities of chicken β-defensin (4 and 10) peptides against pathogenic bacteria and fungi. Front. Cell. Infect. Microbiol. 2015, 5, 36. [Google Scholar] [CrossRef]
- Lee, M.O.; Romanov, M.N.; Plemyashov, K.V.; Dementieva, N.V.; Mitrofanova, O.V.; Barkova, O.Y.; Womack, J.E. Haplotype structure and copy number polymorphism of the beta-defensin 7 genes in diverse chicken breeds. Anim. Genet. 2017, 48, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, T.; Coorens, M.; van Dijk, A.; Haagsman, H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013, 41, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, A.; Veldhuizen, E.J.A.; Kalkhove, S.I.C.; Bokhoven, J.L.M.T.-V.; Romijn, R.A.; Haagsman, H.P. The β-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob. Agents Chemother. 2006, 51, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Coble, D.J.; Sandford, E.E.; Ji, T.; Abernathy, J.; Fleming, D.; Zhao, H.; Lamont, S.J. Impacts of Salmonella enteritidis infection on liver transcriptome in broilers. Genesis 2013, 51, 357–364. [Google Scholar] [CrossRef]
- Bullis, K.L. The history of avian medicine in the U.S. II. Pullorum disease and fowl typhoid. Avian Dis. 1977, 21, 422. [Google Scholar] [CrossRef]
- Lister, S.A.; Barrow, P. Bacterial diseases. In Poultry Diseases, 6th ed.; Pattison, M., McMullin, P., Bradbury, J.M., Alexander, D., Eds.; W.B. Saunders Ltd.: Edinburgh, UK, 2008; pp. 110–145. ISBN 978-0-7020-2862-5. [Google Scholar]
- Soria, M.A.; Bonnet, M.A.; Bueno, D.J. Relationship of Salmonella infection and inflammatory intestinal response with hema-tological and serum biochemical values in laying hens. Vet. Immunol. Immunopathol. 2015, 165, 145–153. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Lehmann, A.; Hornby, P.J. Intestinal SGLT1 in metabolic health and disease. Am. J. Physiol. Liver Physiol. 2016, 310, G887–G898. [Google Scholar] [CrossRef]
- Fernández, M.; Passalacqua, K.; Arias, J.; Arias, J. Partial biomimetic reconstitution of avian eggshell formation. J. Struct. Biol. 2004, 148, 1–10. [Google Scholar] [CrossRef]
- Gautron, J.; Hincke, M.T.; Mann, K.; Panhéleux, M.; Bain, M.; McKee, M.D.; Solomon, S.E.; Nys, Y. Ovocalyxin-32, a novel chicken eggshell matrix protein. J. Biol. Chem. 2001, 276, 39243–39252. [Google Scholar] [CrossRef]
- Gautron, J.; Murayama, E.; Vignal, A.; Morisson, M.; McKee, M.D.; Réhault, S.; Labas, V.; Belghazi, M.; Vidal, M.-L.; Nys, Y.; et al. Cloning of ovocalyxin-36, a novel chicken eggshell protein related to lipopolysaccharide-binding proteins, bactericidal permeability-increasing proteins, and plunc family proteins. J. Biol. Chem. 2007, 282, 5273–5286. [Google Scholar] [CrossRef] [PubMed]
- Hincke, M.T.; Gautron, J.; Mann, K.; Panhéleux, M.; McKee, M.D.; Bain, M.; Solomon, S.E.; Nys, Y. Purification of ovocalyxin-32, a novel chicken eggshell matrix protein. Connect. Tissue Res. 2003, 44, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wellman-Labadie, O.; Gautron, J.; Hincke, M.T. Recombinant eggshell ovocalyxin-32: Expression, purification and biological activity of the glutathione S-transferase fusion protein. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Ellakany, H.F.; El-Hamid, A.A.; Bovera, F.; Ghazaly, S.A. Control of Salmonella enteritidis infection in male layer chickens by acetic acid and/or prebiotics, probiotics and antibiotics. Arch Geflügelk 2012, 76, 239–245. [Google Scholar]
- Fan, S.; Zheng, J.; Duan, Z.; Yang, N.; Xu, G. The influences of SE infection on layers’ production performance, egg quality and blood biochemical indicators. J. Anim. Sci. Biotechnol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amalaradjou, M.A.R.; Bhunia, A.K. Modern approaches in probiotics research to control foodborne pathogens. Adv. Food Nutr. Res. 2012, 67, 185–239. [Google Scholar] [CrossRef]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal bacteria: An emerging player in defense against respiratory pathogens. Front. Immunol. 2019, 10, 1203. [Google Scholar] [CrossRef]
- Laptev, G.Y.; Bolshakov, V.N.; Soldatova, V.V. Feed additive “Mix-Oil” in feeding pigs. Sel’skokhozyaystvennyye Vesti 2012, 1, 24. [Google Scholar]
- Bagno, O.A.; Prokhorov, O.N.; Shevchenko, S.A.; Shevchenko, A.I.; Dyadichkina, T.V. Use of phytobiotics in farm animal feeding (review). Selskokhoziaistvennaia Biol. 2018, 53, 687–697. [Google Scholar] [CrossRef]
- Laptev, G.Y.; Filippova, V.A.; Kochish, I.I.; Yildirim, E.A.; Ilina, L.A.; Dubrovin, A.V.; Braznik, E.; Novikova, N.I.; Dmitrieva, M.E.; Smolensky, V.I.; et al. Examination of the expression of immunity genes and bacterial profiles in the caecum of growing chickens infected with Salmonella Enteritidis and fed a phytobiotic. Animals 2019, 9, 615. [Google Scholar] [CrossRef]
- Bai, S.P.; Huang, Y.; Luo, Y.H.; Wang, L.L.; Ding, X.M.; Wang, J.P.; Zeng, Q.F.; Zhang, K.Y. Alteration in lymphocytes responses, cytokine and chemokine profiles in laying hens infected with Salmonella Typhimurium. Veter. Immunol. Immunopathol. 2014, 160, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of dietary supplementation of nitrocompounds on Salmonella colonization and ileal immune gene expression in laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2017, 96, 4280–4286. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef]
- Ministry of Health of the USSR, Chief Veterinary Directorate at the State Commission for Food and Procurement of the Council of Ministers of the USSR. Laboratory Diagnostics of Human and Animal Salmonellosis, Detection of Salmonella in Feed, Food and Environmental Objects (Methodological Recommendations); Central Research Institute of Epidemiology of the Ministry of Health of the USSR: Moscow, Russia, 1990.
- Titov, V.Y.; Vertiprakhov, V.G.; Kosenko, O.V.; Fisinin, V.I.; Dmitrieva, M.E.; Novikova, O.B.; Petrov, V.A. Concentrations of nitrite and non-thiol nitroso compounds in tissues as a high sensitive marker of leukocyte activity. Russ. Agric. Sci. 2017, 4, 58–61. [Google Scholar] [CrossRef]
- Zeka, F.; Vanderheyden, K.; de Smet, E.; Cuvelier, C.A.; Mestdagh, P.; Vandesompele, J. Straightforward and sensitive RT-qPCR based gene expression analysis of FFPE samples. Sci. Rep. 2016, 6, 21418. [Google Scholar] [CrossRef]
- El Khoury, R.; Atoui, A.; Verheecke-Vaessen, C.; Maroun, R.G.; el Khoury, A.; Mathieu, F. Essential oils modulate gene expression and ochratoxin a production in Aspergillus carbonarius. Toxins 2016, 8, 242. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sadovnikov, N.V.; Pridybailo, N.D.; Vereshchak, N.A.; Zaslonov, A.S. General and Special Methods of Testing the Blood of Poultry of Industrial Crosses; Ural State Agricultural Academy; AVIVAK Research and Production Enterprise: Yekaterinburg—Saint Petersburg, Russia, 2009; pp. 18–25. ISBN 978-5-87203-260-6. [Google Scholar]
- Kostyna, M.A. Determination of classes of immunoglobulins by the method of discrete sedimentation. In Problems of Increasing the Resistance of Newborn Animals: Collection of Scientific Papers of VNIINBZh; VNIINBZh: Voronezh, Russia, 1983; pp. 76–80. [Google Scholar]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Lakin, G.F. Biometrics; Vysshaya Shkola: Moscow, Russia, 1990; 352p, ISBN 5-06-000471-6. [Google Scholar]
- R. Documentation. Tukey HSD. Available online: https://www.rdocumentation.org/packages/stats/versions/3.6.1/topics/TukeyHSD (accessed on 28 November 2020).
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hautamaki, V.; Fränti, P. Knee point detection in BIC for detecting the number of clusters. In Lecture Notes in Computer Science, International Conference on Advanced Concepts for Intelligent Vision Systems—ACIVS 2008, Juan-les-Pins, France, 20–24 October 2008; Blanc-Talon, J., Bourennane, S., Philips, W., Popescu, D., Scheunders, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5259, pp. 664–673. ISBN 978-3-540-88457-6. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Version 1.0.5. 22 August 2017. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 28 November 2020).
- Skopina, M.Y.; Vasileva, A.A.; Pershina, E.V.; Pinevich, A.V. Diversity at low abundance: The phenomenon of the rare bacterial biosphere. Microbiology 2016, 85, 272–282. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Hammer, Ø. Past 3.x—The Past of the Future. Natural History Museum, University of Oslo. Available online: http://folk.uio.no/ohammer/past/ (accessed on 6 November 2020).
- Hammer, Ø. PAST: PAleontological STatistics. Version 3.22: Reference Manual. Natural History Museum University of Oslo, 1999–2018; pp. 159–160. Available online: http://folk.uio.no/ohammer/past/past3manual.pdf (accessed on 23 July 2020).
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. GigaScience 2013, 2, 16. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Plotly. Plotly R Open Source Graphing Library, 2019. Available online: https://plot.ly/r/ (accessed on 28 November 2020).
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN Community Edition—interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. Version 1.1.453; RStudio Inc.: Boston, MA, USA, 2018. [Google Scholar]
- Van Hemert, S. Gene Expression Profiling of Chicken Intestinal Host. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007. [Google Scholar]
- Kaiser, M.G.; Block, S.S.; Ciraci, C.; Fang, W.; Sifri, M.; Lamont, S.J. Effects of dietary vitamin E type and level on lipopolysaccharide-induced cytokine mRNA expression in broiler chicks. Poult. Sci. 2012, 91, 1893–1898. [Google Scholar] [CrossRef]
- Kannaki, T.; Verma, P.; Reddy, M.R. Differential gene expression of antimicrobial peptides β defensins in the gastrointestinal tract of Salmonella serovar Pullorum infected broiler chickens. Veter. Res. Commun. 2011, 36, 57–62. [Google Scholar] [CrossRef]
- Sadeyen, J.-R.; Trotereau, J.; Velge, P.; Marly, J.; Beaumont, C.; Barrow, P.A.; Bumstead, N.; Lalmanach, A.-C. Salmonella carrier state in chicken: Comparison of expression of immune response genes between susceptible and resistant animals. Microbes Infect. 2004, 6, 1278–1286. [Google Scholar] [CrossRef]
- Moldawer, L.L.; Gelin, J.; Schersten, T.; Lundholm, K.G. Circulating interleukin 1 and tumor necrosis factor during inflammation. Am. J. Physiol. 1987, 253, R922–R928. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.; Tompkins, R.G.; Gelfand, J.A.; Michie, H.R.; Stanford, G.G.; van der Meer, J.W.; Endres, S.; Lonnemann, G.; Corsetti, J.; Chernow, B. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 1990, 161, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Lotze, M. (Eds.) The Cytokine Handbook, Two-Volume Set, 4th ed.; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2003; p. 1572. ISBN 978-0-12-689663-3. [Google Scholar]
- Fong, Y.; Moldawer, L.L.; Marano, M.; Wei, H.; Barber, A.; Manogue, K.; Tracey, K.J.; Kuo, G.A.; Fischman, D.; Cerami, A. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am. J. Physiol. 1989, 256, 659. [Google Scholar] [CrossRef] [PubMed]
- Moldawer, L.L.; Andersson, C.; Gelin, J.; Lundholm, K.G. Regulation of food intake and hepatic protein synthesis by recom-binant derived cytokines. Am. J. Physiol. 1988, 254, G450–G456. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Wei, H.; Manogue, K.R.; Fong, Y.; Hesse, D.G.; Nguyen, H.T.; Kuo, G.C.; Beutler, B.; Cotran, R.S.; Cerami, A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J. Exp. Med. 1988, 167, 1211–1227. [Google Scholar] [CrossRef]
- Ferraccioli, G.; Bracci-Laudiero, L.; Alivernini, S.; Gremese, E.; Tolusso, B.; de Benedetti, F. Interleukin-1β and interleukin-6 in arthritis animal models: Roles in the early phase of transition from acute to chronic inflammation and relevance for human rheumatoid arthritis. Mol. Med. 2010, 16, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Dewald, B.; Moser, B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 1994, 55, 97–179. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. Inflammation: Friend or foe for animal production? Poult. Sci. 2018, 97, 510–514. [Google Scholar] [CrossRef]
- Setta, A.; Barrow, P.; Kaiser, P.; Jones, M.A. Early immune dynamics following infection with Salmonella enterica serovars Enteritidis, Infantis, Pullorum and Gallinarum: Cytokine and chemokine gene expression profile and cellular changes of chicken cecal tonsils. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 397–410. [Google Scholar] [CrossRef]
- Berndt, A.; Wilhelm, A.; Jugert, C.; Pieper, J.; Sachse, K.; Methner, U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 2007, 75, 5993–6007. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A.; Turk, E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology 2004, 19, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Li, J.; Wang, C.; Liu, X.; Zhang, H.; Mei, Y.; Ling, F.; Li, S.; Chen, S.; et al. Molecular characterization, expression profile and polymorphisms of the porcine TNNC2 gene. Hereditas 2008, 145, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Duan, Z.; Qu, L.; Zheng, J.; Yang, N.; Xu, G. Expression analysis for candidate genes associated with eggshell me-chanical property. J. Integr. Agric. 2016, 15, 397–402. [Google Scholar] [CrossRef]
- Matulova, M.; Varmuzova, K.; Sisak, F.; Havlickova, H.; Babak, V.; Stejskal, K.; Zdrahal, Z.; Rychlik, I. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet. Res. 2013, 44, 37. [Google Scholar] [CrossRef] [PubMed]
- Gautron, J.; Nys, Y. Function of eggshell matrix proteins. In Bioactive Egg Compounds; Huopalahti, R., Lopez-Fandino, R., Anton, M., Schade, R., Eds.; Springer: Berlin, Germany, 2007; pp. 109–115. ISBN 978-3-540-37883-9. [Google Scholar]
- Dunn, I.C.; Wilson, P.W.; Lu, Z.; Bain, M.M.; Crossan, C.L.; Talbot, R.T.; Waddington, D. New hypotheses on the function of the avian shell gland derived from microarray analysis comparing tissue from juvenile and sexually mature hens. Gen. Comp. Endocrinol. 2009, 163, 225–232. [Google Scholar] [CrossRef]
- Chung, G.; Lo, K. RARRES1 (retinoic acid receptor responder (tazarotene induced) 1). Atlas Genet. Cytogenet. Oncol. Haematol. 2011, 11, 121–123. [Google Scholar] [CrossRef]
- RARRES1 Retinoic Acid Receptor Responder 1 Gallus Gallus (Chicken). Gene ID: 395209, Updated on 24-Jun-2020. National Center for Biotechnology Information, U.S. National Library of Medicine: Bethesda, MD, USA. Available online: https://www.ncbi.nlm.nih.gov/gene/395209 (accessed on 28 November 2020).
- Yang, K.-T.; Lin, C.-Y.; Liou, J.-S.; Fan, Y.-H.; Chiou, S.-H.; Huang, C.-W.; Wu, C.-P.; Lin, E.-C.; Chen, C.-F.; Lee, Y.-P.; et al. Differentially expressed transcripts in shell glands from low and high egg production strains of chickens using cDNA microarrays. Anim. Reprod. Sci. 2007, 101, 113–124. [Google Scholar] [CrossRef]
- Dunn, I.C.; Joseph, N.T.; Bain, M.; Edmond, A.; Wilson, P.W.; Milona, P.; Nys, Y.; Gautron, J.; Schmutz, M.; Preisinger, R.; et al. Polymorphisms in eggshell organic matrix genes are associated with eggshell quality measurements in pedigree Rhode Island Red hens. Anim. Genet. 2009, 40, 110–114. [Google Scholar] [CrossRef]
- Chiang, S.-C.; Veldhuizen, E.J.; Barnes, F.A.; Craven, C.J.; Haagsman, H.P.; Bingle, C.D. Identification and characterisation of the BPI/LBP/PLUNC-like gene repertoire in chickens reveals the absence of a LBP gene. Dev. Comp. Immunol. 2011, 35, 285–295. [Google Scholar] [CrossRef]
- Fudge, A.M. Laboratory Medicine: Avian and Exotic Pets, 1st ed.; W. B. Saunders: Philadelphia, PA, USA, 2000; 486p, ISBN 0-7216-7679-0. [Google Scholar]
- Doubek, J.; Bouda, J.; Doubek, M.; Fürll, M.; Knotková, Z.; Pejřilová, S.; Pravda, D.; Scheer, P.; Svobodová, Z.; Vodička, R. Veterinární Hematologie, 1st ed.; Noviko A.S.: Brno, Czech Republic, 2003; 464 s; ISBN 80-86542-02-5. [Google Scholar]
- Meyer, D.J.; Coles, E.H.; Rich, L.J. Medicina de Laboratório Veterinária: Interpretação e Diagnóstico; Roca: Sao Paulo, Brazil, 1995; 308p, ISBN 857241133X. [Google Scholar]
- Suzuki, S. Pathogenicity of Salmonella enteritidis in poultry. Int. J. Food Microbiol. 1994, 21, 89–105. [Google Scholar] [CrossRef]
- Itoh, N.; Kikuchi, N.; Hiramune, T. Biochemical changes in fowl serum during infection with Salmonella Typhimurium. J. Veter. Med. Sci. 1996, 58, 1021–1023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ognik, K.; Cholewińska, E.; Czech, A.; Kozłowski, K.; Wlazło, Ł.; Nowakowicz-Dębek, B.; Szlązak, R.; Tutaj, K. Effect of silver nanoparticles on the immune, redox, and lipid status of chicken blood. Czech J. Anim. Sci. 2016, 61, 450–461. [Google Scholar] [CrossRef]
- Lust, G.; Squibb, R.L. Alkaline phosphatase changes in chicken tissues during Newcastle disease virus infection. Appl. Microbiol. 1967, 15, 677. [Google Scholar] [CrossRef]
- Rivetz, B.; Bogin, E.; Hornstein, K.; Merdinger, M. Biochemical changes in chicken serum during infection with strains of Newcastle disease virus of differing virulence. I. Enzyme study. Avian Pathol. 1975, 4, 189–197. [Google Scholar] [CrossRef][Green Version]
- Groves, P.J.; Sharpe, S.M.; Cox, J.M. Response of layer and broiler strain chickens to parenteral administration of a live Sal-monella Typhimurium vaccine. Poult. Sci. 2015, 94, 1512–1520. [Google Scholar] [CrossRef]
- Sotirov, L.; Koinarski, V. Lysozyme and complement activity in broiler-chickens with coccidiosis. Rev. Med. Vet. 2003, 154, 780–784. [Google Scholar]
- Koinarski, V.; Sotirov, L. Lisozyme and complement activities in broiler-chickens with coccidiosis. II. Experiment with E. acervulina. Rev. Med. Vet. 2005, 156, 199–201. [Google Scholar]
- Koinarski, V.; Sotirov, L.K.; Denev, S. Serum lysozyme concentrations in broilers treated with Sel-Plex® and sodium selenite and infected with Eimeria tenella. Turk. J. Vet. Anim. Sci. 2012, 36, 433–437. [Google Scholar] [CrossRef]
- Valtchev, I.; Koynarski, T.; Sotirov, L.; Nikolov, Y.; Petkov, P. Effect of aflatoxin B1 on Moulard duck’s natural immunity. Pak. Vet. J. 2015, 35, 67–70. [Google Scholar]
- Videnska, P.; Sisak, F.; Havlickova, H.; Faldynova, M.; Rychlik, I. Influence of Salmonella enterica serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Veter. Res. 2013, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Thiennimitr, P.; Winter, S.E.; Bäumler, A.J. Salmonella, the host and its microbiota. Curr. Opin. Microbiol. 2012, 15, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Juricova, H.; Videnska, P.; Lukac, M.; Faldynova, M.; Babak, V.; Havlickova, H.; Sisak, F.; Rychlik, I. Influence of Salmonella enterica serovar Enteritidis infection on the development of the cecum microbiota in newly hatched chicks. Appl. Environ. Microbiol. 2012, 79, 745–747. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Cheng, G.; Liu, C.; Ahmed, S.; Shabbir, M.A.B.; Hussain, H.I.; Dai, M.; Yuan, Z. Microbial shifts in the intestinal microbiota of Salmonella infected chickens in response to enrofloxacin. Front. Microbiol. 2017, 8, 1711. [Google Scholar] [CrossRef]
- Timoshko, M.A. The Microflora of the Digestive Tract of Farm Animals; Shtiintsa: Chisinau, Moldova, 1990. [Google Scholar]
- Fasina, Y.O.; Newman, M.M.; Stough, J.M.; Liles, M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016, 95, 247–260. [Google Scholar] [CrossRef]
- Tarakanov, B.V. Methods for Studying the Microflora of the Digestive Tract of Farm Animals and Poultry; Nauchnyi mir: Moscow, Russia, 2006; 188p, ISBN 5-89176-386-9. [Google Scholar]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2013, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W. Chicken gut microbiota: Importance and detection technology. Front. Veter. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, K.L.; Wood, D.L.A.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.O.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S. Bacteroides. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Eds.; Academic Press: London, UK, 2015; Volume 2, pp. 917–944. ISBN 978-0-12-397169-2. [Google Scholar]
- Potempa, M.; Potempa, J.; Kantyka, T.; Nguyen, K.A.; Wawrzonek, K.; Manandhar, S.P.; Popadiak, K.; Riesbeck, K.; Eick, S.; Blom, A.M. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009, 5, e1000316. [Google Scholar] [CrossRef]
- Gibson, G.; Wang, X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 1994, 77, 412–420. [Google Scholar] [CrossRef]
- Svetoch, E.A.; Eruslanov, B.V.; Levchuk, V.P.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Stepanshin, J.; Dyatlov, I.; Seal, B.S.; Stern, N.J. Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl. Environ. Microbiol. 2011, 77, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A., Jr.; Corrier, D.; de Loach, J. In vitro inhibition of Salmonella typhimurium and Escherichia coli 0157: H7 by an an-aerobic gram-positive coccus isolated from the cecal contents of adult chickens. J. Food Prot. 1992, 55, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Murry, A., Jr.; Hinton, A., Jr.; Morrison, H. Inhibition of growth of Escherichia coli, Salmonella typhimurium, and Clostridia perfringens on chicken feed media by Lactobacillus salivarius and Lactobacillus plantarum. Int. J. Poult. Sci. 2004, 3, 603–607. [Google Scholar] [CrossRef]
- Stern, N.J.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Pokhilenko, V.D.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 2006, 50, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- García-Amado, M.A.; Michelangeli, F.; Gueneau, P.; Perez, M.E.; Domínguez-Bello, M.G. Bacterial detoxification of saponins in the crop of the avian foregut fermenter Opisthocomus hoazin. J. Anim. Feed. Sci. 2007, 16, 82–85. [Google Scholar] [CrossRef]
- Miller, T.L. Ecology of methane production and hydrogen sinks in the rumen. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Engelhardt, W.V., Leonhard-Marek, S., Breves, G., Giesecke, D., Eds.; Ferdinand Enke: Stuttgart, Germany, 1995; pp. 317–331. ISBN 3-432-26851-3. [Google Scholar]
- Rouvière, P.E.; Wolfe, R.S. Novel biochemistry of methanogenesis. J. Biol. Chem. 1988, 263, 7913–7916. [Google Scholar] [CrossRef]
- Bie, X.; Meale, S.J.; Valle, E.; Guan, L.L.; Zhou, M.; Kelly, W.J.; Henderson, G.; Attwood, G.T.; Janssen, P.H. RUMINANT NUTRITION SYMPOSIUM: Use of genomics and transcriptomics to identify strategies to lower ruminal methanogenesis. J. Anim. Sci. 2015, 93, 1431–1449. [Google Scholar] [CrossRef]
- An HSUS Report: The Impact of Animal Agriculture on Global Warming and Climate Change. The Humane Society of the United States, Animal Studies Repository. Available online: https://www.wellbeingintlstudiesrepository.org/hsus_reps_environment_and_human_health/3/ (accessed on 28 November 2020).
- Russell, J.B. Rumen Microbiology and Its Role in Ruminant Nutrition; James B. Russell Publishing: Ithaca, NY, USA, 2002. Available online: https://www.ars.usda.gov/research/software/download/?softwareid=409 (accessed on 28 November 2020).
- Lan, P.T.N.; Sakamoto, M.; Sakata, S.; Benno, Y. Bacteroides barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. Int. J. Syst. Evol. Microbiol. 2006, 56, 2853–2859. [Google Scholar] [CrossRef]
- Carron, M.; Chang, Y.-M.; Momanyi, K.; Akoko, J.; Kiiru, J.; Bettridge, J.; Chaloner, G.; Rushton, J.; O’Brien, S.; Williams, N.; et al. Campylobacter, a zoonotic pathogen of global importance: Prevalence and risk factors in the fast-evolving chicken meat system of Nairobi, Kenya. PLoS Negl. Trop. Dis. 2018, 12, e0006658. [Google Scholar] [CrossRef]
- Hird, S.M.; Sánchez, C.; Carstens, B.C.; Brumfield, R.T. Comparative gut microbiota of 59 Neotropical bird species. Front. Microbiol. 2015, 6, 1403. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Kuehbacher, T.; Rehman, A.; Lepage, P.; Hellmig, S.; Fölsch, U.R.; Schreiber, S.; Ott, S.J. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J. Med. Microbiol. 2008, 57, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.C.S.; Ozawa, S.; Hasegawa, K. Composition of the cockroach gut microbiome in the presence of parasitic nematodes. Microbes Environ. 2016, 31, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Cho, E.S.R.; Bang, H.-T.; Shim, K.S. Effects of oxygenated or hydrogenated water on growth performance, blood parameters, and antioxidant enzyme activity of broiler chickens. Poult. Sci. 2016, 95, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Musa, H.H.; Chen, G.H.; Cheng, J.H.; Yousif, G.M. Relation between abdominal fat and serum cholesterol, triglycerides, and lipoprotein concentrations in chicken breeds. Turk. J. Vet. Anim. Sci. 2007, 31, 375–379. [Google Scholar]


| Subgroups | OTUs | Shannon Index | ||
|---|---|---|---|---|
| 1 dpi 1 | 7 dpi 1 | 1 dpi | 7 dpi | |
| S-I | 601.7 ± 44.8 a | 385.7 ± 56.6 a | 6.6 ± 0.02 a | 6.4 ± 0.29 a |
| S-II | 610.7 ± 35.8 a | 428.3 ± 49.1 a | 6.1 ± 0.39 a,b | 6.5 ± 0.06 a |
| S-III | 570.0 ± 25.2 a | 390.7 ± 36.3 a | 6.3 ± 0.13 a,b | 6.3 ± 0.22 a |
| S-IV | 521.3 ± 19.7 a | 435.3 ± 16.2 a | 6.1 ± 0.07 b | 6.4 ± 0.21 a |
| Subgroups 1 | Egg Weight, g | No. of Laid Eggs | Egg Mass, g |
|---|---|---|---|
| S-I | 64.94 ± 1.91 a | 8.44 ± 1.76 a | 547.54 ± 111.03 a |
| S-II | 64.29 ± 2.43 a,c | 7.48 ± 2.10 a,b | 479.19 ± 134.37 b |
| S-III | 66.10 ± 2.19 b,c | 8.56 ± 1.40 a,c | 564.81 ± 91.2 a,c |
| S-IV | 66.33 ± 1.71 b | 7.52 ± 2.10 a,b | 498.86 ± 141.17 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laptev, G.Y.; Yildirim, E.A.; Ilina, L.A.; Filippova, V.A.; Kochish, I.I.; Gorfunkel, E.P.; Dubrovin, A.V.; Brazhnik, E.A.; Narushin, V.G.; Novikova, N.I.; et al. Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens. Animals 2021, 11, 360. https://doi.org/10.3390/ani11020360
Laptev GY, Yildirim EA, Ilina LA, Filippova VA, Kochish II, Gorfunkel EP, Dubrovin AV, Brazhnik EA, Narushin VG, Novikova NI, et al. Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens. Animals. 2021; 11(2):360. https://doi.org/10.3390/ani11020360
Chicago/Turabian StyleLaptev, Georgi Yu., Elena A. Yildirim, Larisa A. Ilina, Valentina A. Filippova, Ivan I. Kochish, Elena P. Gorfunkel, Andrei V. Dubrovin, Evgeni A. Brazhnik, Valeriy G. Narushin, Natalia I. Novikova, and et al. 2021. "Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens" Animals 11, no. 2: 360. https://doi.org/10.3390/ani11020360
APA StyleLaptev, G. Y., Yildirim, E. A., Ilina, L. A., Filippova, V. A., Kochish, I. I., Gorfunkel, E. P., Dubrovin, A. V., Brazhnik, E. A., Narushin, V. G., Novikova, N. I., Novikova, O. B., Dunyashev, T. P., Smolensky, V. I., Surai, P. F., Griffin, D. K., & Romanov, M. N. (2021). Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens. Animals, 11(2), 360. https://doi.org/10.3390/ani11020360












