Simple Summary
Weaning is a vital process for weaned pigs since piglets are exposed to psychologic and environmental stresses. These stresses converge on the pig to cause low feed consumption and weight gain meanwhile increased risk of diarrhea and mortality during the early postweaning period. The use of antibiotic growth promoters to help prevent weaning stress in weaned pigs has been forbidden in the European Union, Korea, Japan and China. Pyrroloquinoline quinone disodium (PQQ·Na2) is increasing interest in use of alternatives to in-feed antibiotics. In this study, we found PQQ·Na2 can improve growth performance meanwhile improves antioxidant status of weaned pigs. A high oral dose of PQQ·Na2 does not appear to have harmful effects on weaned pigs.
Abstract
The research was implemented to assess the safety of feeding excess of pyrroloquinoline quinone disodium (PQQ·Na2) to 108 Duroc × Landrace × Large White weaned pigs (BW = 8.38 ± 0.47 kg). Pigs were weaned at 28 d and randomly distributed to one of three diets with six replicates and six pigs per replicate (three males and three females). Pigs in the control group were fed a corn-soybean meal-based diet (without growth promoter) while the two experimental diets were supplied with 7.5 and 75.0 mg/kg PQQ·Na2, respectively. Average daily gain (ADG), average daily feed intake (ADFI), feed conversion (F:G), diarrhea incidence, hematology, serum biochemistry, organ index and general health were determined. Diets supplementation with 7.5 mg/kg PQQ·Na2 in weaned pigs could increase ADG during the entire experimental period (p < 0.05). And there was a tendency to decrease F:G (p = 0.063). The F:G of weaned pigs fed 7.5 and 75.0 mg/kg PQQ·Na2 supplemented diets was decreased by 9.83% and 8.67%, respectively, compared to the control group. Moreover, pigs had reduced diarrhea incidence (p < 0.01) when supplemented with PQQ·Na2. No differences were observed between pigs supplemented with 0.0, 7.5 and 75.0 mg/kg PQQ·Na2 diets on hematological and serum biochemical parameters as well as histological assessment of heart, liver, spleen, lung and kidney. At day 14, pigs had increased activity of glutathione peroxidase (GSH-Px) (p < 0.05), catalase (CAT) (p < 0.05) and total antioxidant capacity (T-AOC) (p < 0.05), and the serum concentration of malondialdehyde (MDA) was decreased (p < 0.01) with PQQ·Na2 supplementation. At day 28, pigs had increased activities of total superoxide dismutase (T-SOD) (p < 0.01), GSH-Px (p < 0.01), CAT (p < 0.05) and T-AOC (p < 0.01), and serum concentration of MDA was lower (p < 0.01) with PQQ·Na2 supplementation. In conclusion, PQQ·Na2 can improve weaned pigs growth performance and serum antioxidant status. Meanwhile high PQQ·Na2 inclusion of 75.0 mg/kg does not appear to result in harmful effects on growth performance of pigs.
1. Introduction
Pyrroloquinoline quinone (PQQ) was initially isolated from a microorganism and is a quinone compound like a coenzyme for methanol dehydrogenase [1,2,3]. PQQ has been found in both plant and animal tissues [4], and can be reversibly transformed into pyrroloquinoline quinol (PQQH2) via a semiquinone intermediate [5]. The ability of PQQH2 to eliminate free radicals is 7.4-fold higher than vitamin C [6], thus PQQ can reduce oxidative stress in organisms. PQQ plays an important mission in a lot of physiological and biological functions [7]. In particular, its role in growth promoting, anti-diabetic effects, anti-oxidative action and neuroprotection is widely concerned [8]. PQQ in mouse and rat models, can improve performance of growth, enhance reproductive and immunity, protect nerves and preserve myocardia function [9,10,11]. Supplementation with 0.2 mg/kg PQQ·Na2 for broiler chicks improved growth performance, dressing percentage, and immunity and plasma status [12,13]. In addition, diet supplementation with 0.1 mg/kg PQQ·Na2 can regulate meat quality and antioxidant ability in broilers [14].
The safety of PQQ has been extensively researched. In rats, the no-observed-adverse-effect-level (NOAEL) of PQQ·Na2 is 400 mg /kg bw/day for both sexes [15]. The median lethal dose of PQQ in rats was shown to be 500–1000 mg/kg body weight in female and 1000–2000 mg/kg body weight in male [16]. A research in human lymphocytes and mice proved that no genotoxic of PQQ was observed in vivo or in vitro [17]. In the United States, a maximum dose of PQQ for healthy adults is 50 mg/day [16].
Our previous study shown dietary supplementation PQQ·Na2 improved weaned pigs growth performance and decreased incidence of diarrhea by improving intestinal development [18]. And PQQ·Na2 can enhance weaned pigs’ immunity by inhibiting the NF-κB pathway meanwhile correcting intestinal microbiota maladjustment [19]. A study shown that PQQ·Na2 can protect weaned pigs’ intestines via modulating NQO1 related genes [20]. Gestating and lactating sows’ diets supplementation with 20 mg/kg PQQ·Na2 can improve their reproductive performance [21].
In pigs, little is known about the effects of PQQ overdoses on their growth and health. Therefore, our objective was to determine the influence of PQQ·Na2 on growth, blood parameters, organ indexes and redox status of weaned pigs. And the hypothesis is that excess PQQ·Na2 will not appear to have harmful effects on weaned pigs.
2. Materials and Methods
The protocol employed in this trial was approved by the China Agricultural University Animal Care and Use Committee (Beijing, China). (China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Form No. AW3004020.)
2.1. Pigs and Experimental Protocol
A total of 108 Duroc × Landrace × Large White weaned pigs (28 ± 2 days of age; BW = 8.38 ± 0.47 kg), were allocated to one of three diets in a randomized complete block design with six replicates and six pigs per replicate (three barrows and three gilts). Pigs in the control group were fed a corn-soybean meal-based diet (without growth promoter) while pigs assigned to the two experimental treatment received the control diet supplemented with 7.5 or 75.0 mg/kg PQQ·Na2, respectively (Table 1). The corn-soybean meal-based diet was formulated to exceed National Research Council (2012) recommendations for weaned pigs [22]. The PQQ·Na2 (purity, ≥ 98%) was chemically synthesized and purchased from Changmao Biochemical Engineering Company (Changzhou, China). PQQ·Na2 was premixed with corn starch to a concentration of 1 g/kg mixture before inclusion in the diet.

Table 1.
Composition and analyzed nutrient composition of experimental diets (as-fed basis).
Pigs were housed in totally slatted floor pens (1.2 × 2.0 m2) containing a nipple drinker and stainless-steel feeder for ad libitum access to water and feed over the 28d trial. Ambient room temperature was maintained at 29 °C for the first week, and lowered by 1 °C each week thereafter. Diarrhea scores were recorded daily for all pigs from d 1 to 28 by the same person and were based on the following scale: 1 = well-formed feces, 2 = sloppy feces, 3 = diarrhea [23]. Pigs with a score of 3 were considered to have diarrhea. The incidence of diarrhea for weaned pigs in each pen was calculated as [(number of weaned pigs with diarrhea × number of days of diarrhea) / (total number of weaned pigs × number of days of experiment)] × 100 [24]. Body weight were measured on day 0, 14 and 28 and feed disappearance on these days were recorded. ADG, ADFI, F:G were calculated based on weight and feed measurements.
2.2. Sample Collection and Processing
On day 14 and d 28, after an overnight fast, 18 pigs (1 pig per pen) were selected for blood sampling according to body weight closest to the mean value within the pen. Blood samples were collected from the jugular vein into a vacuum tube containing EDTAK2 (ethylenediaminetetraacetic acid disodium salt) and no anticoagulant for whole blood and serum, respectively. Whole blood was assayed for hematological parameters within 1 h after sampling. Serum were separated by centrifugation for 10 min at 3000× g and 4 °C, and stored at −20°C until analysis.
On day 28, 18 weaned pigs (1 pig per pen) were weighed, electrically stunned and immediately euthanized by exsanguination. Heart, liver, spleen, lung and kidney were harvested and weighed. Relative weights of heart, liver, spleen, lung and kidney were expressed as a ratio to whole body mass (g/kg). After weighed, immediately take tissue samples fixed in 4% formalin buffer solution.
2.3. Hematological and Serum Biochemical Parameters Analysis
Hematological parameters including red blood cells (RBC), white blood cells (WBC), hematocrit (HCT), hemoglobin (HGB), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT) and red blood cell distribution width-coefficient of variance (RDW-CV) were determined using a Sysmex Microcell Counter CL-180 (Tokyo, Japan).
Serum biochemical parameters including glucose (GLU), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin (ALB), total bilirubin (TBILI), urea nitrogen (UN), total protein (TP) and creatinine (CRE) were measured by corresponding commercial kits (BioSino Bio-technology and Science Incorporated, Beijing, China) using an Automatic Biochemical Analyzer (Hitachi 7160, Hitachi High Technologies Corporation, Tokyo, Japan).
2.4. Histopathology Analysis
Tissue samples from the heart, liver, spleen, lung and kidney were fixed in 4% formalin buffer solution for 48 h, cleaning and embedded in paraffin. Paraffin sections of 5μm thickness were stained with hematoxylin and eosin.
2.5. Antioxidative Physiological Analyses
The T-SOD, GSH-Px, CAT, T-AOC and MDA activities in serum were determined using assay kits in strictly according to the manufacturer instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Statistical Analysis
All experimental data except for diarrhea incidence were analyzed using the GLM Procedure of SAS as a randomized complete block design (SAS Inst. Inc., Cary, NC, USA). Differences in diarrhea incidence among treatments were tested by a Chi-square test. Pen was the experimental unit for the performance traits, and individual pig was the experimental unit for all other traits studied. Differences among means were evaluated by the Student-Newman-Keuls test. And all data except the diarrhea incidence of the experiment followed a normal distribution. Treatment effects were considered to be significant if p < 0.05.
3. Results
3.1. Performance and Diarrhea Incidence
The effects of PQQ·Na2 supplementation on ADG, ADFI and F:G are presented in Table 2. During the entire experimental period, weaned pigs fed 7.5 mg/kg PQQ·Na2 supplemented diets displayed increased ADG (p < 0.05) compared to control. No difference was noted in ADFI among the different groups (p > 0.05). And there was a tendency to decrease F:G (p = 0.063). Overall, weaned pigs fed 7.5 and 75.0 mg/kg PQQ·Na2 supplemented diets was decreased by 9.83% and 8.67%, respectively, compared to the control group but these differences were not statistically significant.

Table 2.
Performance of weaned pigs fed graded levels of PQQ·Na2 1 for 28 day 2.
Diarrhea incidence was exhibited in Table 3, weaned pigs fed diets supplemented with 7.5 or 75.0 mg/kg PQQ·Na2 had decreased diarrhea incidence (p < 0.01) than weaned pigs fed diets non-supplemented with PQQ·Na2 during days 0 to 14, days 15 to 28 and the entire experimental period.

Table 3.
Incidence of diarrhea in weaned pigs fed graded levels of PQQ·Na2 1 for 28 day 2.
3.2. Hematological and Serum Biochemical
All hematologic and serum biochemical parameters were present in Table 4 and Table 5. Normal hematologic and serum biochemical parameters reference values is from Merck Veterinary Manual (Merck, 2010). There were no significant differences observed in hematologic and serum biochemical parameters that weaned pigs supplemented with 0.0, 7.5 or 75.0 mg/kg PQQ·Na2.

Table 4.
Effects of graded levels of PQQ·Na2 1 on hematological parameters of weaned piglets 2.

Table 5.
Effects of graded levels of PQQ·Na2 1 on serum biochemical parameters of weaned piglets 2.
3.3. Organ Index and Histological Structures
Effects of graded levels of PQQ·Na2 on organ index in weaned pigs are presented in Table 6. Histological structures of heart, liver, spleen, lung and kidney were observed in pigs fed different graded levels of PQQ·Na2 diets for 28 days are presented in Figure 1.

Table 6.
Effects of graded levels of PQQ·Na21 on organ index of weaned piglets (g/kg BW) 2.
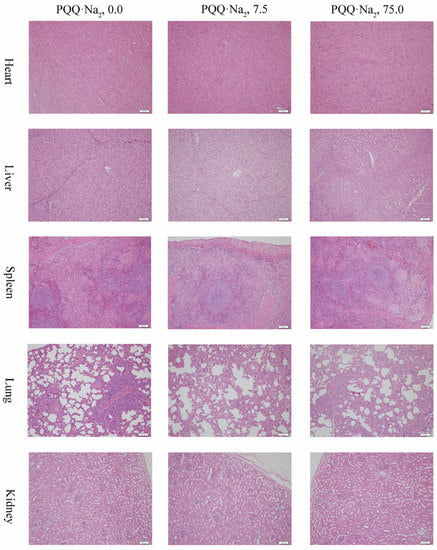
Figure 1.
Effects of graded levels of PQQ·Na2 on heart, liver, spleen, lung and kidney morphology in weaned piglets. Hematoxylin-eosin staining, scale bars = 100 μm.
When weaned pigs fed diets supplemented with 0.0 (control), 7.5 (low-dose) or 75.0 (high-dose) mg/kg PQQ·Na2, normal histological structures in the heart, liver, spleen and kidney were observed. The lungs of all experimental animals showed interstitial pneumonia characterized by massive infiltration of lymphoid cells.
3.4. Redox Status
Data for antioxidative enzymes in serum are presented in Table 7. At day 14, pigs fed diets supplemented with 7.5 mg/kg PQQ·Na2 had increased activity of CAT (p < 0.05) and T-AOC (p < 0.05), and the serum concentration of MDA (p < 0.01) was decreased compared with control. And pigs fed diets supplemented with 75.0 mg/kg PQQ·Na2 had increased activity of GSH-Px (p < 0.05) and T-AOC (p < 0.05) compared with control. At d 28, pigs fed diets supplemented with 7.5 mg/kg PQQ·Na2 had increased activities of T-SOD (p < 0.01), GSH-Px (p < 0.01) and CAT (p < 0.05), and serum concentration of MDA (p < 0.01) was lower compared with pigs fed no PQQ·Na2. Pigs fed diets supplemented with 75.0 mg/kg PQQ·Na2 had increased activities of T-SOD (p < 0.01), GSH-Px (p < 0.01) and T-AOC (p < 0.01), and serum concentration of MDA (p < 0.01) was lower compared with control.

Table 7.
Antioxidant indexes in serum of weaned pigs fed graded levels of PQQ·Na2 1 for 28 d 2.
4. Discussion
Weaning is a necessary step in pig production. During weaning, piglets are subjected to a variety of psychological and environmental stresses. Among these stressors, the weaning diet is plant-based, less digestible and contains antinutritional factors. Furthermore, the pig is accustomed to a liquid diet before weaning and is abruptly changed to a solid diet after weaning. These stresses converge on the pig to cause low feed consumption and weight gain meanwhile increased risk of diarrhea and mortality during the early postweaning period. The use of antibiotic growth promoters to help prevent weaning stress in weaned pigs has been forbidden in the European Union, Korea, and Japan [25]. And in China the use of antibiotic growth promoters also has been forbidden in 2020 [26]. Consequently, here is increasing interest in use of alternatives to in-feed antibiotics. It is reported that, PQQ·Na2 improved growth performance and decreased diarrhea by enhancing the intestinal morphology and improving the redox status in weaned pigs [18]. And whether PQQ·Na2 can be used as a substitute for in-feed antibiotics, we need to consider adding the positive control (with standard animal growth promoter added) in our future research.
In 2009, PQQ·Na2 was commercialized in the United States after approval through the Food and Drug Administration. The safety of PQQ has been wide researched. A research found that the oral median lethal dose of PQQ·Na2 for male and female rats are 1000–2000 mg/kg body weight and 500–1000 mg/kg body weight, respectively. And the NOAEL of PQQ·Na2 is 100 mg /kg bw/d for both sexes [16]. Thereafter, another research indicates that the NOAEL of PQQ·Na2 is 400 mg /kg bw/d for both sexual rats [15]. The neuroprotective effect of PQQ has also attracted wide attention. In order to treat human psychological and neurological disorders with PQQ, research found that the NOAEL of PQQ intracephalic injection to mice cortical neurons is 1 μM [27]. A research demonstrated that PQQ·Na2 does not have genotoxic potential in vivo [17]. Other studies report that the pharmacokinetic behavior of PQQ Na2 is similar to other water-soluble B vitamin compounds. This suggests that PQQ will not accumulate in the body to produce serious damage [28]. However, intraperitoneal injection of PQQ Na2 at a dose of 11.5 mg/kg body weight for 4 consecutive days, morphologic and functional changes of the kidney were observed [29]. Future more, PQQ can against CTX-induced nephrotoxicity [30]. From these studies, oral administration of PQQ appears to have minimal negative effects, whereas it is necessary to monitor for super dosage.
The hematologic and serum biochemical profiles correlated with pigs’ health. And supplementation with 7.5 and 75.0 mg/kg PQQ·Na2 have no significant differences compared with control group in hematologic and serum biochemical parameters. Histological structures in the lung have a certain proportion of lesion when pigs fed the different levels of PQQ·Na2 for 28 days. Probably during the experiment, pigs were generally infected with pathogenic microorganisms, the proportion in control group, low-dose group and high-dose group were similar. And supplementation with 7.5 and 75.0 mg/kg PQQ·Na2 can obviously improve the structure of liver and promote the development of splenic white medulla lymphatic follicles. Our results obtained from the current research reflected that weaned pigs take excess PQQ·Na2 will not lead to harmful influence to health.
A study proved that weaning will cause oxidative stress injury which plays an important role in free radical metabolism [31]. Free radicals are molecules produced during aging, tissue damage and oxidative stress, if not removed by antioxidants, they may be toxic to cellular components which could induce diarrhea [32,33]. Superoxide dismutase (SOD) provides competent dismutation of O2− and converts it to hydrogen peroxide, which is removed by catalase and GSH-Px [34]. Therefore, increases in GSH-Px, CAT and SOD enzyme levels may be suggesting alleviation of oxidative stress. Some researchers have proved that reduced forms of PQQ (PQQH2) have obvious antioxidant ability [35,36,37,38]. The ability of PQQH2 to eliminate free-radicals was 7.4-fold higher than vitamin C, which is known as the most active water-soluble antioxidant [6]. The T-AOC is an oxidative stress and antioxidant defense biomarker that forms the first step in search for a healthy organism status [39]. In this experiment, we examined the levels of T-SOD, GSH-Px, CAT and T-AOC in serum, and determined that PQQ·Na2 supplementation increased activity of antioxidant enzymes and T-AOC, which suggests alleviation of oxidant stress may have occurred. MDA is a naturally occurring product of lipid peroxidation and prostaglandin biosynthesis [40] and is an indicator of lipid peroxidation. Supplementation of PQQ·Na2 reduced serum concentration of MDA which suggests lower levels of oxidative stress in PQQ·Na2-fed pigs.
In the present study, whether higher dose of PQQ·Na2 supplementation in diet has a positive effect on piglets’ growth performance and health state has been researched. We found diets supplemented 7.5 mg/kg PQQ·Na2 in weaned pigs can improve ADG compared to control. And compared to the control group, supplemented with 7.5 mg/kg PQQ·Na2 and a dosage 10 times higher (75.0 mg/kg PQQ·Na2) has a tendency to decrease F:G, but no impact on ADFI. However, in this research, we found diets supplemented with 7.5 and 75.0 mg/kg PQQ·Na2 in weaned pigs has no significant difference in weaned pigs growth performance. Similarly, the redox status of weaned pigs has no significant difference when diets supplemented with 7.5 and 75.0 mg/kg PQQ·Na2. Meanwhile, weaned pigs hematological and serum biochemical parameters, organ index and histological structures has no differences among the three treatment.
5. Conclusions
In conclusion, diets supplemented with 7.5 and 75.0 mg/kg PQQ·Na2 can improve growth performance meanwhile improves antioxidant status of weaned pigs. And there are no differences diets between supplemented with 7.5 and 75.0 mg/kg PQQ·Na2. A high oral dose of PQQ·Na2 (75.0 mg/kg PQQ·Na2) does not appear to have harmful effects on weaned pigs.
Author Contributions
D.M.: Conceptualization, investigation, methodology, data curation, formal analysis, writing—original draft. C.H.: methodology, data curation. W.W.: methodology, data curation. Z.W.: methodology, data curation. C.S.: methodology, data curation. X.Y.: methodology, data curation. L.S.: formal analysis, investigation. Y.G.: methodology, funding acquisition. F.W.: conceptualization, writing—review, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 31672459.
Institutional Review Board Statement
China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection, No. AW30040202-1.
Data Availability Statement
Some or all data, models, or code generated or used during the study are available in a repository or online in accordance with funder data retention policies (Provide full citations that include URLs or DOIs.).
Acknowledgments
The writers express their real thanks to R. P. She (China Agricultural University), D. M. Zhao (China Agricultural University), Lee. J. Johnston (University of Minnesota) and B. Dong (China Agricultural University) for giving proposals on the experiment design and revision. We also thank all employee of the Fengning Experimental Base (China Agricultural University, Beijing, China) for helpful technical assistance.
Conflicts of Interest
There are no benefits related to the developments of the products from “Shanghai Medical Life Science Research Center Company” and “Changmao Biochemical Engineering Company” as an outcome of the work in this manuscript. We confirm that there are no relevant financial or non-financial competing interests to report.
References
- Salisbury, S.A.; Forrest, H.S.; Cruse, W.B.T.; Kennard, O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nat. Cell Biol. 1979, 280, 843–844. [Google Scholar] [CrossRef]
- Anthony, C. Pyrroloquinoline Quinone (PQQ) and Quinoprotein Enzymes. Antioxidants Redox Signal. 2001, 3, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Umezawa, K.; Takeda, K.; Sugimoto, N.; Ishida, T.; Samejima, M.; Ohno, H.; Yoshida, M.; Igarashi, K.; Nakamura, N. Discovery of a Eukaryotic Pyrroloquinoline Quinone-Dependent Oxidoreductase Belonging to a New Auxiliary Activity Family in the Database of Carbohydrate-Active Enzymes. PLoS ONE 2014, 9, e104851. [Google Scholar] [CrossRef] [PubMed]
- Rucker, R.; Chowanadisai, W.; Nakano, M. Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. 2009, 14, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.A.; Martin, P.; Flückiger, R.; Mah, J.; Gallop, P.M. The Catalysis of Redox Cycling by Pyrroloquinoline Quinone (PQQ), PQQ Derivatives, and Isomers and the Specificity of Inhibitors. Anal. Biochem. 1996, 238, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, A.; Nakano, M.; Nagaoka, S.-I.; Mukai, K. Kinetic Study of the Antioxidant Activity of Pyrroloquinolinequinol (PQQH2, a Reduced Form of Pyrroloquinolinequinone) in Micellar Solution. J. Agric. Food Chem. 2009, 57, 450–456. [Google Scholar] [CrossRef]
- Misra, H.; Rajpurohit, Y.S.; Khairnar, N.P. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 2012, 37, 313–325. [Google Scholar] [CrossRef]
- Akagawa, M.; Nakano, M.; Ikemoto, K. Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci. Biotechnol. Biochem. 2016, 80, 13–22. [Google Scholar] [CrossRef]
- Steinberg, F.; Stites, T.E.; Anderson, P.; Storms, D.; Chan, I.; Eghbali, S.; Rucker, R. Pyrroloquinoline quinone im-proves growth and reproductive performance in mice fed chemically defined diets. Exp. Biol. Med. 2003, 228, 160–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Feustel, P.J.; Kimelberg, H.K. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006, 1094, 200–206. [Google Scholar] [CrossRef]
- Tao, R.; Karliner, J.S.; Simonis, U.; Zheng, J.; Zhang, J.; Honbo, N.; Alano, C.C. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2007, 363, 257–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samuel, K.; Zhang, H.; Wang, J.; Wu, S.; Yue, H.; Sun, L.; Qi, G. Effects of dietary pyrroloquinoline quinone disodium on growth performance, carcass yield and antioxidant status of broiler chicks. Animal 2015, 9, 409–416. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.J.; Samuel, K.G.; Long, C.; Wu, S.G.; Yue, H.Y.; Sun, L.L.; Qi, G.-H. Effects of dietary pyrroloquinoline quinone disodium on growth, carcass characteristics, redox status, and mitochondria metabolism in broilers. Poult. Sci. 2015, 94, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, G.; Liao, X.; Huang, J.; Guo, M.; Zhang, L.; Guo, Y.; Lu, L.; Luo, X. Effect of dietary supplementation of pyrroloquin-oline quinone disodium on growth performance, meat quality and antioxidative ability of broilers. J. Integr. Agric. 2020, 19, 1850–1856. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Wang, W.; Song, Y.; Jia, X. A subchronic oral toxicity study on pyrroloquinoline quinone (PQQ) disodium salt in rats. Food Chem. Toxicol. 2015, 75, 146–150. [Google Scholar] [CrossRef]
- Nakano, M.; Takahashi, H.; Koura, S.; Chung, C.; Tafazoli, S.; Roberts, A. Acute and subchronic toxicity studies of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) in rats. Regul. Toxicol. Pharmacol. 2014, 70, 107–121. [Google Scholar] [CrossRef]
- Nakano, M.; Suzuki, H.; Imamura, T.; Lau, A.; Lynch, B. Genotoxicity of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™). Regul. Toxicol. Pharmacol. 2013, 67, 189–197. [Google Scholar] [CrossRef]
- Yin, X.; Ming, D.; Bai, L.; Wu, F.; Liu, H.; Chen, Y.; Sun, L.; Wan, Y.; Thacker, P.A.; Wu, G.; et al. Effects of pyrroloquinoline quinone supplementation on growth performance and small intestine characteristics in weaned pigs1,2. J. Anim. Sci. 2019, 97, 246–256. [Google Scholar] [CrossRef]
- Huang, C.; Ming, D.; Wang, W.; Wang, Z.; Hu, Y.; Ma, X.; Wang, F. Pyrroloquinoline Quinone Alleviates Jejunal Mucosal Barrier Function Damage and Regulates Colonic Microbiota in Piglets Challenged with Enterotoxigenic Escherichia coli. Front. Microbiol. 2020, 11, 1754. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Cao, C.; Zhang, B.; Yang, W.; Shi, B.; Shan, A. Pyrroloquinoline quinone inhibits the production of inflammatory cytokines via the SIRT1/NF-κB signal pathway in weaned piglet jejunum. Food Funct. 2020, 11, 2137–2153. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, C.; Yang, W.; Zhang, H.; Meng, Q.; Shi, B.; Shan, A. Transcriptome analysis of the effect of pyrroloquinoline quinone disodium (PQQ·Na2) on reproductive performance in sows during gestation and lactation. J. Anim. Sci. Biotechnol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Marquardt, R. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol. Med Microbiol. 1999, 23, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Li, D.; Cao, Y.; Li, X.; Yin, J.; Qiao, S.; Wu, G. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. J. Nutr. Biochem. 2007, 18, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-L.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Cheng, Y.; Chen, Y.; Li, J.; Zhao, Y.; Zhou, Y. Effects of Dietary Zeolite Supplementation as an Antibiotic Alternative on Growth Performance, Intestinal Integrity, and Cecal Antibiotic Resistance Genes Abundances of Broilers. Animal 2019, 9, 909. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, D.; Mao, S.; Zhou, X. Neurotoxicity and apoptosis induced by pyrroloquinoline quinone and its ester deriva-tive on primary cortical neurons. Neurotoxicology 2020, 78, 47–56. [Google Scholar] [CrossRef]
- Harris, C.B.; Chowanadisai, W.; Mishchuk, D.O.; Satre, M.A.; Slupsky, C.M.; Rucker, R.B. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J. Nutr. Biochem. 2013, 24, 2076–2084. [Google Scholar] [CrossRef]
- Watanabe, A.; Hobara, N.; Ohsawa, T.; Higashi, T.; Tsuji, T. Nephrotoxicity of pyrroloquinoline quinone in rats. Hiroshima J. Med. Sci. 1989, 38, 49–51. [Google Scholar]
- Lin, X.; Yang, F.; Huang, J.; Jiang, S.; Tang, Y.; Li, J. Ameliorate effect of pyrroloquinoline quinone against cyclophospha-mide-induced nephrotoxicity by activating the Nrf2 pathway and inhibiting the NLRP3 pathway. Life Sci. 2020, 256, 117901. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Özcan, M.M. Inhibition of Aspergillus parasiticus NRRL 2999 by Pollen and Propolis Extracts. J. Med. Food 2004, 7, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants–quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant en-zymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Urakami, T.; Abeta, H.; Shi, H.; Noguchi, N.; Niki, E. Action of Pyrroloquinolinequinol As an Antioxidant Against Lipid Peroxidation in Solution. Antioxidants Redox Signal. 1999, 1, 547–554. [Google Scholar] [CrossRef]
- He, K.; Nukada, H.; Urakami, T.; Murphy, M.P. Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): Implications for its function in biological systems. Biochem. Pharmacol. 2003, 65, 67–74. [Google Scholar] [CrossRef]
- Ouchi, A.; Ikemoto, K.; Nakano, M.; Nagaoka, S.-I.; Mukai, K. Kinetic Study of Aroxyl Radical Scavenging and α-Tocopheroxyl Regeneration Rates of Pyrroloquinolinequinol (PQQH2, a Reduced Form of Pyrroloquinolinequinone) in Dimethyl Sulfoxide Solution: Finding of Synergistic Effect on the Reaction Rate due to the Coexistence of α-Tocopherol and PQQH2. J. Agric. Food Chem. 2013, 61, 11048–11060. [Google Scholar] [CrossRef]
- Mukai, K.; Ouchi, A.; Nakano, M. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Pyrroloquinolinequinol (PQQH2, a Reduced Form of Pyrroloquinolinequinone) in Micellar Solution. J. Agric. Food Chem. 2011, 59, 1705–1712. [Google Scholar] [CrossRef]
- Kusano, C.; Ferrari, B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Ellah, M.R.A.; Okada, K.; Goryo, M.; Yasuda, J. Levels of malondialdehyde and 4-hydroxyalkenals in bovine liver biopsy. Comp. Haematol. Int. 2012, 22, 823–827. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).