Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of “Shi Ying Zi” Powder
2.2. Oocyst
2.3. Experimental Design
2.4. Ethical Approval
2.5. Clinical Symptoms
2.6. Bloody Diarrheal Score
2.7. Lesion Score of the Cecum
2.8. Oocyst Counts
2.9. Anti-Coccidiosis Index
2.10. Biochemical Indexes
2.11. Histopathology
2.12. Statistical Analysis
3. Results
3.1. Clinical Symptoms and Mortality
3.2. Anticoccidial Efficacy in Chickens
3.3. Biochemical Indexes
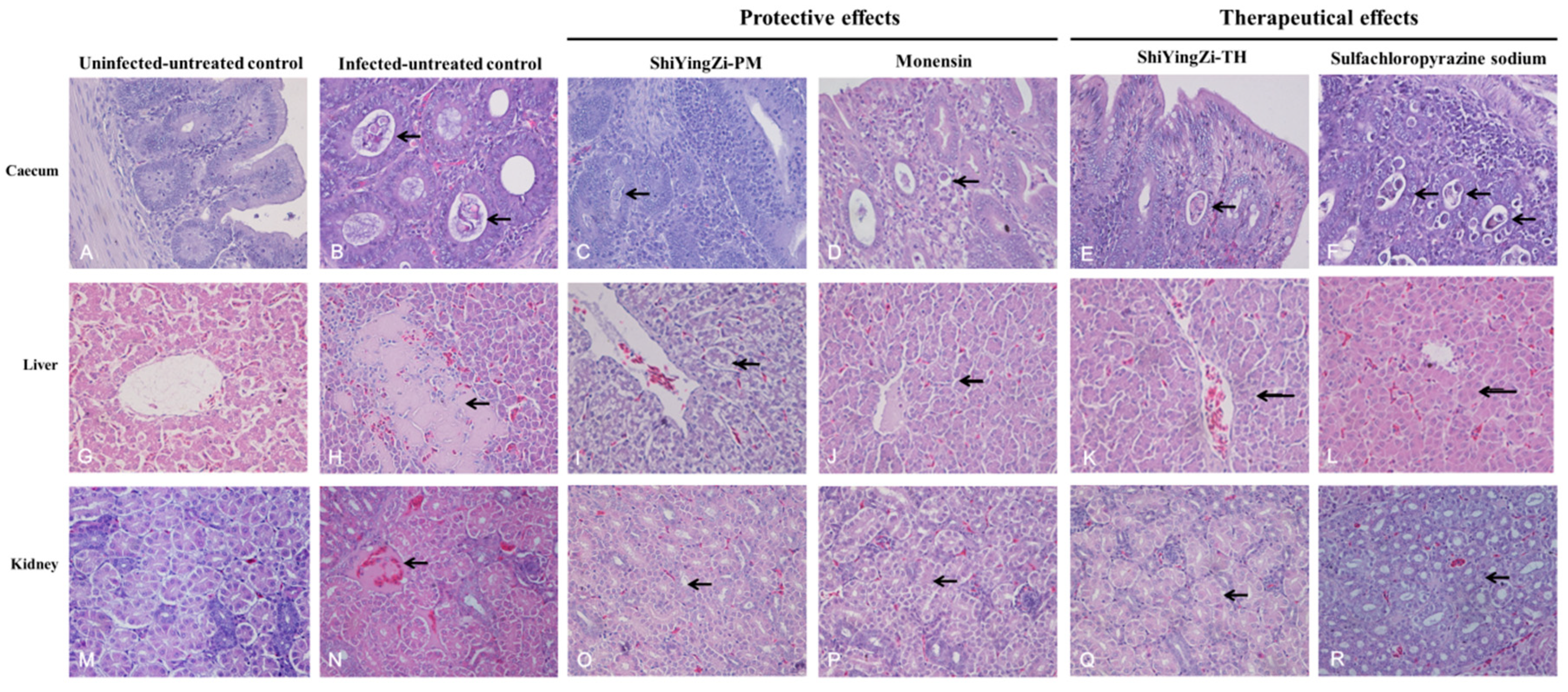
3.4. Histopathological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fatoba, A.; Adeleke, M.A. Diagnosis and control of chicken coccidiosis: A recent update. J. Parasit. Dis. 2018, 4, 483–493. [Google Scholar] [CrossRef]
- Morris, G.M.; Gasser, R.B. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol. Adv. 2006, 6, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.W.; Smith, A.L.; Blake, D.P. Challenges in the successful control of the avian coccidia. Vaccine 2007, 30, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Jarujareet, W.; Kobayashi, M.; Taira, K.; Ooi, H.K. The role of the American cockroach (Periplaneta americana) as transport host of Eimeria tenella to chickens. Parasitol. Res. 2019, 118, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.; Kang, S.S.; Kim, D.W.; Kim, S.H.; Lillehoj, H.S.; Min, W. Protective effects of Aloe vera-based diets in Eimeria maxima-infected broiler chickens. Exp. Parasitol. 2011, 1, 322–325. [Google Scholar] [CrossRef]
- Chen, T.; Huang, B.; Zhao, Q.; Dong, H.; Zhu, S.; Zhao, Z.; Lv, L.; Yan, M.; Han, H. Molecular characterization and functional analysis of Eimeria tenella malate dehydrogenase. Parasitol. Res. 2018, 117, 2053–2063. [Google Scholar] [CrossRef]
- Akhtar, M.; Hai, A.; Awais, M.M.; Iqbal, Z.; Muhammad, F.; ul Haq, A.; Anwar, M.I. Immunostimulatory and protective effects of Aloe vera against coccidiosis in industrial broiler chickens. Vet. Parasitol. 2012, 186, 170–177. [Google Scholar] [CrossRef]
- El-Abasy, M.; Motobu, M.; Na, K.J.; Shimura, K.; Nakamura, K.; Koge, K.; Onodera, T.; Hirota, Y. Protective Effects of Sugar Cane Extracts (SCE) on Eimeria tenella Infection in Chickens. J. Vet. Med. Sci. 2003, 65, 865–871. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Pegg, E.; Macdonald, S.E.; Tomley, F.M.; Blake, D.P.; Marugán-Hernández, V. Laboratory Growth and Genetic Manipulation of Eimeria tenella. Curr. Protoc. Microbiol. 2019, 53, e81. [Google Scholar] [CrossRef]
- Debry, R.W. Identifying conflicting signal in a multigene analysis reveals a highly resolved tree: The phylogeny of Rodentia (Mammalia). Syst. Biol. 2003, 52, 604–617. [Google Scholar] [CrossRef]
- Youn, H.J.; Noh, J.W. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet. Parasitol. 2011, 96, 257–263. [Google Scholar] [CrossRef]
- Nweze, N.E.; Obiwulu, I.S. Anticoccidial effects of Ageratum conyzoides. J. Ethnopharmacol. 2009, 122, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Castaneda, R.E.; Dantan-Gonzalez, E. Control of avian coccidiosis: Future and present natural alternatives. Biomed. Res. Int. 2015, 2015, 430–610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Jia, M.; Li, H.Q.; Zhang, N.D.; Wen, X.; Rahman, K.; Zhang, Q.Y.; Qin, L.P. Cnidium monnieri: A review of traditional uses, phytochemical and ethnopharmacological properties. Am. J. Chin. Med. 2015, 43, 835–877. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, Y.; Zhai, X.; Yang, Y.; Li, C.; Zhang, Q.; Qin, L. Qualitative Analysis and Quality Evaluation of Cnidium monnieri Using UHPLC-ESI-Q-TOF/MS. Chin. Herb. Med. 2016, 8, 323–330. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355. [Google Scholar] [CrossRef]
- Hu, C. Taraxacum: Phytochemistry and health benefits. Chin. Herb. Med. 2018, 10, 353–361. [Google Scholar] [CrossRef]
- Su, H.; Liu, X.; Yan, W.; Shi, T.; Zhao, X.; Blake, D.P.; Tomley, F.M.; Suo, X. piggyBac transposon-mediated transgenesis in the apicomplexan parasite Eimeria tenella. PLoS ONE 2012, 7, e40075. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, R.; Moftah, A.; Clark, E.L.; Macdonald, S.E.; Chaudhry, A.S.; Sparagano, O.; Banerjee, P.S.; Kundu, K.; Tomley, F.M.; et al. An optimised protocol for molecular identification of Eimeria from chickens. Vet. Parasitol. 2014, 199, 24–31. [Google Scholar] [CrossRef]
- Hodgson, J.N. Coccidiosis: Oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970, 28, 99–102. [Google Scholar] [CrossRef]
- Long, P.L.; Millard, B.J.; Joyner, L.P.; Norton, C.C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia. Vet. Lat. 1976, 6, 201–217. [Google Scholar] [PubMed]
- Jiang, S.; Mohammed, A.A.; Jacobs, J.A.; Cramer, T.A.; Cheng, H.W. Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poult. Sci. 2019, 99, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ványi, A.; Sályi, G.; Majoros, G.; Glávits, R.; Sándor, G.; Bagó, G. Interaction of T-2 fusariotoxin and monensin in broiler chickens infected with Coccidia. Acta Vet. Hung. 1989, 37, 327–333. [Google Scholar] [PubMed]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- De Pablos, L.M.; dos Santos, M.F.; Montero, E.; Garcia-Granados, A.; Parra, A.; Osuna, A. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol. Res. 2010, 107, 601–604. [Google Scholar] [CrossRef]
- Pop, L.M.E.; Varga, M.; Coroian, M.E.; Nedișan, V.; Mircean, M.O.; Dumitrache, L.; Farczádi, I.; Fülöp, M.D.; Fazakas, C.M.; Györke, A. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasit. Vectors. 2019, 12, 343. [Google Scholar] [CrossRef]
- Adams, C.; Vahl, H.A.; Veldman, A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: Diet compositions that improve fat digestion during Eimeria acervulina infection. Br. J. Nutr. 1996, 75, 875–880. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Fuller, A.L.; McDougald, L.R. Coccidiosis in the chukar partridge (Alectoris chukar): A survey of coccidiosis outbreaks and a test of anticoccidial drugs against Eimeria kofoidi. Avian. Dis. 2016, 60, 752–757. [Google Scholar] [CrossRef]
- Kawazoe, U.; Fabio, J.D. Resistance to diclazuril in field isolates of Eimeria species obtained from commercial broiler flocks in Brazil. Avian. Pathol. 1994, 23, 305–311. [Google Scholar] [CrossRef]
- Dutta, G.P.; Mohan, A.; Tripathi, R. Study of the Gametocytocidal/Sporontocidal Action of Qinghaosu (Artemisinin) by Electron Microscopy. J. Parasitol. 1990, 76, 849–852. [Google Scholar] [CrossRef]
- Kettunen, H.; Tiihonen, K.; Peuranen, S.; Saarinen, M.T.; Remus, J.C. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and coccidia-infected broiler chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 759–769. [Google Scholar] [CrossRef]
- Hosseini-Mansoub, N.; Bahrami, Y. Influence of dietary fish oil supplementation on humoral immune response and some selected biochemical parameters of broiler chickens. J. Agrobiol. 2011, 28, 67–77. [Google Scholar] [CrossRef]
- Flora, S.J.; Dubey, R.; Kannan, G.M.; Chauhan, R.S.; Pant, B.P.; Jaiswal, D.K. Meso 2,3-dimercaptosuccinic acid (DMSA) and monoisoamyl DMSA effect on gallium arsenide induced pathological liver injury in rats. Toxicol. Lett. 2002, 132, 9–17. [Google Scholar] [CrossRef]
- Cerrato, P.; Lentini, A.; Baima, C.; Grasso, M.; Azzaro, C.; Bosco, G.; Destefanis, E.; Benna, P.; Bergui, M.; Bergamasco, B. Hypogeusia and hearing loss in a patient with an inferior collicular infarction. Neurology 2005, 65, 1840–1841. [Google Scholar] [CrossRef]
- Mondal, D.K.; Chattopadhyay, S.; Batabyal, S.; Bera, A.K.; Bhattacharya, D. Plasma biochemical indices at various stages of infection with a field isolate of Eimeria tenella in broiler chicken. Vet. World. 2011, 4, 404–409. [Google Scholar] [CrossRef]
- Jafari, R.A.; Razi-Jalali, M.; Kiani, R. Effect of fresh dietary garlic powder on some of the serum biochemical parameters in broiler chicks. Compara. Clin. Pathol. 2011, 20, 295–297. [Google Scholar] [CrossRef]
- Hirani, N.D.; Hasnani, J.J.; Dhami, A.J.; Khanna, K. Haemato-biochemical profile of broilers affected with coccidiosis. J. Vet. Parasitol. 2007, 21, 25–28. [Google Scholar]
- Dwyer, B.K.; Gorman, M.; Carroll, I.R.; Druzin, M. Urinalysis vs urine protein-creatinine ratio to predict significant proteinuria in pregnancy. J. Perinatol. 2008, 28, 461–467. [Google Scholar] [CrossRef]
- Liu, K.D.; Thompson, B.T.; Ancukiewicz, M.; Steingrub, J.S.; Douglas, I.S.; Matthay, M.A.; Wright, P.; Peterson, M.W.; Rock, P.; Hyzy, R.C.; et al. Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit. Care. Med. 2011, 39, 2665–2671. [Google Scholar] [CrossRef]
- Dar, S.A.; Verma, P.; Ashfaque, M.; Mir, I.A. Effect of garlic extract on haematobiochemical changes in Eimeria tenella infected broiler chicken. Natl. Acad. Sci. Lett. 2014, 37, 311–316. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, W.; Li, H. Application of metabolomics for unveiling the therapeutic role of traditional Chinese medicine in metabolic diseases. J. Ethnopharmacol. 2019, 242, 112057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Effects of Chinese herbal medicine on sporulation of rabbit coccidia oocysts. Xinjiang Anim. Husbandry. 2012, 7, 16–18. [Google Scholar]
- Ou, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.T.; Lee, J.; Cho, H.Y.; Jun, W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ. Toxicol. 2016, 31, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Empirical formula for treatment of livestock and poultry diseases. Guizhou Anim. Sci. Vet. Med. 2001, 1, 27. [Google Scholar]
- Zhang, J.; Xue, J.; Wang, H.; Zhang, Y.; Xie, M. Osthole improves alcohol-induced fatty liver in mice by reduction of hepatic oxidative stress. Phytother. Res. 2011, 25, 638–643. [Google Scholar] [CrossRef]
| Groups | 4 dpi | 5 dpi | 6 dpi | 7 dpi | 8 dpi | Total Mortality |
|---|---|---|---|---|---|---|
| Uninfected-untreated control | 0 | 0 | 0 | 0 | 0 | 0 a |
| Infected-untreated control | 2 | 2 | 1 | 0 | 0 | 5 b |
| ShiYingZi-PL | 0 | 1 | 0 | 0 | 0 | 1 ac |
| ShiYingZi-PM | 0 | 0 | 0 | 0 | 0 | 0 a |
| ShiYingZi-PH | 1 | 0 | 0 | 0 | 0 | 1 ac |
| Monensin | 0 | 0 | 0 | 0 | 0 | 0 a |
| ShiYingZi-TL | 1 | 1 | 0 | 0 | 0 | 2 ac |
| ShiYingZi-TM | 0 | 1 | 0 | 0 | 0 | 1 ac |
| ShiYingZi-TH | 0 | 0 | 0 | 0 | 0 | 0 a |
| Sulfachloropyrazine sodium | 0 | 0 | 0 | 0 | 0 | 0 a |
| Groups | 4 dpi | 5 dpi | 6 dpi | 7 dpi | 8 dpi |
|---|---|---|---|---|---|
| Uninfected-untreated control | 0 a | 0 a | 0 a | 0 a | 0 a |
| Infected-untreated control | 1 a | 2 b | 3 b | 3 b | 1 a |
| ShiYingZi-PL | 1 a | 2 b | 3 b | 2 c | 1 a |
| ShiYingZi-PM | 0 a | 2 b | 2 c | 2 c | 0 a |
| ShiYingZi-PH | 1 a | 2 b | 3 b | 3 b | 0 a |
| Monensin | 0 a | 2 b | 2c | 2 c | 0 a |
| ShiYingZi-TL | 0 a | 2 b | 3 b | 2 c | 0 a |
| ShiYingZi-TM | 0 a | 1 a | 2 c | 1 a | 1 a |
| ShiYingZi-TH | 1 a | 2 b | 2 c | 2 c | 0 a |
| Sulfachloropyrazine sodium | 0 a | 1 a | 2 c | 1 a | 0 a |
| Groups | 4 dpi | 5 dpi | 6 dpi | 7 dpi | 8 dpi |
|---|---|---|---|---|---|
| Uninfected-untreated control | 0 | 0 | 0 | 0 | 0 |
| Infected-untreated control | 0.27 | 2.78 | 1.93 | 1.54 | 1.15 |
| ShiYingZi-PL | 0.09 | 2 | 1.71 | 0.85 | 0.55 |
| ShiYingZi-PM | 0.14 | 2.13 | 1.24 | 1.03 | 0.60 |
| ShiYingZi-PH | 0 | 3.12 | 1.75 | 1.06 | 0.83 |
| Monensin | 0 | 1.56 | 1.69 | 0.75 | 0.42 |
| ShiYingZi-TL | 0.21 | 2.17 | 1.78 | 1.13 | 0.90 |
| ShiYingZi-TM | 0.33 | 2.33 | 1.51 | 1.23 | 0.95 |
| ShiYingZi-TH | 0.25 | 2.49 | 1.88 | 1.07 | 0.46 |
| Sulfachloropyrazine sodium | 0.19 | 2.12 | 1.25 | 0.99 | 0.78 |
| Indicators | Uninfected-Untreated Control | Infected-Untreated Control | Protective Effect | Therapeutic Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ShiYingZi-PL | ShiYingZi-PM | ShiYingZi-PH | Monensin | ShiYingZi-TL | ShiYingZi-TM | ShiYingZi-TH | Sulfachloropyrazine Sodium | |||
| Average initial weight/g | 165.27 ± 20.5 | 157.07 ± 25.1 | 164.07 ± 20.72 | 155.13 ± 14.17 | 160.43 ± 14.98 | 146.33 ± 12.56 | 160.13 ± 18.12 | 152.4 ± 18.72 | 149.33 ± 19.29 | 159.2 ± 19.93 |
| Average final weight/g | 225 ± 26.06 b | 189.14 ± 29.44 a | 210.27 ± 22.29 ab | 204.82 ± 18.07 ab | 209.41 ± 16.57 ab | 191.6 ± 14.9 a | 204 ± 21.01 a | 188.73 ± 23.08 a | 189.91 ± 23.4 a | 207.44 ± 17.9 ab |
| Relative weight gain rate (%) | 100 | 53 | 78 | 86 | 83 | 78 | 74 | 71 | 77 | 80 |
| Survival rate (%) | 100 | 75 | 95 | 100 | 95 | 100 | 90 | 95 | 100 | 100 |
| Gross lesions score | 0 ± 0 a | 35 ± 5.27 c | 14 ± 11.74 b | 11 ± 9.94 b | 14 ± 11.74 b | 9 ± 8.76 b | 19 ± 13.7 b | 15 ± 10.8 b | 13 ± 4.83 b | 11 ± 7.38 b |
| Cecum oocyst index | 0 | 40 | 20 | 10 | 10 | 10 | 20 | 20 | 20 | 10 |
| ACI | - | - | 139 | 165 | 154 | 159 | 125 | 131 | 144 | 159 |
| Therapeutic evaluation | - | - | low | medium | low | low | low | low | low | low |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 24.56 ± 1.43 a | 25.26 ± 1.16 c | 25.48 ± 1.23 b |
| Infected-untreated control | 25.48 ± 2.35 a | 21.58 ± 1a | 20.94 ± 0.98 a |
| ShiYingZi-PL | 24.8 ± 1.02 a | 23.52 ± 0.82 b | 27.4 ± 2.33 bc |
| ShiYingZi-PM | 23.66 ± 2.19 a | 24.28 ± 1.01 bc | 28.62 ± 1.42 c |
| ShiYingZi-PH | 24.3 ± 0.92 a | 25.22 ± 1.35 c | 28.08 ± 1.98 c |
| Monensin | 23.6 ± 1.98 a | 23.64 ± 1.43 b | 28.14 ± 0.8 c |
| ShiYingZi-TL | 23.67 ± 2.23 a | 20.67 ± 1.72 a | 24.13 ± 1.43 b |
| ShiYingZi-TM | 23.1 ± 0.95 a | 21.73 ± 1.40 a | 23.63 ± 1.68 b |
| ShiYingZi-TH | 24.40 ± 0.92 a | 21.47 ± 3.00 a | 25.23 ± 1.22 b |
| Sulfachloropyrazine sodium | 24.27 ± 3.40 a | 22.67 ± 0.57 a | 25.80 ± 2.19 b |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 11.78 ± 0.79 a | 12.34 ± 0.84 c | 13.74 ± 1.03 c |
| Infected-untreated control | 11.78 ± 1.11 a | 4.64 ± 0.61 a | 4.82 ± 1.36 a |
| ShiYingZi-PL | 10.9 ± 1.51 a | 6.1 ± 0.68 b | 10.42 ± 0.87 b |
| ShiYingZi-PM | 10.48 ± 2.02 a | 7.44 ± 0.8 b | 11.04 ± 0.87 b |
| ShiYingZi-PH | 11.3 ± 0.89 a | 7.24 ± 1.39 b | 11.26 ± 0.63 b |
| Monensin | 10.8 ± 0.99 a | 6.6 ± 1.44 b | 11.46 ± 1.33 b |
| ShiYingZi-TL | 10.73 ± 1.58 a | 4.10 ± 0.92 a | 8.40 ± 0.92 b |
| ShiYingZi-TM | 10.90 ± 0.56 a | 4.57 ± 0.61 a | 7.80 ± 0.61 b |
| ShiYingZi-TH | 11.67 ± 1.80 a | 6.00 ± 1.25 a | 7.20 ± 1.25 b |
| Sulfachloropyrazine sodium | 11.53 ± 2.87 a | 6.20 ± 1.00 a | 9.43 ± 1.00 b |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 0.57 ± 0.06 a | 0.59 ± 0.04 c | 0.57 ± 0.07 c |
| Infected-untreated control | 0.57 ± 0.08 a | 0.38 ± 0.04 a | 0.34 ± 0.05 a |
| ShiYingZi-PL | 0.56 ± 0.03 a | 0.44 ± 0.03 b | 0.47 ± 0.03 b |
| ShiYingZi-PM | 0.54 ± 0.05 a | 0.49 ± 0.04 b | 0.48 ± 0.03 b |
| ShiYingZi-PH | 0.56 ± 0.07 a | 0.47 ± 0.04 b | 0.47 ± 0.04 b |
| Monensin | 0.57 ± 0.06 a | 0.48 ± 0.05 b | 0.45 ± 0.04 b |
| ShiYingZi-TL | 0.55 ± 0.05 a | 0.39 ± 0.04 a | 0.40 ± 0.06 ab |
| ShiYingZi-TM | 0.54 ± 0.06 a | 0.36 ± 0.03 a | 0.41 ± 0.03 ab |
| ShiYingZi-TH | 0.53 ± 0.09 a | 0.38 ± 0.07 a | 0.46 ± 0.04 b |
| Sulfachloropyrazine sodium | 0.56 ± 0.05 a | 0.37 ± 0.04 a | 0.45 ± 0.04 b |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 12.78 ± 0.85 a | 12.92 ± 0.69 a | 11.76 ± 0.87 a |
| Infected-untreated control | 12.78 ± 0.86 a | 16.94 ± 1.38 b | 16.10 ± 0.58 b |
| ShiYingZi-PL | 13.9 ± 2.09 a | 17.42 ± 0.53 b | 16.98 ± 1.65 b |
| ShiYingZi-PM | 13.18 ± 1.45 a | 16.84 ± 0.3 b | 17.58 ± 0.89 b |
| ShiYingZi-PH | 13.00 ± 0.74 a | 17.96 ± 0.8 b | 16.82 ± 1.5 b |
| Monensin | 12.8 ± 1.31 a | 17.04 ± 0.78 b | 16.68 ± 0.9 b |
| ShiYingZi-TL | 12.93 ± 1.77 a | 16.57 ± 2.57 b | 15.73 ± 1.65 b |
| ShiYingZi-TM | 12.20 ± 0.46 a | 17.17 ± 0.83 b | 15.83 ± 2.94 b |
| ShiYingZi-TH | 12.70 ± 1.97 a | 15.47 ± 1.76 b | 18.03 ± 0.80 b |
| Sulfachloropyrazine sodium | 12.73 ± 1.83 a | 16.47 ± 1.56 b | 16.37 ± 0.86 b |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 4.18 ± 0.31 a | 4.08 ± 0.22 a | 4.14 ± 0.23 a |
| Infected-untreated control | 4.08 ± 0.53 a | 5.6 ± 0.5 b | 6.06 ± 0.21 c |
| ShiYingZi-PL | 4.1 ± 0.37 a | 4.66 ± 0.15 a | 4.78 ± 0.23 b |
| ShiYingZi-PM | 4.34 ± 0.49 a | 4.64 ± 0.32 a | 4.34 ± 0.15 a |
| ShiYingZi-PH | 4.14 ± 0.45 a | 4.66 ± 0.21 a | 4.34 ± 0.15 a |
| Monensin | 4.16 ± 0.54 a | 4.5 ± 0.25 a | 4.24 ± 0.26 a |
| ShiYingZi-TL | 4.30 ± 0.78 a | 5.53 ± 0.32 b | 5.13 ± 0.15 b |
| ShiYingZi-TM | 4.16 ± 0.35 a | 5.16 ± 0.67 b | 4.93 ± 0.35 b |
| ShiYingZi-TH | 4.57 ± 0.61 a | 5.33 ± 0.41 b | 4.80 ± 0.60 a |
| Sulfachloropyrazine sodium | 4.60 ± 0.35 a | 5.03 ± 067 b | 4.83 ± 0.60 a |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 18.02 ± 2.27 a | 17.48 ± 1.28 a | 17.08 ± 2.16 a |
| Infected-untreated control | 17.98 ± 2.16 a | 26.62 ± 3.46 c | 28 ± 3.17 c |
| ShiYingZi-PL | 18.34 ± 1.4 a | 23.1 ± 3.49 b | 22.36 ± 1.67 b |
| ShiYingZi-PM | 18.34 ± 1.78 a | 22.18 ± 2.04 b | 20.98 ± 1.2 b |
| ShiYingZi-PH | 18.18 ± 2.53 a | 21.4 ± 1.78 b | 21.36 ± 1.53 b |
| Monensin | 18.52 ± 1.43 a | 23.42 ± 1.54 b | 21.04 ± 1.23 b |
| ShiYingZi-TL | 17.50 ± 1.90 a | 25.03 ± 1.77 b | 22.73 ± 1.71 b |
| ShiYingZi-TM | 19.13 ± 1.72 a | 24.86 ± 3.22 b | 23.66 ± 1.59 b |
| ShiYingZi-TH | 18.56 ± 2.00 a | 24.46 ± 1.60 b | 23.70 ± 1.83 b |
| Sulfachloropyrazine sodium | 19.76 ± 1.04 a | 25.40 ± 2.52 b | 21.56 ± 2.33 b |
| Groups | 1 dpi | 4 dpi | 8 dpi |
|---|---|---|---|
| Uninfected-untreated control | 220.46 ± 11.77 a | 223.22 ± 12.06 a | 230.62 ± 23.95 a |
| Infected-untreated control | 222.56 ± 14.18 a | 263.46 ± 9.28 b | 275.9 ± 10.79 c |
| ShiYingZi-PL | 216.3 ± 13.94 a | 256.66 ± 11.08 b | 259.28 ± 7.49 b |
| ShiYingZi-PM | 214.38 ± 17.06 a | 254.54 ± 7.25 b | 250.12 ± 5.36 b |
| ShiYingZi-PH | 214.1 ± 14.39 a | 251.3 ± 7.11 b | 251.32 ± 12.03 b |
| Monensin | 224.34 ± 8.5 a | 263.3 ± 11.9 b | 253.08 ± 6.31 b |
| ShiYingZi-TL | 224.20 ± 9.78 a | 258.33 ± 10.69 b | 264.03 ± 9.18 b |
| ShiYingZi-TM | 232.33 ± 8.88 a | 263.73 ± 16.22 b | 267.37 ± 20.15 b |
| ShiYingZi-TH | 220.50 ± 11.05 a | 264.50 ± 9.97 b | 264.43 ± 21.63 b |
| Sulfachloropyrazine sodium | 230.50 ± 9.10 a | 263.43 ± 19.90 b | 254.07 ± 0.36 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Li, Y.; Chen, S.; Jia, R.; Huang, Y.; Zou, Y.; Li, L.; Zhao, X.; Yin, Z. Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella. Animals 2020, 10, 1484. https://doi.org/10.3390/ani10091484
Song X, Li Y, Chen S, Jia R, Huang Y, Zou Y, Li L, Zhao X, Yin Z. Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella. Animals. 2020; 10(9):1484. https://doi.org/10.3390/ani10091484
Chicago/Turabian StyleSong, Xu, Yunhe Li, Shufan Chen, Renyong Jia, Yongyuan Huang, Yuanfeng Zou, Lixia Li, Xinxin Zhao, and Zhongqiong Yin. 2020. "Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella" Animals 10, no. 9: 1484. https://doi.org/10.3390/ani10091484
APA StyleSong, X., Li, Y., Chen, S., Jia, R., Huang, Y., Zou, Y., Li, L., Zhao, X., & Yin, Z. (2020). Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella. Animals, 10(9), 1484. https://doi.org/10.3390/ani10091484





