Molecular Cloning of Dynein Heavy Chain and the Effect of Dynein Inhibition on the Testicular Function of Portunus trituberculatus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissues
2.2. SOV Injection and Sample Collection
2.3. Total RNA Extraction and Reverse Transcription of cDNA
2.4. Full-Length cDNA Cloning of Pt-dhc
2.5. Sequence Analysis and Structure Prediction
2.6. Quantitative mRNA Analysis
2.7. Immunofluorescence and Apoptosis Detection
2.8. Enzyme Activity Test
3. Results
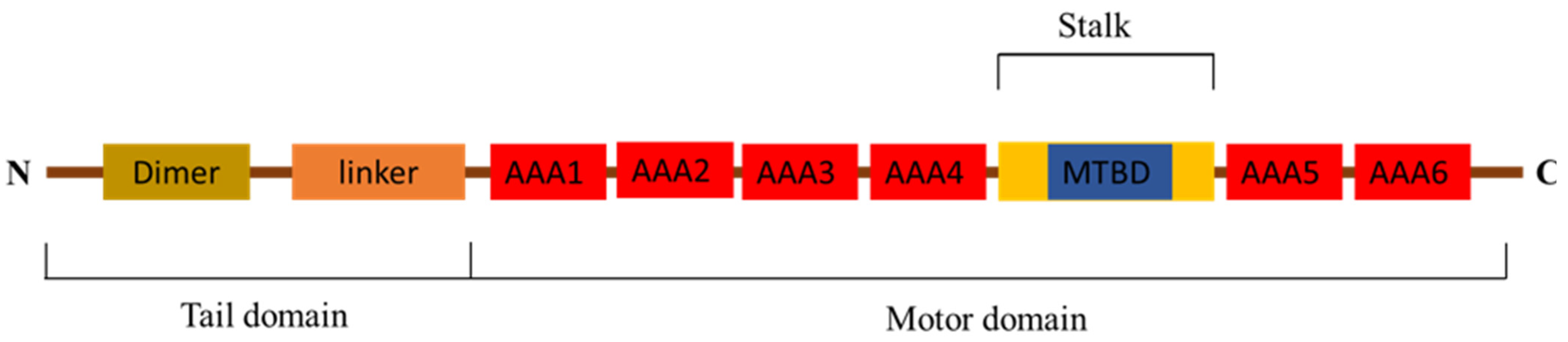
3.1. Full Length of Pt-dhc cDNA Sequence and Protein Structure
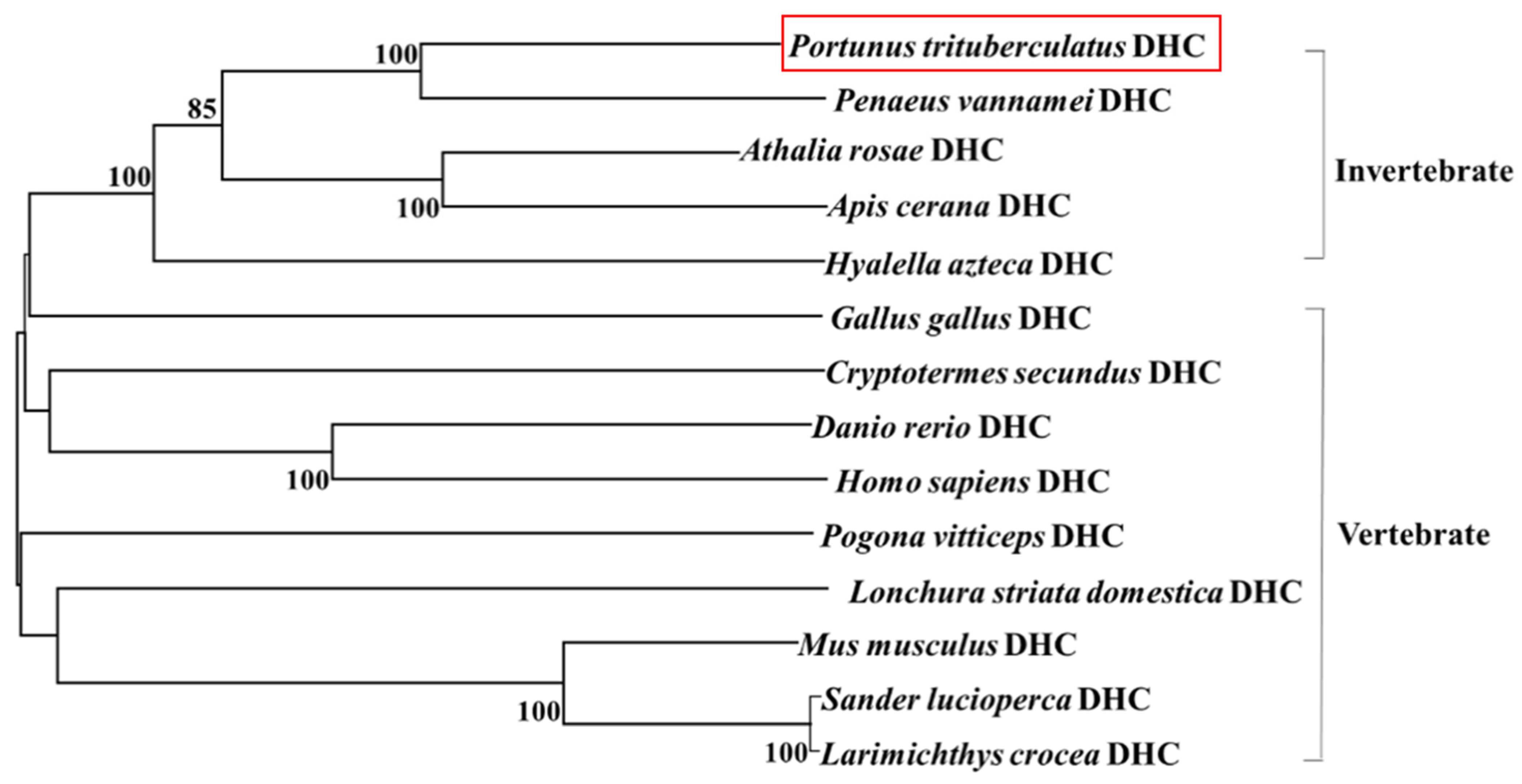
3.2. Sequence Alignment and Phylogenetic Analysis
3.3. The Expression of Pt-dhc in Different Tissues of P. trituberculatus
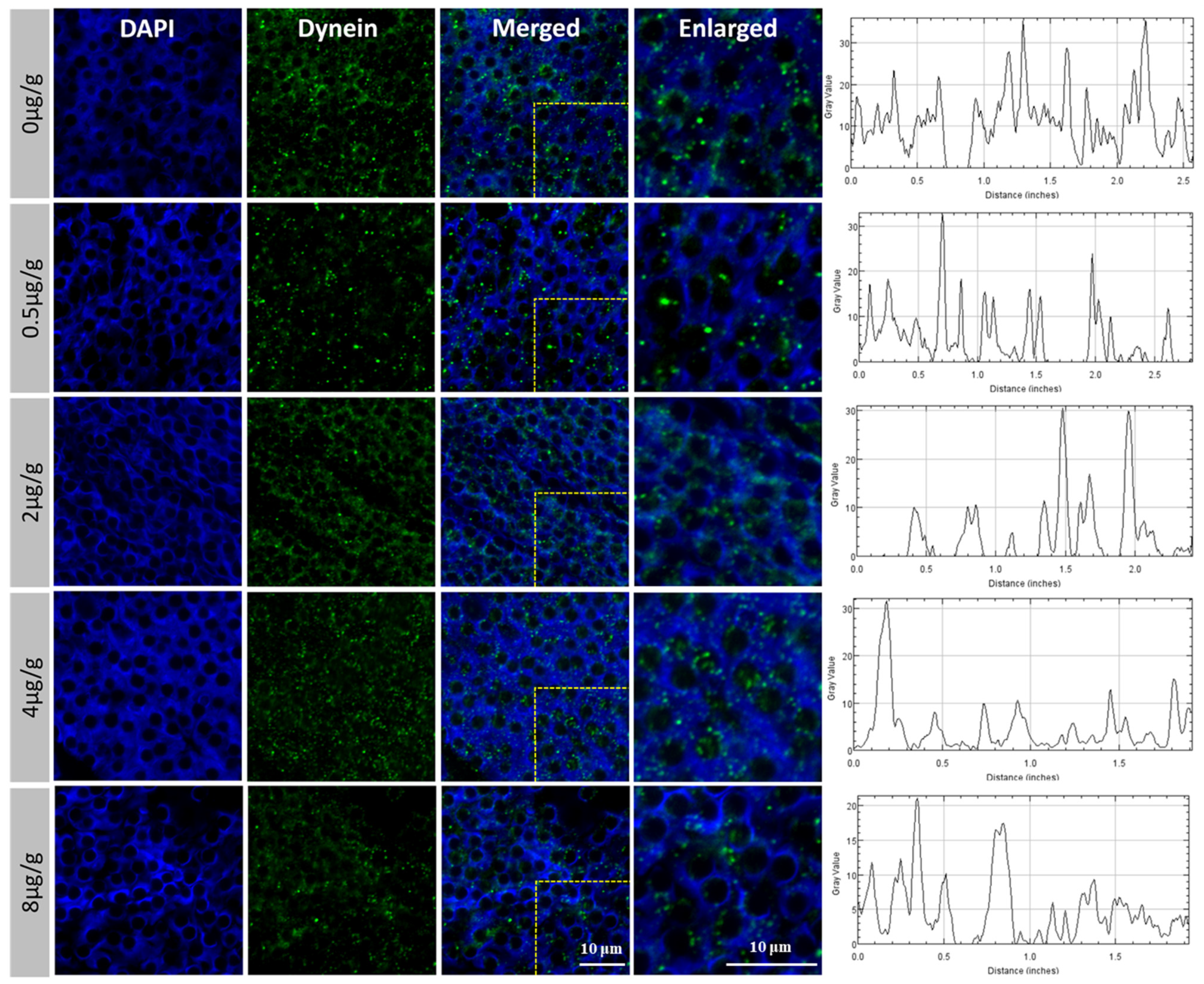
3.4. The Expression and Distribution of Pt-DHC in the Testis of P. trituberculatus Changed after Injection of SOV
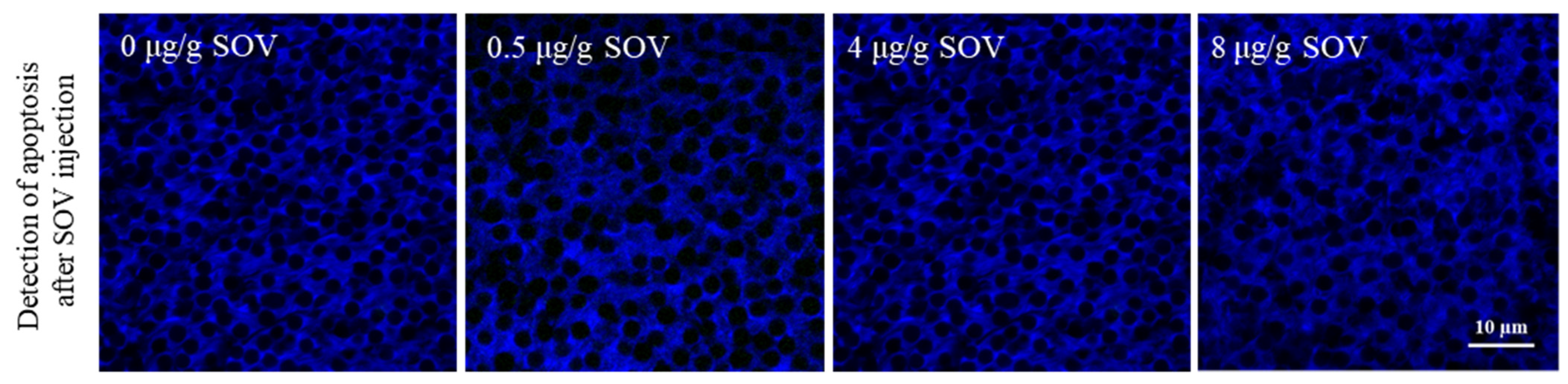
3.5. Detection of Testicular Cell Apoptosis in the Testis of P. trituberculatus after SOV Injection
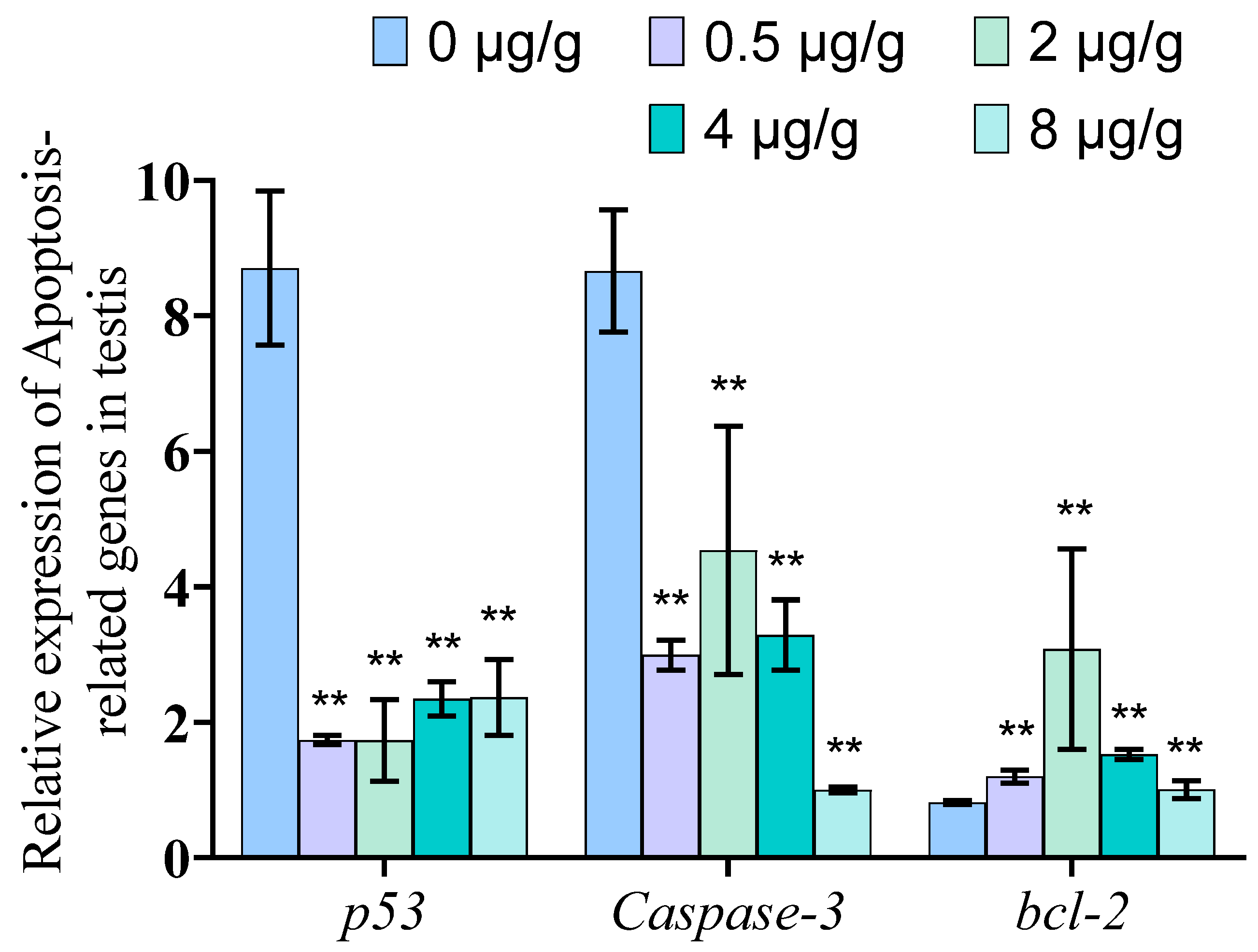
3.6. Dynein Inhibited by SOV Significantly Reduced the mRNA Expression Level of Genes Related to Apoptosis in Testis of P. trituberculatus
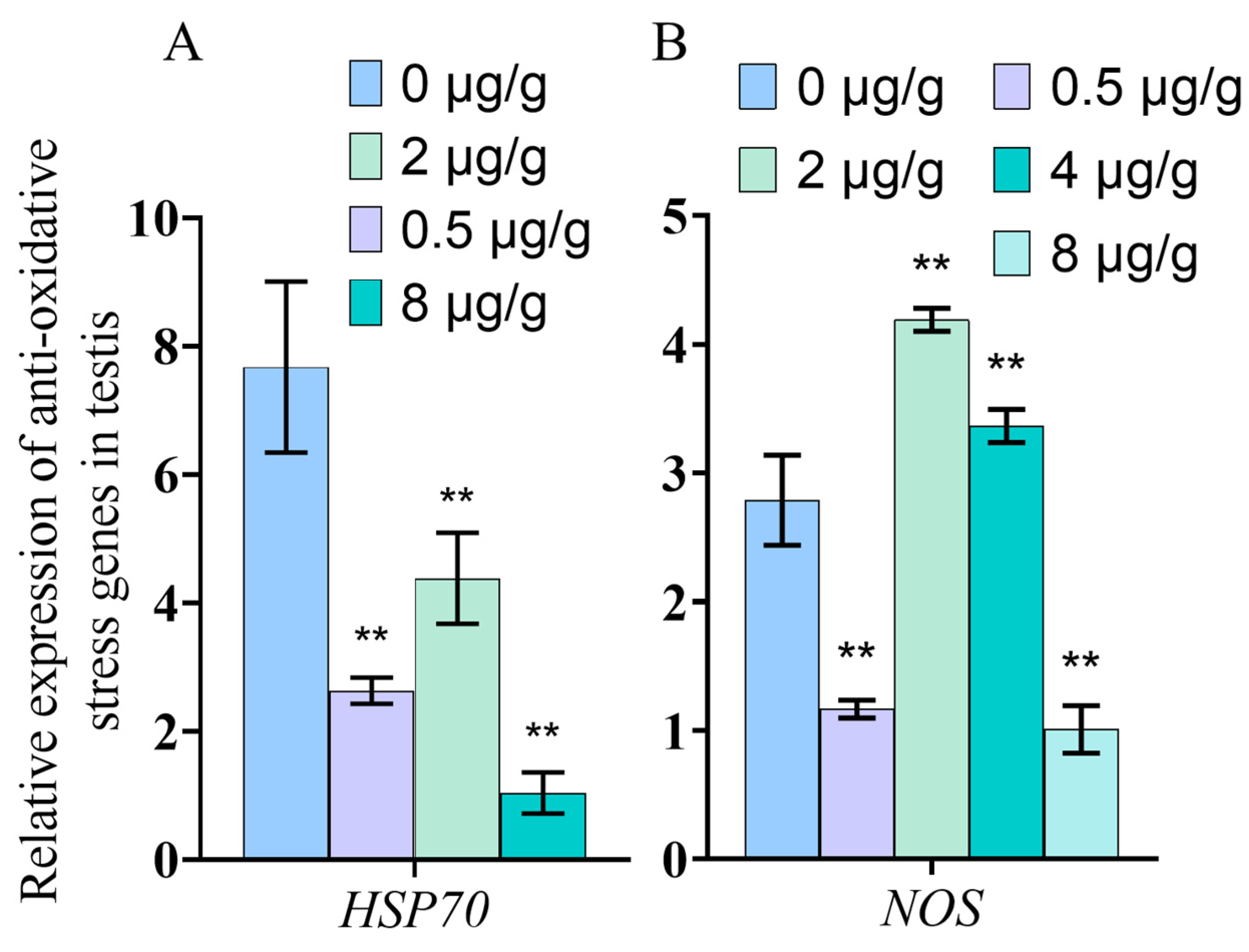
3.7. Dynein Inhibited by SOV Changes the Expression of Anti-Oxidative Stress Genes
3.8. Dynein Inhibited by SOV Induced the Changes in SOD and AKP Enzyme Activities of P. trituberculatus Tissues
4. Discussion
4.1. Protein Structure and mRNA Expression Characteristics of Pt-dhc
4.2. The Function of Dynein Was Inhibited after SOV Injection
4.3. Dynein May Indirectly Participate in Cell Apoptosis in the Testis
4.4. Inhibition of Dynein Function May Lead to Cell Dysfunction in Testis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamieson, B. A comparison of the spermatozoa of OratosquiMa stephensoni and Squilla mantis (Crustacea, Stomatopoda) with comments on the phylogeny of the Malacostraca. Zool. Scr. 1989, 18, 509–517. [Google Scholar] [CrossRef]
- Rorandelli, R.; Paoli, F.; Cannicci, S.; Mercati, D.; Giusti, F. Characteristics and fate of the spermatozoa of Inachus phalangium (Decapoda, Majidae): Des.cription of novel sperm structures and evidence for an additional mechanism of sperm competition in Brachyura. J. Morphol. 2008, 269, 259–271. [Google Scholar] [CrossRef]
- Erickson, R.P.; Jia, Z.; Gross, S.P.; Yu, C.C. How molecular motors are arranged on a cargo is important for vesicular transport. PLoS Comput. Biol. 2011, 7, e1002032. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.X.; Sperry, A.O. C-terminal kinesin motor KIFC1 participates in acrosome biogenesis and vesicle transport. Biol. Reprod. 2003, 69, 1719–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ou, Y.; Cheng, M.; Saadi, H.S.; Thundathil, J.C.; van der Hoorn, F.A. KLC3 is involved in sperm tail midpiece formation and sperm function. Dev. Biol. 2012, 366, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.C.; Yang, W.X. Acroframosome-dependent KIFC1 facilitates acrosome formation during spermatogenesis in the caridean shrimp Exopalaemon modestus. PLoS ONE 2013, 8, e76065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehti, M.S.; Kotaja, N.; Sironen, A. KIF3A is essential for sperm tail formation and manchette function. Mol. Cell. Endocrinol. 2013, 377, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.R.; Liu, M.; Hou, C.C.; She, Z.Y.; Wang, D.H.; Hao, S.L.; Zhang, Y.P.; Yang, W.X. Gene expression pattern of KIFC3 during spermatogenesis of the skink Eumeces chinensis. Gene 2015, 556, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Yang, W.X. Myosin superfamily: The multi-functional and irreplaceable factors in spermatogenesis and testicular tumors. Gene 2016, 576, 195–207. [Google Scholar] [CrossRef]
- Burgess, S.A.; Walker, M.L.; Sakakibara, H.; Knight, P.J.; Oiwa, K. Dynein structure and power stroke. Nature 2003, 421, 715–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.J.; Kon, T.; Knight, P.J.; Sutoh, K.; Burgess, S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 1998, 279, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Grotjahn, D.A.; Lander, G.C. Setting the dynein motor in motion: New insights from electron tomography. J. Biol. Chem. 2019, 294, 13202–13217. [Google Scholar] [CrossRef] [Green Version]
- Allan, V.J. Cytoplasmic dynein. Biochem. Soc. Trans. 2011, 39, 1169–1178. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Li, P.L.; Nguyen, T.; Li, X.; Zhang, Y. Regulation of dynein-mediated autophagosomes trafficking by ASM in CASMCs. Front. Biosci. (Landmark Ed.) 2016, 21, 696–706. [Google Scholar] [CrossRef] [Green Version]
- Lipka, J.; Kuijpers, M.; Jaworski, J.; Hoogenraad, C.C. Mutations in cytoplasmic dynein and its regulators cause malformations of cortical development and neurodegenerative diseases. Biochem. Soc. Trans. 2013, 41, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, A.; Takei, Y.; Kanai, Y.; Tanaka, Y.; Nonaka, S.; Hirokawa, N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 1998, 141, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Ioshii, S.O.; Imanaka-Yoshida, K.; Izutsu, K. Association of cytoplasmic dynein with manchette microtubules and spermatid nuclear envelope during spermiogenesis in rats. J. Cell Sci. 1994, 107 Pt 3, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Li, M.G.; Serr, M.; Newman, E.A.; Hays, T.S. The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Mol. Biol. Cell 2004, 15, 3005–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, R. Drosophila Dynein intermediate chain gene, Dic61B, is required for spermatogenesis. PLoS ONE 2011, 6, e27822. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Huang, T.H.; Xie, Q.D.; Zhang, Q.J.; Ruan, Y. Effect of dynein inhibitor on mouse oocyte in vitro maturation and its cyclin B1 mRNA level. Biomed. Environ. Sci. 2004, 17, 341–349. [Google Scholar] [PubMed]
- Kobayashi, T.; Martensen, T.; Nath, J.; Flavin, M. Inhibition of dynein ATPase by vanadate, and its possible use as a probe for the role of dynein in cytoplasmic motility. Biochem. Biophys. Res. Commun. 1978, 81, 1313–1318. [Google Scholar] [CrossRef]
- Reck-Peterson, S.L.; Redwine, W.B.; Vale, R.D.; Carter, A.P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 2018, 19, 382–398. [Google Scholar] [CrossRef]
- Schiavo, G.; Greensmith, L.; Hafezparast, M.; Fisher, E.M. Cytoplasmic dynein heavy chain: The servant of many masters. Trends Neurosci. 2013, 36, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, M. Big steps toward understanding dynein. J. Cell Biol. 2013, 202, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Q.; Tang, E.I.; Lui, W.Y.; Lee, W.M.; Wong, C.K.C.; Silvestrini, B.; Cheng, C.Y. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E924–E948. [Google Scholar] [CrossRef] [Green Version]
- Eschbach, J.; Dupuis, L. Cytoplasmic dynein in neurodegeneration. Pharmacol. Ther. 2011, 130, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Xu, H.; Cooper, H.M.; Liu, Y. Cytoplasmic dynein: A key player in neurodegenerative and neurodevelopmental diseases. Sci. China Life Sci. 2014, 57, 372–377. [Google Scholar] [CrossRef]
- Xiang, D.F.; Zhu, J.Q.; Hou, C.C.; Yang, W.X. Identification and expression pattern analysis of Piwi genes during the spermiogenesis of Portunus trituberculatus. Gene 2014, 534, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yang, H.; Ren, Z.; Han, S.; Mu, C.; Li, R.; Ye, Y.; Song, W.; Shi, C.; Liu, L.; et al. Characterization of PHB in the gonadal development of the swimming crab Portunus trituberculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 240, 110338. [Google Scholar] [CrossRef]
- Ma, D.D.; Pan, M.Y.; Hou, C.C.; Tan, F.Q.; Yang, W.X. KIFC1 and myosin Va: Two motors for acrosomal biogenesis and nuclear shaping during spermiogenesis of Portunus trituberculatus. Cell Tissue Res. 2017, 369, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.G.; Mu, D.L.; Tang, D.J.; Zhu, J.Q.; Hou, C.C. Expression and functional analysis of cytoplasmic dynein during spermatogenesis in Portunus trituberculatus. Cell Tissue Res. 2021, 386, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Racedo, S.E.; Rawe, V.Y.; Niemann, H. Dynamic changes of the Golgi apparatus during bovine in vitro oocyte maturation. Reproduction 2012, 143, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Alvarez Sedo, C.; Schatten, H.; Combelles, C.M.; Rawe, V.Y. The nuclear mitotic apparatus (NuMA) protein: Localization and dynamics in human oocytes, fertilization and early embryos. Mol. Hum. Reprod. 2011, 17, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Slee, E.A.; Keogh, S.A.; Martin, S.J. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: A potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 2000, 7, 556–565. [Google Scholar] [CrossRef]
- Kirsch, D.G.; Doseff, A.; Chau, B.N.; Lim, D.S.; de Souza-Pinto, N.C.; Hansford, R.; Kastan, M.B.; Lazebnik, Y.A.; Hardwick, J.M. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 1999, 274, 21155–21161. [Google Scholar] [CrossRef] [Green Version]
- Giannakakou, P.; Sackett, D.L.; Ward, Y.; Webster, K.R.; Blagosklonny, M.V.; Fojo, T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat. Cell Biol. 2000, 2, 709–717. [Google Scholar] [CrossRef]
- Lo, K.W.; Kan, H.M.; Chan, L.N.; Xu, W.G.; Wang, K.P.; Wu, Z.; Sheng, M.; Zhang, M. The 8-kDa dynein light chain binds to p53-binding protein 1 and mediates DNA damage-induced p53 nuclear accumulation. J. Biol. Chem. 2005, 280, 8172–8179. [Google Scholar] [CrossRef] [Green Version]
- Galigniana, M.D.; Harrell, J.M.; O’Hagen, H.M.; Ljungman, M.; Pratt, W.B. Hsp90-binding Immunophilins Link p53 to Dynein During p53 Transport to the Nucleus. J. Biol. Chem. 2004, 279, 22483–22489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.L.; Fernandez, A.; Solas, M.T. Mitochondria, motor neurons and aging. J. Neurol. Sci. 2013, 330, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Weber, A.; Hacker, G. The established and the predicted roles of dynein light chain in the regulation of mitochondrial apoptosis. Cell Cycle 2018, 17, 1037–1047. [Google Scholar] [CrossRef] [Green Version]
- Morthorst, T.H.; Olsen, A. Cell-nonautonomous inhibition of radiation-induced apoptosis by dynein light chain 1 in Caenorhabditis elegans. Cell Death Dis. 2013, 4, e799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harders, R.H.; Morthorst, T.H.; Lande, A.D.; Hesselager, M.O.; Mandrup, O.A.; Bendixen, E.; Stensballe, A.; Olsen, A. Dynein links engulfment and execution of apoptosis via CED-4/Apaf1 in C. elegans. Cell Death Dis. 2018, 9, 1012. [Google Scholar] [CrossRef]
- Singh, P.K.; Roukounakis, A.; Frank, D.O.; Kirschnek, S.; Das, K.K.; Neumann, S.; Madl, J.; Romer, W.; Zorzin, C.; Borner, C.; et al. Dynein light chain 1 induces assembly of large Bim complexes on mitochondria that stabilize Mcl-1 and regulate apoptosis. Genes Dev. 2017, 31, 1754–1769. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Todgham, A.E.; Ackerman, P.A.; Bibeau, M.R.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Huang, Y.; Yan, G.; Pan, C.; Zhang, J. Antioxidative status, immunological responses, and heat shock protein expression in hepatopancreas of Chinese mitten crab, Eriocheir sinensis under the exposure of glyphosate. Fish Shellfish Immunol. 2019, 86, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, J.; Wang, Y.; Chen, J.; Liang, C.; Li, R.; Li, Q. Non-specific immune factors differences in coelomic fluid from polian vesicle and coelom of Apostichopus japonicus, and their early response after evisceration. Fish Shellfish Immunol. 2020, 98, 160–166. [Google Scholar] [CrossRef] [PubMed]
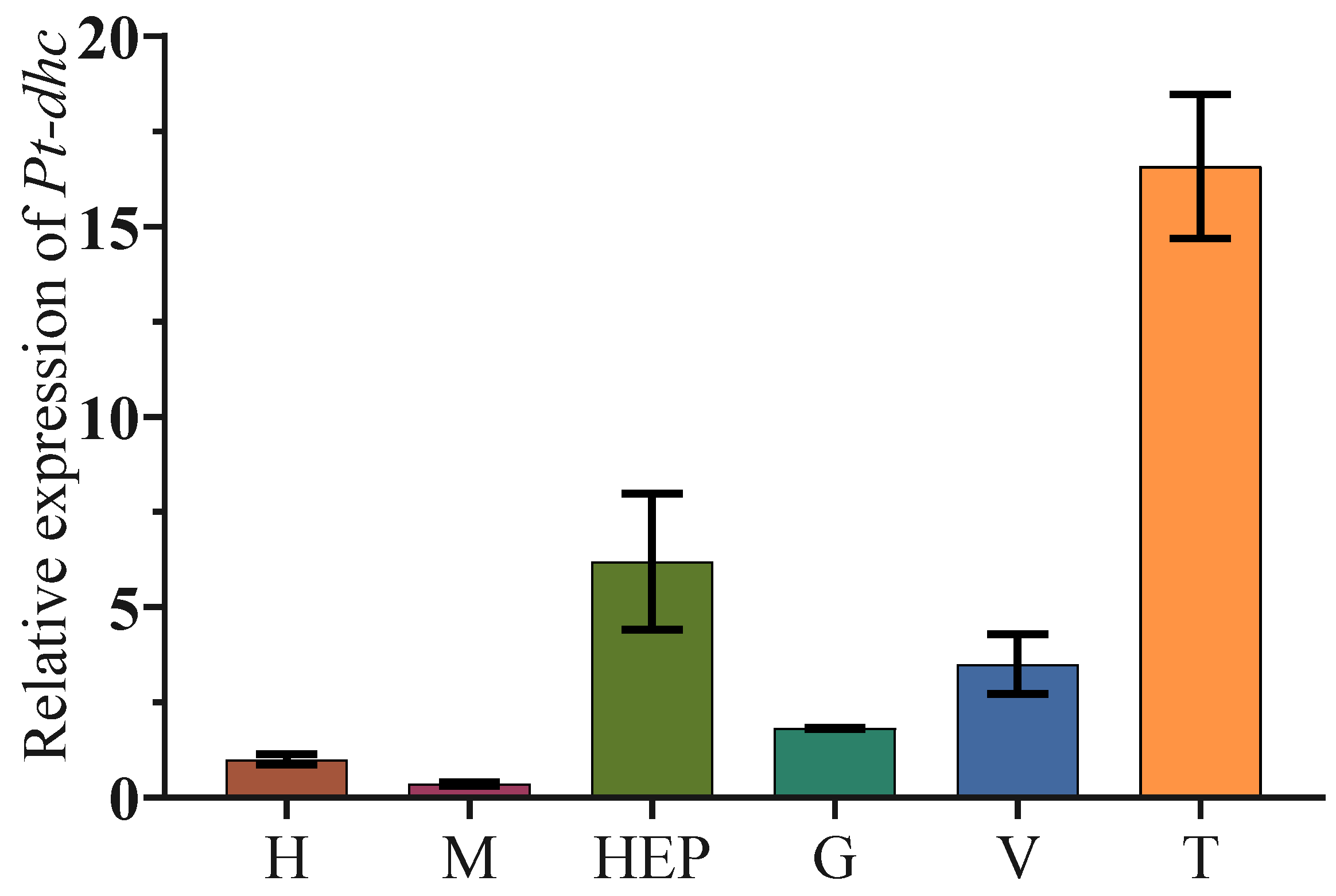

| Primer | Sequence (5′–3′) | Purpose |
|---|---|---|
| DHCF1 | CGCAAGTTCCTGTCCGAYCCNCARRT | PCR |
| DHCF2 | CCAACCTGCCCGACAAYYTNAARAA | PCR |
| DHCF3 | CTTTGTCCAAGGAAGTGCGG | PCR |
| DHCF4 | GCTGATCAAGGGCTACATGAAGRTNAAYATGYT | PCR |
| DHCF5 | CGACACCGTGCTGATGGARCARCCNCC | PCR |
| DHCF6 | GGAGCAGGGAGGAGGCAG | PCR |
| DHCF7 | GACTCCGGCTTCCTGGAGMGNATGAAYAC | PCR |
| DHCF8 | GCGGCAGACATTTCCCTCA | PCR |
| DHCF9 | TGGCCGCCGAGCARAAYAMNCA | PCR |
| DHCR1 | AGCACGCTGGATGGGGTA | PCR |
| DHCR2 | CTCGACCTTCTCGCAGGTNCKYTCRTA | PCR |
| DHCR3 | CGTCCTCCTCGAACACCTTRTARTANGG | PCR |
| DHCR4 | CATAGCCAGGGAGCGGAAC | PCR |
| DHCR5 | GTGGCGTACTTCAGGTCCTGNACYTCRAACA | PCR |
| DHCR6 | CACGGGCACCTTGGGRTTDATYTCCA | PCR |
| DHCR7 | TGAGGGAAATGTCTGCCGC | PCR |
| DHCR8 | TCCTGGATGATGGCGTGRAACCANGC | PCR |
| DHCR9 | AGGACGGCCACGCCNCKYTCRTA | PCR |
| 5′DHCF1 | TCACAGACTTCTCCCAGCGT | 5′RACE |
| 5′DHCF2 | TTCACAGCAAGTGGCTCAGT | 5′RACE |
| 5′DHC5F3 | AGAATGTAACGGTGTGGCTG | 5′RACE |
| 3′DHC3R1 | CGGAGCAAAGGCATTGGCTACATA | 3′RACE |
| 3′DHC3R2 | CTCCATCCCTTTCAATCTTGCCAGT | 3′RACE |
| 3′DHC3R3 | AATAGGTAATGAGATGTCTGGGATGTCG | 3′RACE |
| P53F | GGGTAACGCCATGAACGAGA | P53 qPCR |
| P53R | GCTGCATCTCCGTGTGTTTC | P53 qPCR |
| CAS3F | TCACAGATTGACAAAGAGCGG | Caspase-3 qPCR |
| CAS3R | TCCTCAGGTCAGTAGTGGAAATG | Caspase-3 qPCR |
| BCL2-F | AGCTTACAACTGGATGCGCT | Bcl-2 qPCR |
| BCL2-R | TCGAGAGTGATTTAGGCGGC | Bcl-2 qPCR |
| NOSF | GGAACCCTTCTGAGCAACGA | NOS qPCR |
| NOSR | CGTGTGTGGAGGTTGTCGTA | NOS qPCR |
| HSP70F | CTCAGATGGAGGCAAGCCAA | HSP70 qPCR |
| HSP70R | CTTGACGGTAGTGCCCAAGT | HSP70 qPCR |
| GAPDHF | TGAGGTGAAGGTAGAGGAT | Positive control of qPCR |
| GAPDHR | CCAGTGAAGTGAGCAGAG | Positive control of qPCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Wei, C.; Gao, X.; Chen, Y.; Tang, D.; Zhu, J.; Hou, C. Molecular Cloning of Dynein Heavy Chain and the Effect of Dynein Inhibition on the Testicular Function of Portunus trituberculatus. Animals 2021, 11, 3582. https://doi.org/10.3390/ani11123582
Xiang Q, Wei C, Gao X, Chen Y, Tang D, Zhu J, Hou C. Molecular Cloning of Dynein Heavy Chain and the Effect of Dynein Inhibition on the Testicular Function of Portunus trituberculatus. Animals. 2021; 11(12):3582. https://doi.org/10.3390/ani11123582
Chicago/Turabian StyleXiang, Qiumeng, Chaoguang Wei, Xinming Gao, Yiner Chen, Daojun Tang, Junquan Zhu, and Congcong Hou. 2021. "Molecular Cloning of Dynein Heavy Chain and the Effect of Dynein Inhibition on the Testicular Function of Portunus trituberculatus" Animals 11, no. 12: 3582. https://doi.org/10.3390/ani11123582
APA StyleXiang, Q., Wei, C., Gao, X., Chen, Y., Tang, D., Zhu, J., & Hou, C. (2021). Molecular Cloning of Dynein Heavy Chain and the Effect of Dynein Inhibition on the Testicular Function of Portunus trituberculatus. Animals, 11(12), 3582. https://doi.org/10.3390/ani11123582





