Survival of Embryos and Fry of Sea Trout (Salmo trutta m. trutta) Growing from Eggs Exposed to Different Concentrations of Selenium during Egg Swelling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Description
2.2. Determination of Selenium Accumulation in the Body of Hatching and Fry
2.3. Statistical Analysis
3. Results
3.1. Survival of the Embryos until Hatching
3.2. Selenium Content in the Body of Hatch and Fry
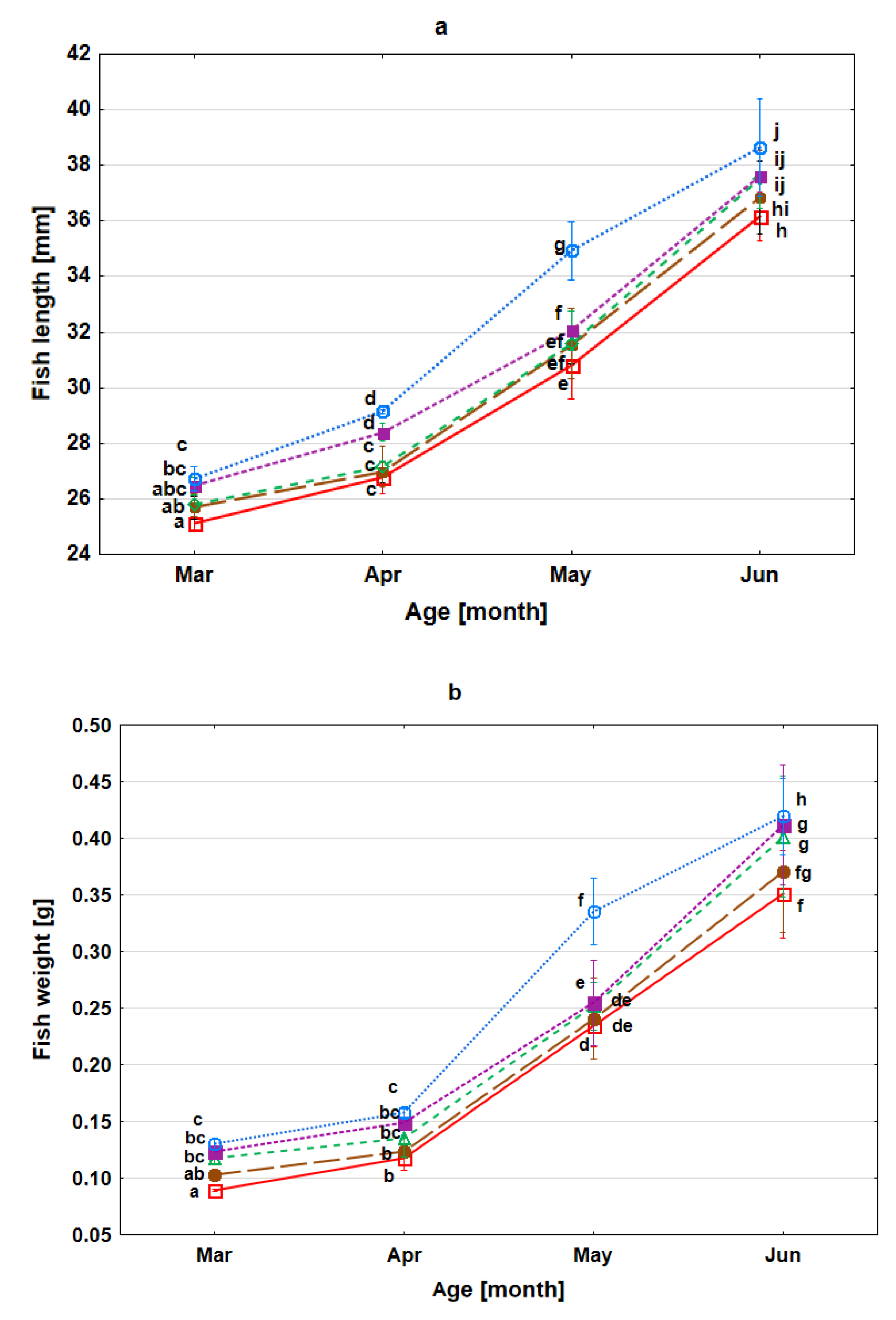
3.3. Survival Rate, Growth and Condition of Fry during Rearing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohmb, D.; Brigelius-Flohéa, R.; Schomburgc, L.; Bechtholdb, A.; Leschik-Bonnetb, E.; Hesekerd, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Allan, B.C.; Lacourcier, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Ann. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Reg. Tox. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Zeng, H.; Combs, G.F. Selenium as an anticancer nutrient: Roles of cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 9, 1–7. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.G.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.D.; Wu, Q.; Zhang, Z.W.; Li, S.; Wang, X.L.; Lei, X.G.; Xu, S.W. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. Biophys. Acta. 2013, 1830, 3112–3120. [Google Scholar] [CrossRef]
- He, Y.; Xian, Y.; Zhou, Y.; Yang, Y.; Zang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Env. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Noël, L.; Guérin, T.; Kolf-Clauw, M. Subchronic dietary exposure of rats to cadmium alters the metabolism of metals essential to bone health. Food. Chem. Toxicol. 2004, 42, 1203–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wang, Y.; Gu, Q.; Li, W. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81. [Google Scholar] [CrossRef]
- Betancor, M.B.; Caballero, M.; Terova, G.; Saleh, R.; Atalah, E.; Benítez-Santana, T.; Bell, J.G.; Izquierdo, M. Selenium inclusion decreases oxidative stress indicators and muscle injuries in sea bass larvae fed high-DHA microdiets. Br. J. Nutr. 2012, 108, 2115–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christophersen, O.A.; Lyons, G.; Haug, A.; Steinnes, E. Selenium. In Heavy Metals in Soil; Alloway, B.J., Ed.; Trace metals and metalloids in soils and their bioavailability; Springer: Dordrecht, The Netherlands, 2013; pp. 429–463. [Google Scholar]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadra, M.; Caron, A.; Planas, D.; Ponton, D.E.; Rosabal, M.; Amyot, M. The fish or the egg: Maternal transfer and subcellular partitioning of mercury and selenium in yellow perch (Perca flavescens). Sci. Total Env. 2019, 675, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Mechlaoui, M.; Dominguez, D.; Robaina, L.; Geraert, P.-A.; Kaushika, S.; Saleh, R.; Briens, M.; Monteroa, D.; Izquierdo, M. Effects of different dietary selenium sources on growth performance, liver and muscle composition, antioxidant status, stress response and expression of related genes in gilthead seabream (Sparus aurata). Aquaculture 2019, 507, 251–259. [Google Scholar] [CrossRef]
- Lorentzen, M.; Maage, A.; Julshamn, K. Effects of dietary selenite or selenomethionine on tissue selenium levels of Atlantic salmon (Salmo salar). Aquaculture 1994, 121, 359–367. [Google Scholar] [CrossRef]
- Attita, Y.A.; Abdalah, A.A.; Zeweil, H.S.; Bovera, F.; Tag El-Din, A.A.; Araft, M.A. Effect of inorganic or organic selenium supplementation on productive performance, egg quality and some physiological traits of dual-purpose breeding hens. Czech J. Anim. Sci. 2010, 55, 505–519. [Google Scholar] [CrossRef] [Green Version]
- Fairweather-Tait, S.J.; Boa, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Diplock, A.T.; Hoekstra, W.G. Metabolic aspects of selenium action and toxicity. CRC Critical Rev. Toxicol. 1976, 5, 271–329. [Google Scholar] [CrossRef]
- Meyer, J.; Moulis, J.-M.; Gaillard, J.; Lutz, M. Replacement of sulfur by selenium in iron-sulfur proteins. Adv. Inorg. Chem. 1992, 38, 73–115. [Google Scholar] [CrossRef]
- Spallholz, J.E.; Hoffman, D.J. Selenium toxicity: Cause and effects in aquatic birds. Aquat. Toxicol. 2002, 57, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, E.M.B.; Cumbie, P.M.; Bauer, T.L.; Bell, J.S.; Harlan, C.W. Histopathological, hematological, condition-factor, and organ weight changes associated with selenium accumulation in fish from Belews Lake, North Carolina. Arch. Environ. Con. Tox. 1984, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lemly, A.D. Symptoms and implications of selenium toxicity in fish: The Belews Lake case example. Aquat. Toxicol. 2002, 57, 39–49. [Google Scholar] [CrossRef]
- Chapman, P.M.; Adams, W.J.; Brooks, M.L.; Delos, C.G.; Luoma, S.N.; Maher, W.A.; Ohlendorf, H.M.; Presser, T.S.; Shaw, D.P. Ecological Assessment of Selenium in the Aquatic Environment; Society of Environmental Toxicology and Chemistry (SETAC) Press: Pensacola, FL, USA, 2010. [Google Scholar]
- Sorensen, E.M.B. Selenium accumulation, reproductive status, and histopathological changes in environmentally exposed redear sunfish. Archiv. Toxicol. 1988, 61, 324–329. [Google Scholar] [CrossRef]
- Rudolph, B.-L.; Andrelle, I.; Kennedy, C.J. Reproductive success, early life stage development, and survival of westslope cutthroat trout (Oncorhynchus clarki lewisi) exposed to elevated selenium in an area of active coal mining. Environ. Sci. Technol. 2008, 42, 3109–3114. [Google Scholar] [CrossRef]
- DeForest, D.K.; Brix, K.V.; Adams, W.J. Critical review of proposed residue-based selenium toxicity thresholds for freshwater fish. Hum. Ecol. Risk Assess. 1999, 5, 1187–1228. [Google Scholar] [CrossRef]
- Hamilton, S.J. Review of residue-based selenium toxicity thresholds for freshwater fish. Ecotoxicol. Env. Saf. 2003, 56, 201–210. [Google Scholar] [CrossRef]
- Muscatello, J.R.; Bennett, P.M.; Himbeault, K.T.; Belknap, A.M.; Janz, D.M. Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environ. Sci. Technol. 2006, 40, 6506–6512. [Google Scholar] [CrossRef]
- Lemly, A.D. Selenium Assessment in Aquatic Ecosystems: A Guide for Hazard Evaluation and Water Quality Criteria; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Env. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Kroll, K.J.; Doroshov, S.I. Vitellogenin: Potential vehicle for selenium bioaccumulation in oocytes of the white sturgeon (Acipenser transmontanus). In Acipenser; Willcot, A.P., Ed.; Cemagref: Bordeaux, France, 1991; pp. 99–106. [Google Scholar]
- Kennedy, C.J.; McDonald, L.E.; Loveridge, R.; Strosher, M.M. The effect of bioaccumulated selenium on mortalities and deformities in the eggs, larvae, and fry of a wild population of cutthroat trout (Oncorhynchus clarki lewisi). Arch. Environ. Contam. Toxicol. 2000, 39, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar] [PubMed]
- Le, K.T.; Fotedar, R. Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquaculture 2011, 420, 57–62. [Google Scholar] [CrossRef] [Green Version]
- EPA. Aquatic Life Ambient Water Quality Criterion for Selenium in Freshwater 2016-Fact Sheet. 2016. Available online: https://www.epa.gov/sites/production/files/2016-06/documents/se_2016_fact_sheet_final.pdf (accessed on 20 August 2021).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Addendum; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 20 August 2021).
- Dungan, R.S.; Frankenberger, W.T. Microbial transformations of selenium and the bioremediation of seleniferous environments. Bioremediat. J. 1999, 3, 171–188. [Google Scholar] [CrossRef]
- Fan, T.W.-M.; Teh, S.J.; Hinton, D.E.; Higashi, R.M. Selenium biotransformations into proteinaceous forms by foodweb organisms of selenium-laden drainage waters in California. Aquat Toxicol. 2002, 57, 65–84. [Google Scholar] [CrossRef]
- Lenz, M.; Lens, P.N.L. The essential toxin: The changing perception of selenium in environmental sciences. Sci. Total Environ. 2009, 407, 3620–3633. [Google Scholar] [CrossRef] [PubMed]
- Plak, A.; Bartmiński, P. The impact of land use on the organic and inorganic selenium content in soils developed from loses. J. Elem. 2017, 22, 1463–1474. [Google Scholar] [CrossRef]
- Oldfield, J.E. Selenium World Atlas; Selenium-Tellurium Development Association: Grimbergen, Belgium, 1999. [Google Scholar]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In Selenium in Plants. Plant Ecophysiology; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer: New York, NY, USA, 2017; pp. 209–230. [Google Scholar]
- Poston, H.A.; Combs, G.F.; Leibovitz, J.L. Vitamin E and selenium interrelations in the diet of Atlantic salmon (Salmo salar): Gross, histological and biochemical deficiency signs. J. Nut. 1976, 106, 892–904. [Google Scholar] [CrossRef]
- Julshamn, K.; Sandnes, K.; Lie, Ø.; Waagbø, R. Effects of dietary selenium supplementation on growth, blood chemistry and trace element levels in serum and liver of adult Atlantic salmon (Salmo salar). Fisk. Dir. Skr. Ser. Ernӕing. 1990, 3, 47–58. [Google Scholar]
- Gatellier, P.; Mercier, Y.; Renerre, M. Effect of diet finishing mode (pasture or mixed diet) on antioxidant status of Charolais bovine meat. Meat Sci. 2004, 67, 385–394. [Google Scholar] [CrossRef]
- Johnston, I.; Xuejun, L.I.; Vieira, L.; Nickel, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculure 2006, 256, 323–336. [Google Scholar] [CrossRef]
- Luo, X.-L.; Rauan, A.; Xing, J.-X.; Sun, J.; Wu, W.-Y.; Ji, H. Influence of dietary Se supplementation on aquaponic system: Focusing on the growth performance, ornamental features and health status of Koi carp (Cyprinus carpio var. Koi), production of lettuce (Lactuca sativa) and water quality. Aquacul. Res. 2021, 52, 505–517. [Google Scholar] [CrossRef]
- Lemly, A.D. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ. Mon. Assess. 1993, 28, 83–100. [Google Scholar] [CrossRef]
- EFSA. Panel on additives and products or substances used in animal feed (FEEDAP). Scientific opinion on the safety and efficacy of DL-selenomethionine as a feed additive for all animal species. EFSA J. 2014, 12, 3567. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2014.3567 (accessed on 20 August 2021).
- Lin, Y.H.; Shiau, S.Y. Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 2005, 250, 356–363. [Google Scholar] [CrossRef]
- Fontagne-Dicharry, S.; Godin, S.; Liu, H.K.; Prabhu, P.A.J.; Bouyssiere, B.; Bueno, M.; Tacon, P.; Medale, F.; Kaushik, S.J. Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br. J. Nutr. 2015, 113, 1876–1887. [Google Scholar] [CrossRef] [Green Version]
- Sele, V.; Ørnsrud, R.; Sloth, J.J.; Berntssen, M.H.G. Selenium and selenium species in feeds and muscle tissue of Atlantic salmon. J. Trace Elem. Med. Biol. 2018, 47, 124–133. [Google Scholar] [CrossRef]
- Bartel, R. Is this the end for salmon and sea trout? Komun. Ryb. 1992, 1, 1–5. (In Polish) [Google Scholar]
- Bartel, R. Guidelines for choosing stocking material for restocking Polish marine areas. Komun. Ryb. 2012, 2, 24–32. (In Polish) [Google Scholar]
- Witkowski, A. The degree of threat to the freshwater ichthyofauna of Poland: Red list of fishes and lampreys-situationin. Chrońmy Przyr. Ojcz. 2009, 65, 33–52. [Google Scholar]
- Dębowski, P. The largest Baltic population of sea trout (Salmo trutta L.): Its decline, restoration attempts, and current status. Fish. Aquat. Life. 2018, 26, 81–100. [Google Scholar] [CrossRef]
- ICES. Report of the Baltic Salmon and Trout Assessment Working Group (WGBAST); ICES CM 2018/ACOM:10; ICES: Copenhagen, Denmark, 2018; p. 369. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. 2019. Available online: www.fishbase.org (accessed on 20 August 2021).
- Wasowicz, W.; Gromadzinska, J.; Rydzynski, K.; Tomczak, J. Selenium status of low-selenium area residents: Polish experience. Toxicol. Lett. 2003, 137, 95–101. [Google Scholar] [CrossRef]
- Niedzielski, P.; Siepak, J.; Siepak, M.; Kraska, M. Occurrence of arsenic, antimony and selenium in surface waters of Drawienski National Park. Polish J. Env. Stud. 2002, 11, 41–45. [Google Scholar]
- Kabata-Pendia, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Kowalska-Góralska, M. Impact of supplementation with selenium during swelling of fish eggs of rainbow trout (Oncorhynchus mykiss R.) on their survival and selenium concentration in eggs. Chem. Agricul. 2007, 8, 142–145. [Google Scholar]
- Kowalska-Góralska, M.; Formicki, K.; Dobrzański, Z.; Wondołowska-Grabowska, A.; Skrzyńska, E.; Korzelecka-Orkisz, A.; Nędzarek, A.; Tański, A. Nutritional composition of Salmonidae and Acipenseridae fish eggs. Ann. Animal Sci. 2020, 20, 629–645. [Google Scholar] [CrossRef]
- Kowalska-Góralska, M.; Senze, M.; Łuczyńska, J.; Czyż, K. Effects of the ionic and nanoparticle forms of Cu and Ag on these metals’ bioaccumulation in the eggs and fry of rainbow trout (Oncorhynchus mykiss W.). Int. J. Env. Res. Public Health 2020, 17, 6392. [Google Scholar] [CrossRef]
- Diaz-Alarcon, J.P.; Navarro-Alarcon, M.; Lopez-Garcia de la Serrana, H.; Lopez-Martinez, M.C. Determination of selenium levels in vegetables and fruits by hydride generation atomic absorption spectrometry. J. Agric. Food Chem. 1994, 42, 2848–2851. [Google Scholar] [CrossRef]
- Elliott, J.M. An experimental study on the natural removal of dead trout fry in a Lake District stream. J. Fish Biol. 1997, 50, 870–877. [Google Scholar] [CrossRef]
- Abdulsahib, H.T.; Mohammed, S.G.; Mohammed, J.J.; Awad, N.A.N. Concentration of selenium and mercury in six species of fishes from Shatt Al-Arab River, Iraq Hassan. Iraqi J. Aquacult. 2012, 9, 223–234. [Google Scholar] [CrossRef]
- Atanasoff, A.; Nikolov, G.; Staykov, Y.G.; Zhelyazkov, G.; Sirakov, I. Proximate and mineral analysis of Atlantic Salmon (Salmo Salar) cultivated in Bulgaria. Biotech. Anim. Husb. 2013, 29, 571–579. [Google Scholar] [CrossRef]
- Brix, K.V.; Toll, J.E.; Tear, L.M.; DeFrost, D.K.; Adams, W.J. Setting site-specific water-quality standards by using tissue residue thresholds and bioaccumulation data. Part 2. Calculating site-specific selenium water-quality standards for protecting fish and birds. Env. Toxicol. Chem. 2005, 24, 231–237. [Google Scholar] [CrossRef]
- Maier, K.J.; Knight, A.W. Ecotoxicology of selenium in freshwater systems. Rev. Environ. Contam. Toxicol. 1994, 134, 31–48. [Google Scholar]
- Stephens, D.; Waddell, B.; DuBois, K.; Peterson, E. Field Screening of Water Quality, Bottom Sediment, and Biota Associated with the Emery and Scofield Project Areas, Central Utah, 1994; Water-Resources Investigations Report, 96-4298; U.S. Fish and Wildlife Service: Washington, DC, USA; Bureau of Reclamation: Washington, DC, USA; Bureau of Indian Affairs: Washington, DC, USA, 1997. [Google Scholar]
- Pakkala, C.J.; White, E.M.; Burdick, G.F.; Harris, E.H.; Lisk, W.H. A survey of the selenium content of fish form 49 New York state waters. Pestic. Monit. J. 1972, 5, 348–355. [Google Scholar]
- Essig, D.A.; Kosterman, M.A. Arsenic, Mercury, and Selenium in Fish Tissue from Idaho Lakes and Reservoirs: A Statewide Assessment. Idaho Department of Environmental Quality: Idaho. 2008. Available online: https://www.deq.idaho.gov/media/639760-arsenic_mercury_fish_tissue_report_0508.pdf (accessed on 20 August 2021).
- DeForest, D. Database of Selenium Concentrations in Fish Tissues from Reference Sites; Parametrix: Bellevue, WA, USA, 2009; Available online: http://www.namc.org/docs/00043670.PDF (accessed on 20 August 2021).
- Pedrero, Z.; Murillo, S.; Camara, C.E.; Schram, E.; Luten, J.B.; Feldmann, I.; Jakubowski, N.; Madrid, Y. Selenium speciation in different organs of African catfish (Clarias gariepinus) enriched through a selenium-enriched garlic based diet. J. Anal. At. Spectrom. 2011, 26, 116–125. [Google Scholar] [CrossRef]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625–640. [Google Scholar] [CrossRef]
- Fordyce, F.M. Fluorine: Human health risks. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, MA, USA, 2011; Volume 2, pp. 776–785. [Google Scholar]
- Berillis, P. Factors that can lead to the development of skeletal deformities in fishes: A review. J. Fish. Sci. 2015, 9, 17–23. [Google Scholar]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V.; et al. Toxic effects of zinc chloride on the bone development in Danio rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef] [Green Version]

| Se in Water [mg L−1] | 0 | 1 | 2 | 8 | 16 |
|---|---|---|---|---|---|
| Selenium concentration in fish body (dw) [µg·g−1] | |||||
| Hatch * | 3.61 ± 0.21 b | 4.49 ± 0.62 c | 4.75 ± 0.32 cd | 5.31 ± 0.32 cd | 5.40 ± 0.34 d |
| four-month-old fry ** | 2.74 ± 0.30 a | 2.96 ± 0.41 a | 3.70 ± 0.16 b | 4.24 ± 0.51 b | 4.94 ± 0.35 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziewulska, K.; Kirczuk, L.; Czerniawski, R.; Kowalska-Góralska, M. Survival of Embryos and Fry of Sea Trout (Salmo trutta m. trutta) Growing from Eggs Exposed to Different Concentrations of Selenium during Egg Swelling. Animals 2021, 11, 2921. https://doi.org/10.3390/ani11102921
Dziewulska K, Kirczuk L, Czerniawski R, Kowalska-Góralska M. Survival of Embryos and Fry of Sea Trout (Salmo trutta m. trutta) Growing from Eggs Exposed to Different Concentrations of Selenium during Egg Swelling. Animals. 2021; 11(10):2921. https://doi.org/10.3390/ani11102921
Chicago/Turabian StyleDziewulska, Katarzyna, Lucyna Kirczuk, Robert Czerniawski, and Monika Kowalska-Góralska. 2021. "Survival of Embryos and Fry of Sea Trout (Salmo trutta m. trutta) Growing from Eggs Exposed to Different Concentrations of Selenium during Egg Swelling" Animals 11, no. 10: 2921. https://doi.org/10.3390/ani11102921
APA StyleDziewulska, K., Kirczuk, L., Czerniawski, R., & Kowalska-Góralska, M. (2021). Survival of Embryos and Fry of Sea Trout (Salmo trutta m. trutta) Growing from Eggs Exposed to Different Concentrations of Selenium during Egg Swelling. Animals, 11(10), 2921. https://doi.org/10.3390/ani11102921






