Very Small Home Ranges of Two Gravid European Brown Bears during Hyperphagia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
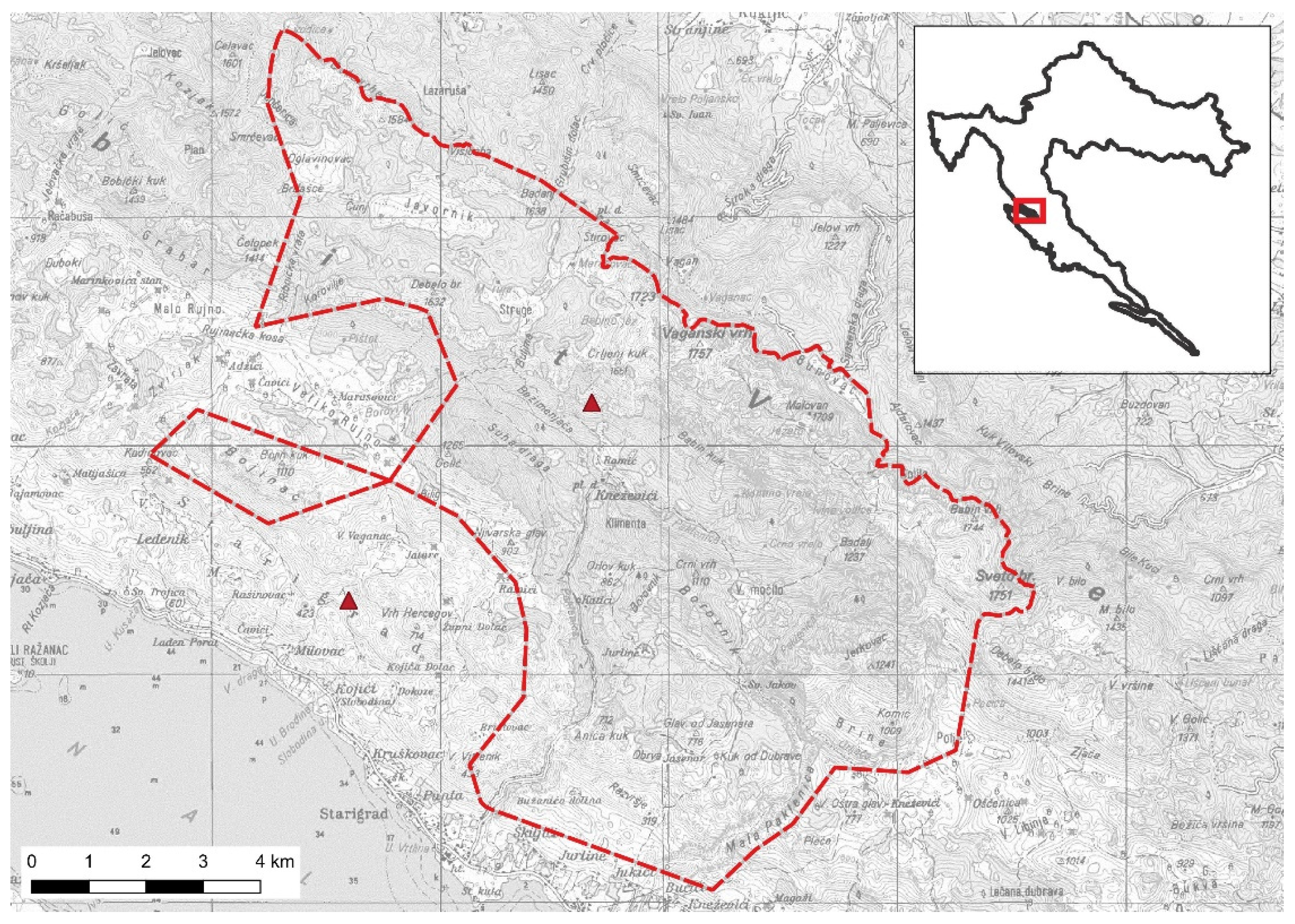
2.1. Study Area and Study Species
2.2. Capturing of Bears
2.3. Estimation of Home Range, Overlapping Territories, Proximity to Feeding Sites and Settlements
3. Results
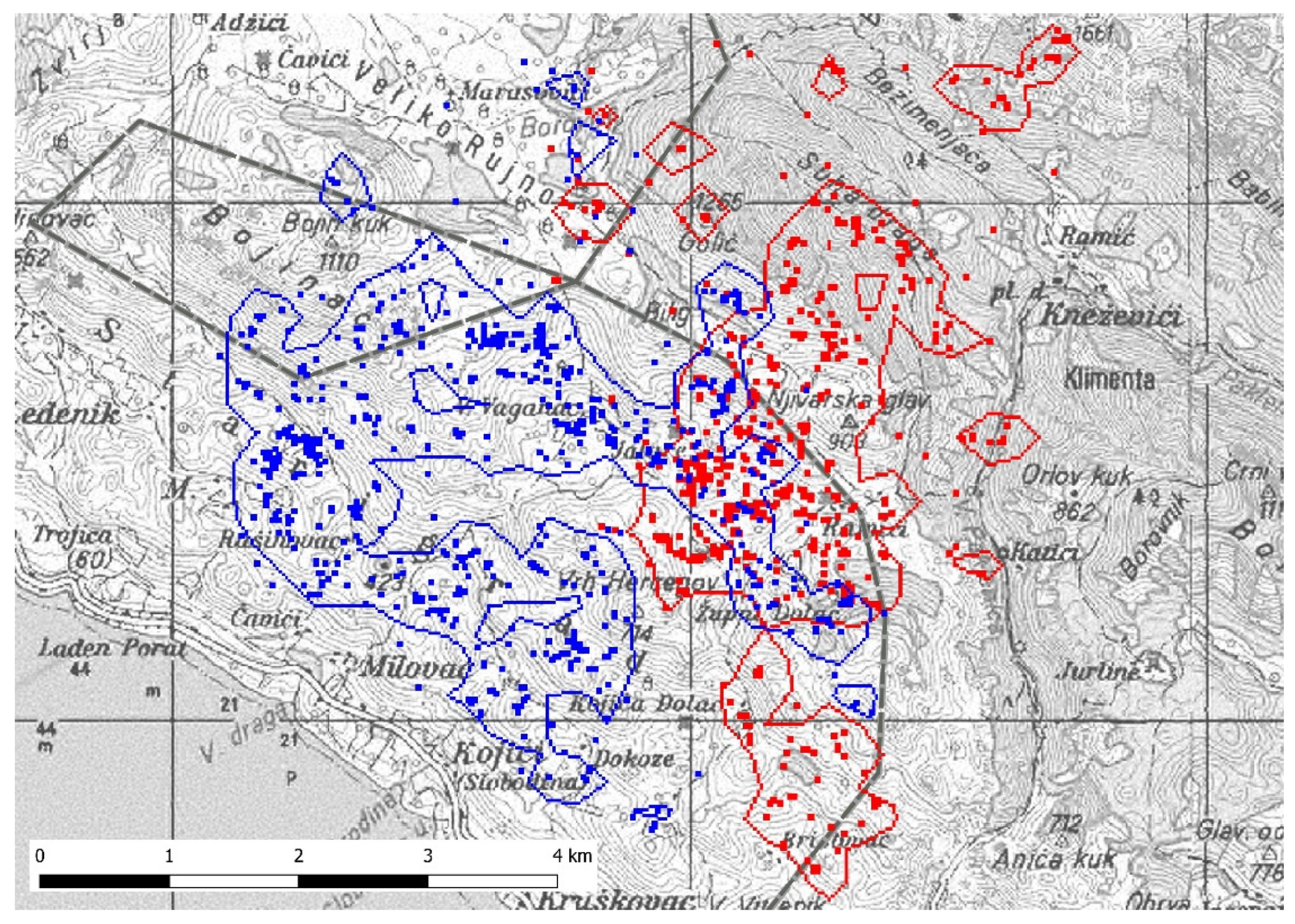
3.1. Home Range
3.2. Overlapping Territories
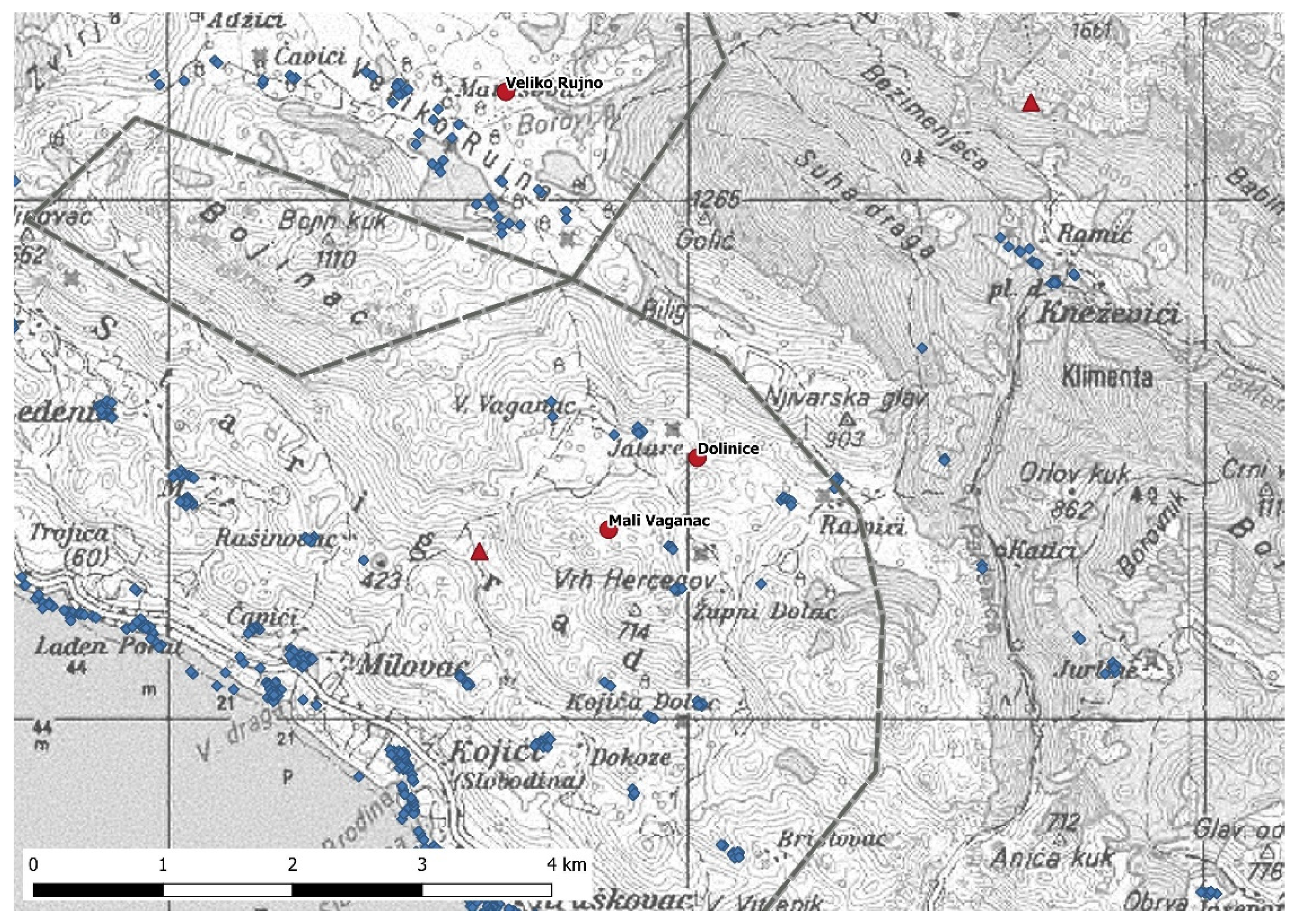
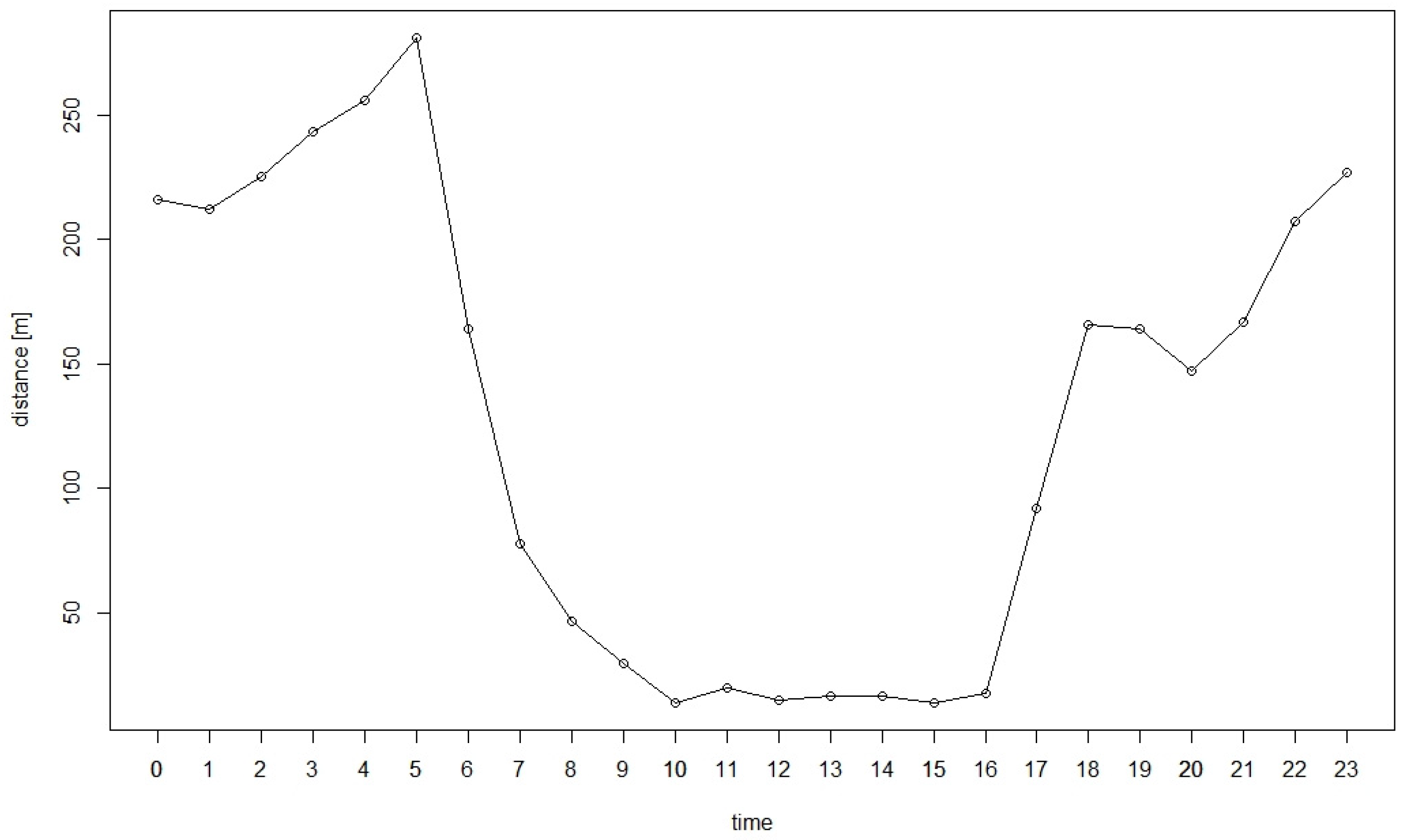
3.3. Proximity to Feeding Sites and Settlements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, C.; Ripple, W.J. Rewilding the world’s large carnivores. R. Soc. Open Sci. 2018, 5, 172235. [Google Scholar] [CrossRef] [PubMed]
- Boitani, L.; Linnell, J.D.C. Bringing large mammals back: Large carnivores in Europe. In Rewilding European Landscapes; Springer: Singapore, 2015; pp. 67–84. [Google Scholar]
- Treves, A.; Karanth, K.U. Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv. Biol. 2003, 17, 1491–1499. [Google Scholar] [CrossRef]
- Duncan, C.; Nilsen, E.B.; Linnell, J.D.C.; Pettorelli, N. Life-history attributes and resource dynamics determine intraspecific home-range sizes in Carnivora. Remote. Sens. Ecol. Conserv. 2015, 1, 39–50. [Google Scholar] [CrossRef]
- Kaczensky, P.; Chapron, G.; von Arx, M.; Huber, Đ.; Andrén, H.; John, L. Status Management and Distribution of Large Carnivores—Bear, Lynx, Wolf & Wolverine—In Europe. Status of Large Carnivores in Europe—Update 2012; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Huber, Đ.; Bišćan, A.; Reljić, S.; Domazetović, Z.; Frković, A.; Majnarić, D.; Majić- Skrbinšek, A.; Sindičić, M.; Šprem, N.; Modrić, M.; et al. Brown Bear Management Plan for the Republic of Croatia; Ministry of Regional Development, Forestry and Water Management, Directorate for the Protection of Nature: Zagreb, Croatia, 2019. [Google Scholar]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science. 2014, 346, 1517–1519. [Google Scholar] [CrossRef]
- Ordiz, A.; Kindberg, J.; Sæbø, S.; Swenson, J.E.; Støen, O.G. Brown bear circadian behaviour reveals human environmental encroachment. Biol. Conserv. 2014, 173, 1–9. [Google Scholar] [CrossRef]
- Martin, J.; Basille, M.; Van Moorter, B.; Kindberg, J.; Allainé, D.; Swenson, J.E. Coping with human disturbance: Spatial and temporal tactics of the brown bear (Ursus arctos). Can. J. Zool. 2010, 88, 875–883. [Google Scholar] [CrossRef]
- Mohammadi, A.; Nayeri, D.; Rosen, T. Conservation challenges of the brown bear (Ursus arctos) at the Southern edge of its global range. Int. Bear News. 2020, 29, 25–26. [Google Scholar]
- Can, Ö.E.; D’Cruze, N.; Garshelis, D.L.; Beecham, J.J.; Macdonald, D.W. Resolving human-bear conflict: A global survey of countries, experts, and key factors. Conserv. Lett. 2014, 7, 501–513. [Google Scholar] [CrossRef]
- Bourbonnais, M.L.; Nelson, T.; Cattet, M.R.L.; Darimont, C.T.; Stenhouse, G.B.; Janz, D.M. Environmental factors and habitat use influence body condition of individuals in a species at risk, the grizzly bear. Conserv. Physiol. 2014, 2, cou043. [Google Scholar] [CrossRef]
- Kusak, J.; Huber, Đ. Brown bear habitat quality in Gorski Kotar Croatia. Ursus 1995, 10, 281–291. [Google Scholar]
- Mangipane, L.S.; Belant, J.L.; Hiller, T.L.; Colvin, M.E.; Gustine, D.D.; Mangipane, B.A.; Hilderbrand, G.V. Influences of landscape heterogeneity on home-range sizes of brown bears. Mamm. Biol. 2018, 88, 1–7. [Google Scholar] [CrossRef]
- Kavčič, I.; Adamič, M.; Kaczensky, P.; Krofel, M.; Kobal, M.; Jerina, K. Fast food bears: Brown bear diet in a hu-man-dominated landscape with intensive supplemental feeding. Wildl. Biol. 2015, 21, 1–8. [Google Scholar] [CrossRef]
- Huber, D.; Roth, H.U. Denning of brown bears in Croatia. Bears Biol. Manag. 1997, 9, 79. [Google Scholar] [CrossRef]
- Dahle, B.; Støen, O.-G.; Swenson, J.E. Factors influencing home-range size in subadult brown bears. J. Mammal. 2006, 87, 859–865. [Google Scholar] [CrossRef]
- Dahle, B.; Swenson, J.E. Seasonal range size in relation to reproductive strategies in brown bears Ursus arctos. J. Anim. Ecol. 2003, 72, 660–667. [Google Scholar] [CrossRef]
- Belant, J.L.; Kielland, K.; Follmann, E.H.; Adams, L.G. Interspecific resource partitioning in sympatric ursids. Ecol. Appl. 2006, 16, 2333–2343. [Google Scholar] [CrossRef]
- Hilderbrand, G.V.; Robbins, C.T. Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial eco-system. Oecologia 1999, 121, 546–550. [Google Scholar] [CrossRef]
- Van Daele, L.J.; Barnes, V.G.; Belant, J.L. Ecological flexibility of brown bears on Kodiak Island, Alaska. Ursus 2012, 23, 21–29. [Google Scholar] [CrossRef]
- Hilderbrand, G.V.; Farley, S.D.; Robbins, C.T.; Hanley, T.A.; Titus, K.; Servheen, C. Use of stable isotopes to determine diets of living and extinct bears. Can. J. Zool. 1996, 74, 2080–2088. [Google Scholar] [CrossRef]
- Geiser, F. Hibernation. Curr. Biol. 2013, 23, 188–193. [Google Scholar] [CrossRef]
- Gunther, K.A.; Bramblett, A.M.; Weselmann, R.J. Grizzly bear consumption of midges in Yellowstone National Park. Ursus 2018, 29, 51–57. [Google Scholar] [CrossRef]
- Pereira, J.; Viličić, L.; Rosalino, L.M.; Reljić, S.; Habazin, M.; Huber, Đ. Brown bear feeding habits in a poor mast year where supplemental feeding occurs. Ursus 2021, 32e1, 1–13. [Google Scholar] [CrossRef]
- Todorov, V.R.; Zlatanova, D.P.; Valchinkova, K.V.; Society, B.W. Sofia University “St. Kliment Ohridski” Home range, mobility and hibernation of brown bears (Ursus arctos, Ursidae) in areas with supplementary feeding. Nat. Conserv. Res. 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Keis, M.; Tammeleht, E.; Valdmann, H.; Saarma, U. Ants in brown bear diet, and discovery of a new ant species for Estonia from brown bear scats. Hystrix It. J. Mamm. 2019, 30, 112–119. [Google Scholar]
- Tosoni, E.; Mei, M.; Ciucci, P. Ants as food for Apennine brown bears. Eur. Zool. J. 2018, 85, 342–348. [Google Scholar] [CrossRef]
- Seryodkin, I.V. Behavior of brown bears during feeding in the Sikhote-Alin. Achiev. Life Sci. 2016, 10, 38–47. [Google Scholar] [CrossRef][Green Version]
- Clevenger, A.P.; Purroy, F.J.; Pelton, M.R. Food habits of brown bears (Ursus arctos) in the Cantabrian Mountains, Spain. J. Mammal. 1992, 73, 415–421. [Google Scholar] [CrossRef]
- De Angelis, D.; Huber, Đ.; Reljic, S.; Ciucci, P.; Kusak, J. Factors affecting the home range of Dinaric-Pindos brown bears. J. Mammal. 2021, 102, 481–493. [Google Scholar] [CrossRef]
- McLoughlin, P.D.; Ferguson, S.H.; Messier, F. Intraspecific variation in home range overlap with habitat quality: A comparison among brown bear populations. Evol. Ecol. 2000, 14, 39–60. [Google Scholar] [CrossRef]
- Mitchell, M.S.; Powell, R.A. A mechanistic home range model for optimal use of spatially distributed resources. Ecol. Model. 2004, 177, 209–232. [Google Scholar] [CrossRef]
- Huber, D.; Roth, H.U. Home ranges and movements of brown bears in Plitvice Lakes National Park, Yugoslavia. Bears Biol. Manag. 1986, 6, 93. [Google Scholar] [CrossRef]
- Gonzáles-Bernardo, E.; Russo, L.F.; Valderrábano, E.; Fernández, Á.; Penteriani, V. Denning in brown bears. Ecol. Evol. 2020, 10, 6844–6862. [Google Scholar] [CrossRef]
- Evans, A.L.; Singh, N.J.; Friebe, A.; Arnemo, J.M.; Laske, T.G.; Fröbert, O.; Swenson, J.E.; Blanc, S. Drivers of hibernation in the brown bear. Front. Zool. 2016, 13, 17. [Google Scholar] [CrossRef]
- Lukač, G. Influence of visitor numbers on breeding birds in the Paklenica National Park, Croatia. Ekologia 2005, 24, 186–199. [Google Scholar]
- Penzar, B.; Penzar, I. Velebit—Klimatska prekretnica. Paklenički Zb. 1995, 1, 11–15. [Google Scholar]
- Adžić, I. (Paklenica National Park, Starigrad, Croatia). unpublished work.
- Kučinić, M.; Ješovnik, A.; Bujan, J.; Zec, M.; Čolić, L.; Pavliš, I.; Penezić, M.; Bračko, G. Inventarizacija mirmekofaune nacionalnog parka Paklenica. In Zbornik Istraživačkih Radova Udruge Studenata Biologije—”BIUS” u Nacionalnom Parku Paklenica; Zagreb, Croatia, 2011; pp. 31–47. [Google Scholar]
- Croatian Bureau of Statistics. Population by Age and Sex, by Settlements, 2011 Census; Croatian Bureau of Statistics: Zagreb, Croatia, 2011. [Google Scholar]
- Franjo, Š.; (Paklenica National Park, Starigrad, Croatia). Personal communication, 2020.
- Skrbinšek, T.; Jelenčič, M.; Luštrik, R.; Konec, M.; Boljte, B.; Jerina, K.; Černe, R.; Jonovič, M.; Bartol, M.; Huber, Đ.; et al. Genetic estimates of census and effective population sizes of brown bears in northern Dinaric mountains and south-eastern alps. Rep. Life 2017. Available online: https://dinalpbear.eu/wp-content/uploads/DAB2015.C5.PopulationSizeEstimateFinalReport_Skrbin%C5%A1ek-et-al.2017.pdf (accessed on 15 December 2021).
- Matson, G.; Van Daele, L.; Goodwin, E.; Aumiller, L.; Reynolds, H.; Hristienko, H. A Laboratory Manual for Cementum Age Determination of Alaska Brown Bear First Premolar Teeth; Alaska Department of Fish and Game Division of Wildlife Conservation: Juneau, AK, USA, 1993.
- Horne, J.S.; Garton, E.O.; Krone, S.M.; Lewis, J.S. Analyzing animal movements using brownian bridges. Ecology 2007, 88, 2354–2363. [Google Scholar] [CrossRef]
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Ćirović, D.; de Gabriel Hernando, M.; Paunović, M.; Karamanlidis, A.A. Home range, movements, and activity patterns of a brown bear in Serbia. Ursus 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Mancinelli, S.; Boitani, L.; Ciucci, P. Determinants of home range size and space use patterns in a protected wolf (Canis lupus) population in the central Apennines, Italy. Can. J. Zool. 2018, 96, 828–838. [Google Scholar] [CrossRef]
- Jenness, J. Calculating landscape surface area from digital elevation models. Wildl. Soc. Bull. 2004, 32, 829–839. [Google Scholar] [CrossRef]
- Friedberg, J.; Kochanny, C.O. Quantifying home-range overlap: The importance of the utilization distribution. J. Wildl. Mang. 2005, 69, 1346–1359. [Google Scholar]
- Ordiz, A.; Støen, O.-G.; Sæbø, S.; Kindberg, J.; Delibes, M.; Swensson, J.E. Do bears know they are being hunted? Biol. Conserv. 2012, 152, 21–28. [Google Scholar] [CrossRef]
- McNab, B.K. Bioenergetics and the determination of home range size. Am. Nat. 1963, 97, 133–140. [Google Scholar] [CrossRef]
- Minta, S.C. Tests of spatial and temporal interaction among animals. Ecol. Appl. 1992, 2, 178–188. [Google Scholar] [CrossRef]
- Seryodkin, I.V.; Pikunov, D.G.; Kostyria, A.V.; Goodrich, J.M. The fall season in the lives of bears in the Skihote-Alin Reserve. In Skihote-Alin Biosphere District: Condition of Ecosystems and Their Components; Dalanauka: Vladivostok, Russia, 2012. [Google Scholar]
- Huber, D.; Roth, H.U. Movements of European brown bears in Croatia. Acta Theériol. 1993, 38, 151–159. [Google Scholar] [CrossRef]
- Seryodkin, I.V.; Leacock, W.B.; Paczkowski, J.; Petrunenko, Y.K. Seasonal home ranges and movements of brown bears Ursos arctos in the Kuril lake basin (Southern Kamchatka). J. Becmник Ceвepo-Bocmoчнoгo Hayчнoгo Цeнmpa Двo Paн 2018, 3, 80–90. [Google Scholar]
- Maher, C.R.; Lott, D.F. A review of ecological determinants of territoriality within vertebrate species. Am. Midl. Nat. 2000, 143, 1–29. [Google Scholar] [CrossRef]
- Frank, S.C.; Leclerc, M.; Pelletier, F.; Rosell, F.; Swenson, J.E.; Bischof, R.; Kindberg, J.; Eiken, H.G.; Hagen, S.B.; Zedrosser, A. Sociodemographic factors modulate the spatial response of brown bears to vacancies created by hunting. J. Anim. Ecol. 2017, 87, 247–258. [Google Scholar] [CrossRef]
- McNellan, B. Density-dependent population regulation of brown bears. Int. Conf. Bear Res. Manag. 1994, 3, 3–34. [Google Scholar]
- Pigeon, K.E.; Côté, S.D.; Stenhouse, G.B. Assessing den selection and den characteristics of grizzly bears. J. Wildl. Manag. 2016, 80, 884–893. [Google Scholar] [CrossRef]
- Gilman, R.T.; Mathews, N.E.; Skinner, B.G.; Julis, V.L.; Frank, E.S.; Paul-Murphy, J. Effects of maternal status on the movement and mortality of sterilized female white-tailed deer. J. Wildl. Manag. 2010, 74, 1484–1491. [Google Scholar] [CrossRef]
- Ciarniello, L.M.; Boyce, M.; Seip, D.R.; Heard, D.C. Grizzly bear habitat selection is scale dependent. Ecol. Appl. 2007, 17, 1424–1440. [Google Scholar] [CrossRef]
| September 2019 | October 2019 | November 2019 | September–November 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bear ID | Age (years) | BBMM (95%) | MCP (95%) | BBMM (95%) | MCP (95%) | BBMM (95%) | MCP (95%) | BBMM (95%) | MCP (95%) |
| B95 | 5 | 6.0 | 9.0 | 5.4 | 8.2 | 2.7 | 3.7 | 9.3 | 16.3 |
| B97 | 7 | 4.8 | 11.1 | 6.6 | 12.6 | 0.2 | 0.2 | 7.5 | 14.5 |
| Bear ID | September 2019 | October 2019 | November 2019 |
|---|---|---|---|
| B95 | 723.0 ± 370.3 | 568.3 ± 282.7 | 534.4 ± 223.6 |
| B97 | 760.8 ± 400.1 | 570.3 ± 326.0 | 562.5 ± 163.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, L.; De Angelis, D.; Babic, N.; Reljić, S. Very Small Home Ranges of Two Gravid European Brown Bears during Hyperphagia. Animals 2021, 11, 3580. https://doi.org/10.3390/ani11123580
Schulte L, De Angelis D, Babic N, Reljić S. Very Small Home Ranges of Two Gravid European Brown Bears during Hyperphagia. Animals. 2021; 11(12):3580. https://doi.org/10.3390/ani11123580
Chicago/Turabian StyleSchulte, Laura, Daniele De Angelis, Natarsha Babic, and Slaven Reljić. 2021. "Very Small Home Ranges of Two Gravid European Brown Bears during Hyperphagia" Animals 11, no. 12: 3580. https://doi.org/10.3390/ani11123580
APA StyleSchulte, L., De Angelis, D., Babic, N., & Reljić, S. (2021). Very Small Home Ranges of Two Gravid European Brown Bears during Hyperphagia. Animals, 11(12), 3580. https://doi.org/10.3390/ani11123580






