Simple Summary
The study aims to define the potential and sustainable use of pre-commercial product ITTINSECT™ APS V1 as fish meal replacement in the diets of rainbow trout Oncorhynchus mykiss. For this, a 60 days feeding trail was performed by using diets with different replacing rate of fishmeal (0 (ITTM0), 25 (ITT25), 50 (ITT50), 75 (ITT75) and 100 (ITT100) %) with ITTINSECT™ APS V1. At the end of the feeding trial, significantly higher growth performance was observed in the group fed with the diet with a 25% and 50% substitution of fish meal by ITTINSECT™ APS V1. Moreover, the growth-related gene expressions analyzed in muscle tissue had significantly higher gene expression levels for these two diets (25% and 50% substitution) when compared to the control. The hematology values were found to be identical, whereas other parameters (such as serum total protein, globulins and glucose levels, or some immune-related gene expression) had different results among the experimental groups. In conclusion, replacement of fish meal with up to 50% ITTINSECT™ APS V1 in diets for rainbow trout is suggested in order to achieve the best growth performance in rainbow trout and enhance sustainable aquaculture practices.
Abstract
The aim of the study was to determine the potential and sustainable use of pre-commercial product ITTINSECT™ APS V1 as a major protein source in rainbow trout (Oncorhynchus mykiss) diets. A 60-day feeding experiment was conducted to potentially use ITTINSECT as fish meal replacement in the diets of rainbow trout. Five isonitrogenous in dry matter (38% crude protein) and isolipidic (15% crude lipid) diets were produced: a control diet (fishmeal-based) (ITT0) and four experimental diets replacing fishmeal by 25 (ITT25), 50 (ITT50), 75 (ITT75) and 100 (ITT100) %, with ITTINSECT™ APS V1. Triplicate tanks, containing 15 fish each (65.81 ± 1.26 g), were hand-fed to apparent satiation twice every day during the experiment. At the end of the feeding trial, significantly higher growth performance was observed in the group fed ITTM25 and ITTM50 diets. This performance was supported by growth-related gene expressions analyzed in muscle; significantly higher GH and IGF-I genes expression levels were determined in ITT25 and ITT50 when compared to control (ITT0) (p < 0.05). While no significant differences were found between the hematology values (p > 0.05), serum total protein, globulins and glucose levels were significantly different between experimental groups (p < 0.05). In addition to this, the immune-related genes such as TNF-α, IL8 and IL1-β expression levels were determined to be significantly different (p < 0.05). In conclusion, in order to achieve the best growth performance in rainbow trout and enhance sustainable aquaculture practices, replacement of fish meal with up to 50% ITTINSECT™ APS V1 in diets for rainbow trout is suggested.
1. Introduction
Fish meal (FM) is a costly raw material that increases the feed costs in aquaculture [1,2]. As a result, research is being conducted to determine the feasibility of using alternative plant or animal sources in fish feeds, which will allow for more cost-effective feed production [3,4]. The rapid increase of the aquaculture industry worldwide also increases the need for such feed. However, it is very difficult to meet the current demand by producing a fish-meal-based feed made of wild-caught fish. On the other hand, the use of these species as human food adversely affects the future of the aquaculture feed industry [5]. In order to ensure sustainable fish production, the use of vegetable protein sources in feeds has become very common. However, in carnivorous species such as rainbow trout, even soy, the richest plant-based protein is problematic regarding flavor, antinutritional factors, and the ability to meet daily nutritional needs [6,7]. The use of plant sources in aquaculture feeds is still seen as an unsolved problem [8]. Apart from vegetable protein sources, the European Commission/Annex II of Regulation 2017/893 of 24 May 2017 approved the use of insect meal as an ingredient in fish feeds. Thus, studies using some insect species in fish feed are available in the literature [9,10,11,12]. Apart from obtaining fish living in nature by hunting, insect meal can be easily grown under controlled conditions and has low ecological impact and ammonia release. It is also very low in consumption as human food [13,14]. In addition, thanks to its balanced protein, amino acid and fatty acid content, insect meal is considered to be a suitable protein source that can be used instead of fish meal in aquaculture feeds [9].
In general, the observation of growth response is not enough to identify the impact of feeding on the health of fish. The changes in blood parameters that are based on feeding activities give a more reliable indication of the health state of fish [15,16].
The ITTINSECT™ APS V1 product line is a pre-commercial, sustainable aquaculture feed especially developed to have zero impact on the ocean. The Italian company Ittinsect s.r.l. is developing the product with the inclusion of alternative protein sources that improve the organic performance of finfish species. The product contains high percentages of yellow mealworm (Tenebrio molitor) and black soldier fly (Hermetia illucens) meals, processed with different methods to balance proteins, lipids and chitin. The nutritional profile of the feed was completed with the inclusion of plant- and animal-based nutrients derived from upcycled by-products, with the aim of replicating the nutritional profile of fishmeal.
As of today, there little scientific information regarding the effects of aquaculture feeds including a major insect protein component. Furthermore, to the best of our knowledge, it is the first time that a feed ingredient has been engineered using more than one species of insect at the same time and using a different processing method for each species of insect.
Thus, the present study aims to report the first results on the effects of the ITTINSECT™ APS V1 (ITTM) ingredient on growth performance, serum biochemical variables, haematological parameters, dietary chemical composition, proximate composition fillet amino acid and the fatty acid contents of rainbow trout (Oncorhynchus mykiss).
2. Materials and Methods
2.1. Experimental Diets, Sustainability and Economic Assessment
Among the insect ingredients, 50% of weight was black soldier fly and 50% of weight was meal worm. Black soldier fly is defatted, while mealworm is full-fat. Full-fat ingredient was used to add a relevant lipid percentage to the overall formula, as well as to reduce the economic and environmental cost of de-fatting insect meals. In a full-fat insect meal, the relative percentage of chitin is smaller than in a de-fatted insect meal, hence more inclusion of insect by weight could be used. Both black soldier fly and mealworm received an enzymatic pre-treatment to increase nutrient absorption. The enzymatic process cannot be given in detail because it is under consideration for a patent. The Ittinsect APS mix was complemented with lyophilised yeasts collected from brewery by-products.
The experimental diets were produced in the laboratory. All dry ingredients were carefully mixed with a laboratory feed mixer for diet preparation. The mixtures were primed with tap water to yield a suitable pulp. Wet diets were assembled into 3-mm pellets by laboratory scale pellet mill (Pellet Mill, SYSLJ-1, Henan, China), dried at 40 °C in a drying cabinet for 18 h and stored at −20 °C until use. Five isonitrogenous (38% protein) and isolipidic (15% crude lipid) diets in dry matter were produced for the experiment. The control diet was FM-based, and four experimental diets replaced FM with ITTM by 25%, 50%, 75% and 100%. The formulation, feed cost and nutrient composition of the experimental diets and major protein components are shown in Table 1. The prices were set according to the local market retail prices (at the current time) of diets. These prices (US $/kg) are as follows: herring FM, 2.035; soybean meal, 0.44; ITTM 1.61; wheat meal, 0.24; corn starch, 0.24; fish oil, 2.05; and vitamin-mineral premix, 0.50.

Table 1.
Ingredients (g kg−1), composition of the experimental diets and essential amino acids (AA) profile (as % of protein) in anchovy fish meal (AFM), ITTINSECT® meal (ITTM), and experimental diets containing graded replacement levels of AFM with ITT on protein unit basis as well as amino acid requirements for Rainbow trout as (% of dietary protein).
Accordingly [17], the following formulae were used to compute the relative utilization values of marine-derived feed components, fish meal (FMU) and fish oil (FOU):
- FMU (g/kg of fish gain) = fish meal share in the diet (g/kg) × (feed intake (g)/body weight gain (g))
- FOU (g/kg of fish gain) = fish oil share in the diet (g/kg) × (feed intake (g)/body weight gain (g))
The currency type for economic evaluations is the US dollar ($). The economic conversion ratio (ECR) was calculated using the following equation:
- The economic conversion ratio (ECR) and economic profit index (EPI) were computed by [18] to measure the economic relative efficiency and advantages of the tested diet cost of feed per unit of fish gain.
- ECR ($/kg of fish gain) = (feed intake (g)/body weight gain (g)) × cost of feed ($/kg)
- EPI ($/fish−1) = [weight gain (kg) × selling price (4 $ kg−1)] − [weight gain (kg) × diet price ($ kg−1)]
- Rainbow trout sale price calculated at 4 $ kg−1.
2.2. Fish and Feeding Trial
A 60-day experiment was carried out on 225 rainbow trout obtained from a private fish farm (Çanakkale, Turkey). At the begining of the experiment, fish were weighted individually (65.81 ± 1.26 g) and divided into fifteen fiberglass tanks of 0.25 m3 equipped with recirculating aquaculture system (RAS), with three replicates per experimental diets. The experimental fish were acclimated to the experimental tanks for one week and fed with extruded 3 mm commercial rainbow trout diet (Agromey, Turkey) prior to the start of the experiment. The experimental fish were fed two times a day until apparent satiation. During the acclimatization and experimentation periods, the fish were held at ambient temperatures of 12 ± 1 °C, dissolved oxygen of 7.02 ± 0.78 mg L−1 and pH of 7.22 ± 0.46.
2.3. Chemical Analyses of Feed and Fillets
Feed and fish (five fish per tank) samples were analyzed for proximate composition according to AOAC [19] at the end of trial. Following AOAC’s methods [18], dry matter (AOAC, 934.01), Ash (AOAC, 942.05) and proteins (N × 6.25; AOAC, 955.04) were determined. All experimental diets and fish fillet samples for amino acid (AA) analysis were first freze-dried and homogenized by mortar. Homogenized samples were hydrolyzed with 6 N HCl (0.1 g 20 mL−1) 24 h at 110 °C under nitrogen atmosphere [20]. Amino acids were determined by Shimadzu LC-MS 8040 (Shimadzu corporation Kyoto, Japan) [21]. The separation column Zorbax Eclipse AAA 4.6 × 150 mm, 3.5 µm was used in the analysis. Mobile phase A was composed of formic acid, water 1/1000 (v/v) and mobile phase B formic acid, methanol 1/1000 (v/v). For flow rates of 1 mL min−1, the column temperature was set at 40 °C, and the injected sample volume was 0.2 μL. The certified L-amino acid mix standard (Sigma-Aldrich 79248) for calibration was used for AA analysis [22].
2.4. Blood Sampling and Analyses
Blood was collected from the fish at the end of the 60-day trial from a total of 15 fish per group, 5 from each tank. The fish were randomly selected from the test tanks and instantly fainted in a bucket containing a natural stunner and commonly used clove oil (20 mg L−1) [23]. A 2.5 mL plastic syringe was used to sample blood from the caudal vein. Blood samples were divided into K3EDTA and cellular serum tubes for hematological and serum biochemical analyses. The gland-plated blood was centrifuged at 5000× g for 10 min for serum analysis. The serum was then stored at −80 °C before the analyses. Red blood cells, haematocrit and haemoglobin concentration were determined by using the method of [24]. RBC was counted with a Thoma hemocytometer with the usage of Dacie’s diluting fluid. The haematocrit was determined by using a capillary hematocrit tube. The haemoglobin concentration was determined with spectrophotometry (540 nm) by using the cyanomethahemoglobin method. Biochemical indices such as glucose (GLU), total protein (TPROT), albumin (ALB), triglyceride (TRI), cholesterol (CHOL), alkaline phosphatase (ALP), glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT) and lactate dehydrogenase (LDH) in serum were analyzed using bioanalytical test kits (PG Instruments, Leicestershire, UK).
2.5. Gene Expression Analysis
To determinate growth-related gene expressions such as GH, and IGF-1 in muscle tissue and immune-related genes such as TNF-α, IL-1ß and IL-8 in liver tissue, samples from three fishes of each tank were kept in RNAlater (Thermo Scientific, Waltham, USA) solution at −20 °C for total RNA extraction for growth- and immune-related gene expression studies. Real-time qPCR data was analyzed using the efficiency corrected relative expression method with β-actin as a reference gene. The GeneJet RNA purification kit was used to isolate the RNA (Thermo Scientific, Waltham, USA). A MultiskanTM FC Microplate Photometer (Thermo Scientific, Waltham, USA) was used to assess the quality of extracted RNAs. DNase-I (Thermo Scientific, Waltham, USA) was used to separate DNA from RNAs, and RevertAid H Minus Single Strand cDNA Synthesis Kit was used to create cDNA (Thermo Scientific, Waltham, USA). NCBI web site; mRNA sequences of the β-actin; GH, IGF-I, IL-8, TNF-α, and IL-1β genes, which are unique to rainbow trout; and FastPCR 6.0 package software were used to create primers [25]. Table 2 lists primer sequences, total base length and gene bank numbers. Real-time PCR (Bio RAD, ABD) device was used with a view to identify the differences in gene expression levels of the experimental groups. PCR analysis was conducted with PCR mix, Maxima SYBR Green qPCR Master Mix and ROX Solution (Thermo Scientific, Waltham, USA). Analysis of real-time PCR results was conducted via CFX Manager 3.1 software. Proportional changes in mRNA expression levels of target genes were calculated through 2−ΔΔCt method based on cycle thresholds (Ct) of amplification curves obtained following amplification process comprising denaturation, annealing of primer and chain extension [26].

Table 2.
Primer sequences used in this study.
2.6. Calculations
Growth performance and feed utilization were calculated using following equations:
FCR (feed conversion ratio) = feed consumed/weight gain
RGR (relative growth rate %) = ((final wet weight − initial wet weight)/initial wet weight) × 100
SGR (specific growth rate %/day) = ((ln final wet weight − ln initial wet weight)/days) × 100
2.7. Statistical Analyses
All values were presented as means ± SD and analyzed by SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) followed by post-hoc Tukey’s test was used to reveal whether there were differences in the measured parameters among the treatments. All analyses were performed at a significance p level of 0.05.
3. Results
3.1. Growth Performance
All the test diets were comparable in terms of nutrients, such as protein, lipid and amino acid (AA) contents for rainbow trout. The AA composition of ITTINSECT and diets is presented in Table 1.
Growth parameters and survival rates are presented in Table 3. No mortality was observed during the experiment. After 60 days of feeding, growth performance, FCR and SGR values showed clear dietary supplementation effects of ITTINSECT (p < 0.05). The ITTM25 and ITTM50 groups showed the best values for WG, FCR and SGR, compared to the other experimental groups (p < 0.05). The relative FM usages in diets decreased per kg of fish gain. On the contrary, although FO usage in the diets did not change, it was found to be higher in groups due to low weight gain. Regarding the economic analyses, the different dietary levels of ITTM affected the diets cost and economic parameters such as ECR, EPI and PRO (p < 0.05) (Table 3).

Table 3.
Growth performance, sustainability and economic assessment of rainbow trout fed with experimental diets for 60 days.
3.2. Fillet Proximate Composition and Amino Acid Profile
The fillet chemical and AA compositions of the experimental fish fed with the experimental diets are shown in Table 4.

Table 4.
Fillet proximate composition and amino acid profile of rainbow trout fed with experimental diets for 60 days.
Based on the chemical composition crude protein, ash and moisture were not affected by the treatment. The inclusion of ITTM significantly increased crude lipid contents in all the four diets containing ITTM25, ITTM50, ITTM75 and ITTM100, compared to the ITTM0. Additionally, the high lipid content of the fillet in fish of the four ITTM diets did not show notable differences among them. Fillet essential amino acid (EAA) and non-essential amino acid (NEAA) content of rainbow trout fillets are presented in Table 4. Among EAAs of fillet leucine, lysine and valine showed significant decreases in the high dietary inclusion levels of ITTM (75 and 100%) (p < 0.05). For NEAAs such as glutamine, proline and serine showed similar trends and tended to decrease due to increase of ITTM levels in diets for rainbow trout (p < 0.05).
3.3. Hematological and Serum Biochemical Parameters
Hematological and serum biochemical parameters results are given in Table 5.

Table 5.
Hematological and serum biochemical parameters of rainbow trout, O. mykiss, fed diets containing different levels of ITTINSECT™ APS V1 meal (ITTM) for 60 days.
The RBC count, Hct and Hgb concentration in all experimental groups were not found significantly different (p > 0.05). Serum TRIG, CHOL, and ALB values showed no significant changes (p > 0.05), but TPROT, and GLO, a serum protein, tended to decline when replacement levels exceeded 75 percent, while significantly lower serum glucose level was determined in the group fed with ITTM75 diet compared control group (p < 0.05).
3.4. Gene Expression
Changes in gene expression responses of the fish after the 60-day feeding experiment with ITTM supplemented and control diet are presented in Figure 1 and Figure 2.
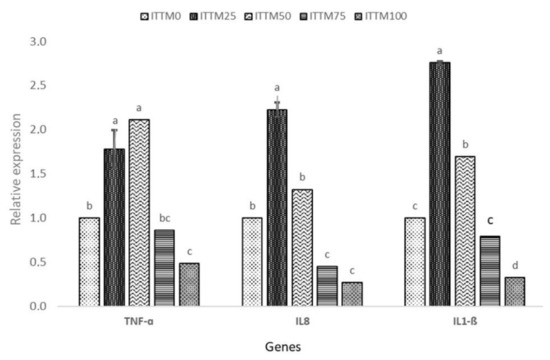
Figure 1.
Relative gene expression (mean ± SD) of TNF-α, IL-8 and IL-1β in the liver of rainbow trout fed with experimental diets. Values with common superscripts (a,b,c) are not significantly different (p > 0.05).
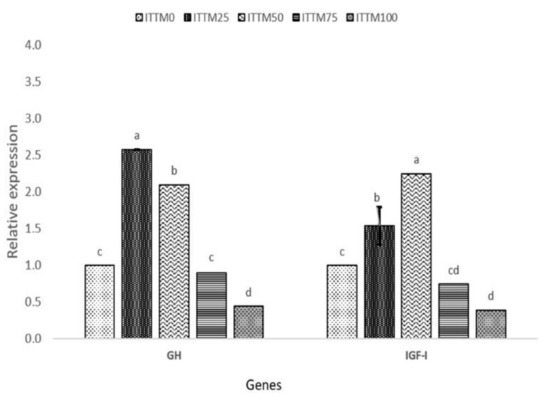
Figure 2.
Relative gene expression (mean ± SD) of GH and IGF-I in the muscle of rainbow trout fed with experimental diets. Values with common superscripts (a,b,c) are not significantly different (p > 0.05).
The fish fed with ITTM25 and ITTM50 diets showed a significantly higher expression of GH, IGF-I, TNF-α, IL-8 and IL-1β in the muscle and liver tissue compared to the other experimental groups (p < 0.05). Significant down-regulation was shown in fish fed with ITTM75 and ITTM100 diets (p < 0.05).
4. Discussion
It is well known that fish farming relies heavily on marine captures for the nutrition of carnivorous fish species. However, such fisheries are typically not sustainable, and an increased demand for livestock and aquaculture feeds has resulted in a rapid reduction in fishmeal availability. This has led to a rapid increase in prices, where feed expenses already absorb between 40 and 70 percent of the cost of farmed fish [9]. Therefore, in the past years several alternatives to FM have been explored, among which insect biomass stands out as a promising alternative protein source that could replace FM. Insects rich in amino acids, lipids, vitamins and minerals leave a significantly smaller ecological footprint, and some species even show anti-fungal activity and/or anti-fungal peptides that may increase the shelf/life of insect-containing feeds [9]. In the current study, a basic economic analysis of feed production revealed that raising the content of ITTM in fish diets reduced the diet cost from 1.36 to 1.17 US$ per kg diet (Table 1). Feed price, feeding rate, stocking density, fish size, fish production and fish sales are all important factors in determining the best profit in aquaculture [27]. According to the findings of this study, using ITTM as a FM replacer in fish diets up to 50% has a favorable economic impact, lowering feed costs by 6.62%. Similar results were reported by [28] when black soldier fly larvae meal was used as a FM replacer in European seabass (Dicentrarchus labrax) diets. The present experimental results showed the high environmental sustainability of ITTM use in rainbow trout diets. Due to improved feed utilization and growth performance in ITTM50 diet in the present study, FM use was decreased for rainbow trout production. Previous study on Siberian sturgeon using black fly meal reported reduction in FM up to 70% and FO up to 96.5% in fish diets [29].
The results of the growth performance showed that the inclusion of ITTM in diet for rainbow trout up to 50% did not lead to any adverse effect on growth performance. Previous studies reported that the use of insect-based meals such as yellow mealworm and black soldier fly as an alternative protein source for FM alone or in combination did not affect growth performance of fish species such as channel catfish (Ictalurus punctatus) and blue tilapia (Oreochromis aureus) [30]. Similarly, [31] demonstrated that defatted black soldier fly could entirely replace FM in Jian carp (Cyprinus carpio var. Jian) diets. According to [32], up to 19.5% of pre-pupae meal of black soldier fly can be successfully used in diets as a raw material of juvenile European seabass with no adverse effect on growth performance, feed utilization, apparent digestibility coefficients or digestive enzyme activity. Growth performance in rainbow trout was unaffected by dietary addition of 25% black soldier meal, while it was decreased by dietary inclusion of 50% black soldier fly meal [33]. Kroeckel et al. [34], on the other hand, found a substantial decline in turbot (Psetta maxima) growth performance for all FM substitutions with black soldiers fly meal. Replacement of FM with diets including yellow feedworm up to 100% did not result in any adverse effect on growth performance except for the case reported by Chemello et al. [35]. The present study is the first attempt to evaluate the ITTM effects as partial or total replacement of FM. The inconsistences in the results provided by Chemello et al. [35] could be due to the differences in fish species and sizes, insect species and cultivation area, and insect processing techniques [36].
In the present study, considering the proximate composition of the fillet of rainbow trout, no significant differences in moisture, protein and ash content emerged by inclusion of ITTM in to the diets. Nevertheless, a significant increase was obtained in fillet lipid composition with the increase of ITTM level in the diets. Melenchónet al. [37] reported similar results for rainbow trout fed with black soldier fly meal and stated that the substitution of fish meal used in rainbow trout diets with a low percentage of commercial black soldier fly meal tended to increases the amount of fillet lipid level. On the contrary, Belforti et al. [38] reported significant decreases of total lipids content, with increasing inclusion of Tenebrio molitor larvae meal diets for rainbow trout. The fluctuations in total lipid levels in the fish body can be connected to variations in synthesis alterations, muscle deposition rate and/or various growth rates in lipids [39]. In addition to this, Table 4 presents the effects of the experimental diets on the aminoacid composition of rainbow trout flesh. In the fillet of fish that consumed the ITTM50 diet, there was a substantial drop in amino acids such as Leu, Lys and Val, but they stayed constant in the ITTM25 feeding group compared to the ITTM0 group. A reducing trend in the same amino acids has been reported in rainbow trout fillet that were fed with mealworm larvae meal [40].
Hemato-biochemical variables are frequently utilized to assess nutritional status, health conditions and fish adaptability to the outside environment [41]. In this study, any changes in the values of several hematological parameters were observed after ITTM inclusion in diets for the rainbow trout (p > 0.05). Similarly, Abdel-Tawwb et al. [28] and Tippayadara et al. [42] reported that replacement of FM by black soldier fly meal had no effects on European sea bass and Nile tilapia (Oreochromis niloticus) hematological parameters, respectively. An increase in serum total protein, albumin and globulin levels is a good indicator of the health and immunological status of fish [43,44]. Insect meal chitin has been related to animal immunopotentiation, and this has been shown in fish and shrimps [11,45,46]. Although, there were significant changes in the total serum protein, and in globulin from ITTM0, ITTM25, ITTM50 and ITTM100 in the current investigation, a modest improvement in fish given low ITTM levels (25%) was seen, indicating a potential immunostimulation of ITTM owing to chitin content. The presence of chitin has been reported to play a role also in triglyceride hydrolysis [47]. Accordingly, in the present study no significant differences among all groups were observed in terms of serum cholesterol and triglyceride values. Although some studies reported significantly lower cholesterol levels in fish fed with insect meal than in those fed with fish-meal-based diets [33], our study found no obvious change in cholesterol of the ITTM groups compared to the control group. These disparities may be also due to the different fish sizes, insect species, insect matrix and processing methods [38]. Cholesterol metabolism in fish differs from that in terrestrial animal models, and there are several processes involved in the regulation of cholesterolemia that require more investigations [48]. Similar results for serum cholesterol levels were reported by Lu et al. [49] in grass carp (Ctenopharyngodon idella) fed with black soldier fly larvae meal.
In salmonids, GH plays an important function in somatic development by promoting a synthesis of protein, feed intake and feeding efficiency [50,51]. Furthermore, GH and IGF-1 participate in many other fish activities, including feeding, predator prevention and osmoregulation [52]. In this study, and in line with growth performance results, differences were observed in the expression of GH receptors or IGF-I transcripts among diets. In our study, rainbow trout received their nutritional requirements according to apparent satiation, but fish fed ITTM75 and ITTM100 diets showed lower feed utilization than other diets. The lower expression of GH and IGF-I in muscle of fish fed with these two diets possibly indicates a lower level of nutrition and growth rate. Immune-related genes, such as TNF-α, Il-8 and IL-1β, showed a significant up-regulation among the test diets ITTM25 and ITTM50. Similar findings were reported by Hender et al. [53] and Zarantoniello et al. [54] in Zebrafish (Danio rerio). Additionally, Kumar et al. [55] found an up-regulation in kidney of rainbow trout when fish were fed with up to 16% black soldier fly supplemented diets. Although, chitin in aquafeeds with positive effects on the fisheries immune system is found in all insect-based diets [10] and may cause intestinal inflammation and decline in the assimilation of nutrition (>30%). The increased incorporation of ITTM in rainbow trout liver in the present investigation has shown significant amounts of chitin that have detrimental impacts on the expression of immune-related genes.
In conclusion, this study demonstrated that at least 50% of FM protein could be replaced with ITTM without any adverse effects on growth performance, fillet composition, blood parameters, growth and immune-related gene expression of rainbow trout. Despite this, it is important to emphasize that the complete life-cycle of rainbow trout from juveniles to adults requires long-term research to produce an increase in ITTM levels to provide additional information about immune-relevant gene expression.
Author Contributions
Conceptualization, Ü.A., D.G., A.R. and F.F.; methodology, Ü.A., O.S.K., S.Y. and R.T.; validation, Ü.A., O.S.K. and S.Y.; formal analysis, Ü.A., O.S.K. and S.Y.; resources, Ü.A., A.G., O.S.K., S.Y. and A.R.; data curation, Ü.A., O.S.K. and S.Y.; writing—original draft preparation, Ü.A., A.G. and O.S.K.; writing—review and editing, Ü.A., D.G.; supervision, Ü.A., A.G., R.T., A.T., K.G. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animals Ethics Committee of Çanakkale Onsekiz Mart University (Çanakkale, Turkey, Approval Number: 2021/03-12).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors are grateful to Ittinsect S.R.L., Italy for donating ITTINSECT™ APS V1 for this experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Zotte, A.D.; De Angelis, A.; Piccolo, G.; Pinotti, L.; Schiavone, A.; et al. Protein hunger of the feed sector: The alternatives offered by the plant world. Ital. J. Anim. Sci. 2020, 19, 1204–1225. [Google Scholar] [CrossRef]
- EUMOFA. Fishmeal and Fish Oil, Maritime Affairs and Fisheries, Production and Trade Flows in the EU; EUMOFA: Brussels, Belgium, 2021. [Google Scholar]
- Staessen, T.W.O.; Verdegem, M.; Koletsi, P.; Schrama, J.W. The effect of dietary protein source (fishmeal vs. plant protein) and non-starch polysaccharide level on fat digestibility and faecal bile acid loss in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2019, 51, 1170–1181. [Google Scholar] [CrossRef] [Green Version]
- Sahin, T.; Yılmaz, S.; Gürkan, M.; Ergün, S. Effects of Rapana venosa meal-supplemented diets on reproduction, histopathology and some blood parameters of rainbow trout (Oncorhynchus mykiss) broodstock. Aquac. Res. 2021, 52, 4897–4910. [Google Scholar] [CrossRef]
- Fréon, P.; Sueiro, J.C.; Iriarte, F.; Evar, O.F.M.; Landa, Y.; Mittaine, J.-F.; Bouchon, M. Harvesting for food versus feed: A review of Peruvian fisheries in a global context. Rev. Fish. Biol. Fish. 2013, 24, 381–398. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Papatryphon, E.; Soares, J.H. The effect of dietary feeding stimulants on growth performance of striped bass, Morone saxatilis, fed-a-plant feedstuff-based diet. Aquaculture 2000, 185, 329–338. [Google Scholar] [CrossRef]
- Schader, C.; Muller, A.; El-Hage Scialabba, N.; Hecht, J.; Isensee, A.; Erb, K.H.; Smith, P.; Makkar, H.P.S.; Klocke, P.; Leiber, F.; et al. Impacts of feding less food-competing feedstuffs to livestock on global food system sustainability. J. R. Soc. Interface 2015, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Èntomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; Van Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed. 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens pre-pupae in the diet. Rev. Fish. Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; Brand, H.V.D.; van Loon, J.J.A.; van Huis, A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.; De Boer, I.J. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acar, U.; Kesbic, O.S.; Yilmaz, S.; Kesbic, F.I.; Gultepe, N. Gibel carp (Carassius auratus gibelio) meal as an alternative major protein in feeds for rainbow trout juveniles (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 2018, 19, 383–390. [Google Scholar]
- Acar, Ü.; Kesbiç, O.S.; Yilmaz, S.; Karabayir, A. Growth performance, haematological and serum biochemical profiles in rainbow trout (Oncorhynchus mykiss) fed diets with varying levels of lupin (Lupinus albus) meal. Aquac. Res. 2018, 49, 2579–2586. [Google Scholar] [CrossRef]
- Sunde, J.; Eiane, S.; Rustad, A.; Jensen, H.; Opstvedt, J.; Nygard, E.; Venturini, G.; Rungruangsak-Torrissen, K. Effect of fish feed processing conditions on digestive protease activities, free amino acid pools, feed conversion efficiency and growth in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2004, 10, 261–277. [Google Scholar] [CrossRef]
- Stejskal, V.; Tran, H.Q.; Prokesova, M.; Gebauer, T.; Giang, P.T.; Gai, F.; Gasco, L. Partially Defatted Hermetia illucens Larva Meal in Diet of Eurasian Perch (Perca fluviatilis) Juveniles. Animals 2020, 10, 1876. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; AOAC: Rockville, MD, USA, 1988. [Google Scholar]
- Rodrigues, A.P.O.; Bicudo, Á.J.A.; Moro, G.V.; Gominho-Rosa, M.D.C.; Gubiani, É.A. Muscle amino acid profile of wild and farmed pirarucu (Arapaima gigas) in two size classes and an estimation of their dietary essential amino acid requirements. J. Appl. Aquac. 2021, in press. [Google Scholar] [CrossRef]
- Sun, Z.; Xing, J.; Khoo, P.Y.; Zhan, Z.A. Combined MRM and SIM Method for Direct Quantitative Determination of Amino Acids in Various Samples on LC/MS/MS. In Proceedings of the American Society for Mass Spectrometry Congress, Sante Fe, NM, USA, 5–9 June 2016. [Google Scholar]
- Roy, C.; Tremblay, P.Y.; Bienvenu, J.F.; Ayotte, P. Quantitative analysis of amino acids and acylcarnitines combined with untargeted metabolomics using ultra-highperformance liquid chromatography and quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2016, 1027, 40–49. [Google Scholar] [CrossRef]
- Iversen, M.; Finstad, B.; McKinley, M.S.; Eliassen, R.A. The efficacy of metomidate, clove oil, Aqui-STM and Benzoak® as anaesthetics in Atlantic salmon (Salmo salar L.) smolts, and their potential stress-reducing capacity. Aquaculture 2003, 221, 549–566. [Google Scholar] [CrossRef]
- Blaxhall, P.C.; Daisley, K.W. Routine hematological methods for use with fish blood. J. Fish. Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Kalendar, R.; Lee, D.; Schulman, A.H. FastPCR Software for PCR Primer and Probe Design and Repeat Search. Genes Genomes Genom. 2009, 3, 1–14. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Hebicha, H.A.; El Naggar, G.O.; Nasr-Allah, A.M. Production Economics of Nile Tilapia (Oreochromis niloticus) Pond Culture in El-Fayum Governorate, Egypt. J. Appl. Aquac. 2013, 25, 227–238. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal Is More Profitable Than Fish Meal and Fish Oil in Siberian Sturgeon Farming: The Effects on Aquaculture Sustainability, Economy and Fish GIT Development. Animals 2021, 11, 604. [Google Scholar] [CrossRef]
- Bondari, K.; Sheppard, D.C. Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac. Res. 1987, 18, 209–220. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly Prepupae as a Feedstuff for Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.-G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Chemello, G.; Renna, M.; Caimi, C.; Guerreiro, I.; Oliva-Teles, A.; Enes, P.; Biasato, I.; Schiavone, A.; Gai, F.; Gasco, L. Partially Defatted Tenebrio molitor Larva Meal in Diets for Grow-Out Rainbow Trout, Oncorhynchus mykiss (Walbaum): Effects on Growth Performance, Diet Digestibility and Metabolic Responses. Animals 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed. 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Melenchón, F.; Larrán, A.; de Mercado, E.; Hidalgo, M.; Cardenete, G.; Barroso, F.; Fabrikov, D.; Lourenço, H.; Pessoa, M.; Tomás-Almenar, C. Potential use of black soldier fly (Hermetia illucens) and mealworm (Tenebrio molitor) insect meals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 491–505. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawwab, M. Effect of feed availability on susceptibility of Nile tilapia, Oreochromis niloticus (L.) to environmental zinc toxicity: Growth performance, biochemical response, and zinc bioaccumulation. Aquaculture 2016, 464, 309–315. [Google Scholar] [CrossRef]
- Iaconisi, V.; Secci, G.; Sabatino, G.; Piccolo, G.; Gasco, L.; Papini, A.M.; Parisi, G. Effect of mealworm (Tenebrio molitor L.) larvae meal on amino acid composition of gilthead sea bream (Sparus aurata L.) and rainbow trout (Oncorhynchus mykiss W.) fillets. Aquaculture 2019, 513, 734403. [Google Scholar] [CrossRef]
- Fazio, F.; Marafioti, S.; Torre, A.; Sanfilippo, M.; Panzera, M.; Faggio, C. Haematological and serum protein profiles of Mugil cephalus: Effect of two different habitats. Ichthyol. Res. 2013, 60, 36–42. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.; Krutmuang, P.; Hoseinifar, S.; Doan, H.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sahu, N.; Pal, A.; Kumar, S. Immunomodulation of Labeo rohita juveniles due to dietary gelatinized and non-gelatinized starch. Fish. Shellfish. Immunol. 2007, 23, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Fawole, F.J.; Sahu, N.P.; Pal, A.K.; Ravindran, A. Haemato-immunological response of Labeo rohita (Hamilton) fingerlings fed leaf extracts and challenged by Aeromonas hydrophila. Aquac. Res. 2016, 47, 3788–3799. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing Fish Meal with Defatted Insect Meal (Yellow Mealworm Tenebrio molitor) Improves the Growth and Immunity of Pacific White Shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, J.; Li, L.; Xia, W. Dietary chitosan improves hypercholesterolemia in rats fed high-fat diets. Nutr. Res. 2008, 28, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Shafaeipour, A.; Yavari, V.; Falahatkar, B.; Maremmazi, J.G.; Gorjipour, E. Effects of canola meal on physiological and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2008, 14, 110–119. [Google Scholar] [CrossRef]
- Lu, R.; Chen, Y.; Yu, W.; Lin, M.; Yang, G.; Qin, C.; Meng, X.; Zhang, Y.; Ji, H.; Nie, G. Defatted black soldier fly (Hermetia illucens) larvae meal can replace soybean meal in juvenile grass carp (Ctenopharyngodon idellus) diets. Aquac. Rep. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Raven, P.; Uh, M.; Sakhrani, D.; Beckman, B.; Cooper, K.; Pinter, J.; Leder, E.; Silverstein, J.; Devlin, R. Endocrine effects of growth hormone overexpression in transgenic coho salmon. Gen. Comp. Endocrinol. 2008, 159, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Oakes, J.; Higgs, D.; Eales, J.; Devlin, R. Influence of ration level on the growth performance and body composition of non-transgenic and growth-hormone-transgenic coho salmon (Oncorhynchus kisutch). Aquacuture 2007, 265, 309–324. [Google Scholar] [CrossRef]
- Campbell, B.; Dickey, J.; Beckman, B.; Young, G.; Pierce, A.; Fukada, H.; Swanson, P. Previtellogenic Oocyte Growth in Salmon: Relationships among Body Growth, Plasma Insulin-Like Growth Factor-1, Estradiol-17beta, Follicle-Stimulating Hormone and Expression of Ovarian Genes for Insulin-Like Growth Factors, Steroidogenic-Acute Regulatory Protein and Receptors for Gonadotropins, Growth Hormone, and Somatolactin1. Biol. Reprod. 2006, 75, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hender, A.; Siddik, M.; Howieson, J.; Fotedar, R. Black Soldier Fly, Hermetia illucens as an Alternative to Fishmeal Protein and Fish Oil: Impact on Growth, Immune Response, Mucosal Barrier Status, and Flesh Quality of Juvenile Barramundi, Lates calcarifer (Bloch, 1790). Biology 2021, 10, 505. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas-Abúndez, J.A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Tulli, F.; et al. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019, 9, 8598. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Fawole, F.J.; Romano, N.; Hossain, S.; Labh, S.N.; Overturf, K.; Small, B.C. Insect (black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish. Shellfish. Immunol. 2021, 109, 116–124. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).