Effects of Reduced Space Allowance and Heat Stress on Behavior and Eye Temperature in Unweaned Lambs: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
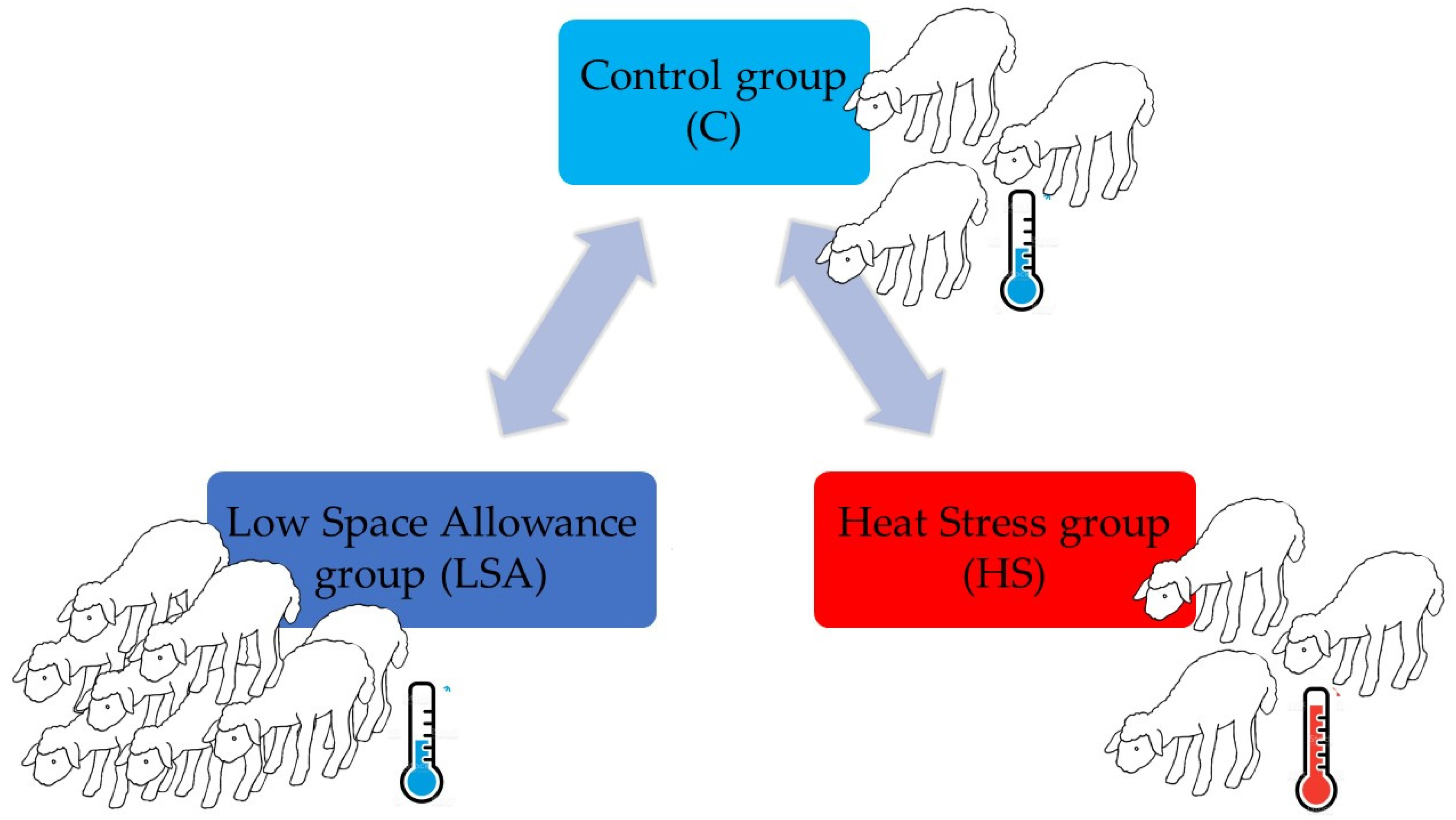
2.1. Experimental Design: Animals and Housing Conditions
2.2. Behavioral Observations
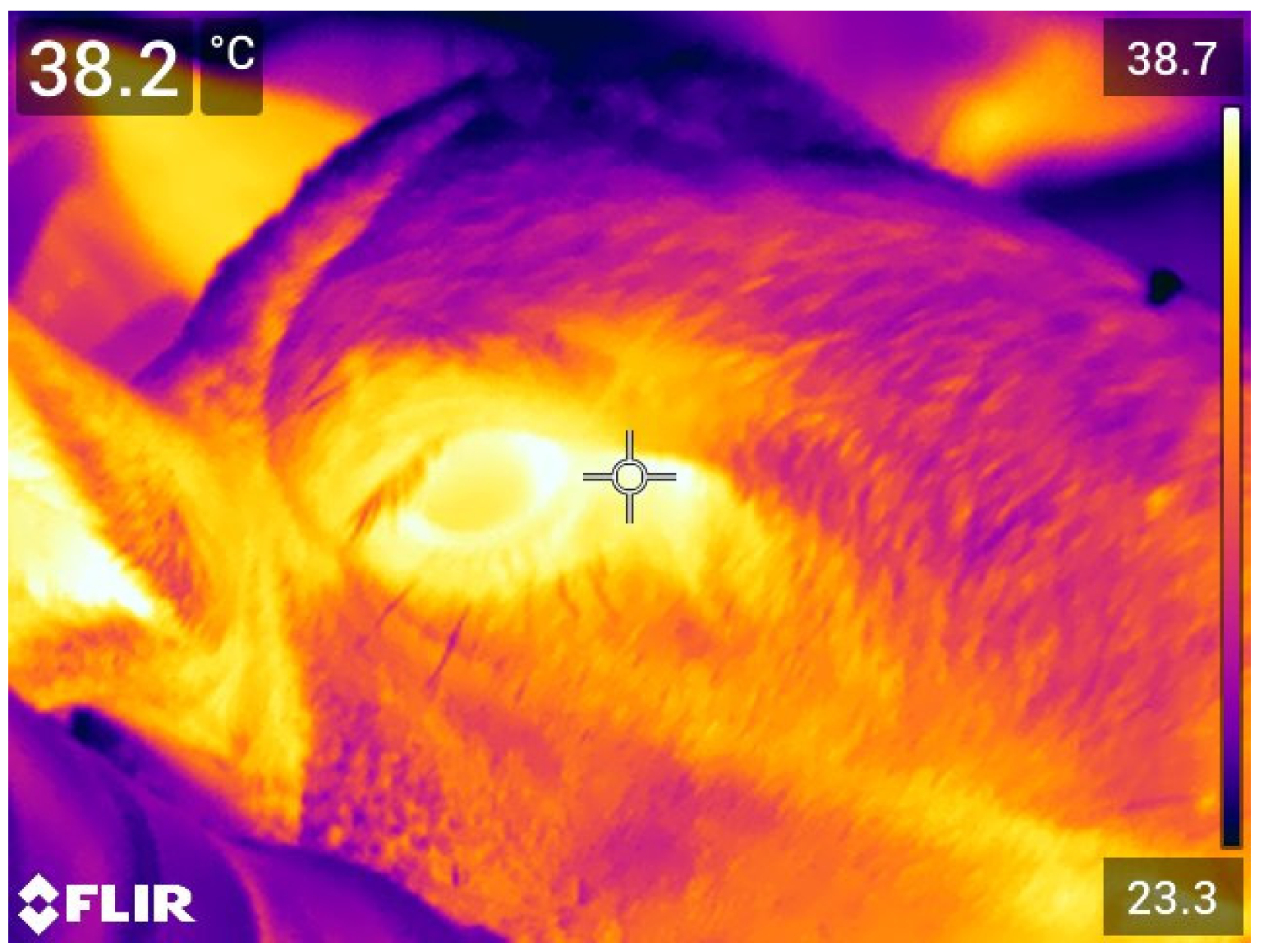
2.3. Eye Temperature (ET)
2.4. Statistical Analysis
3. Results
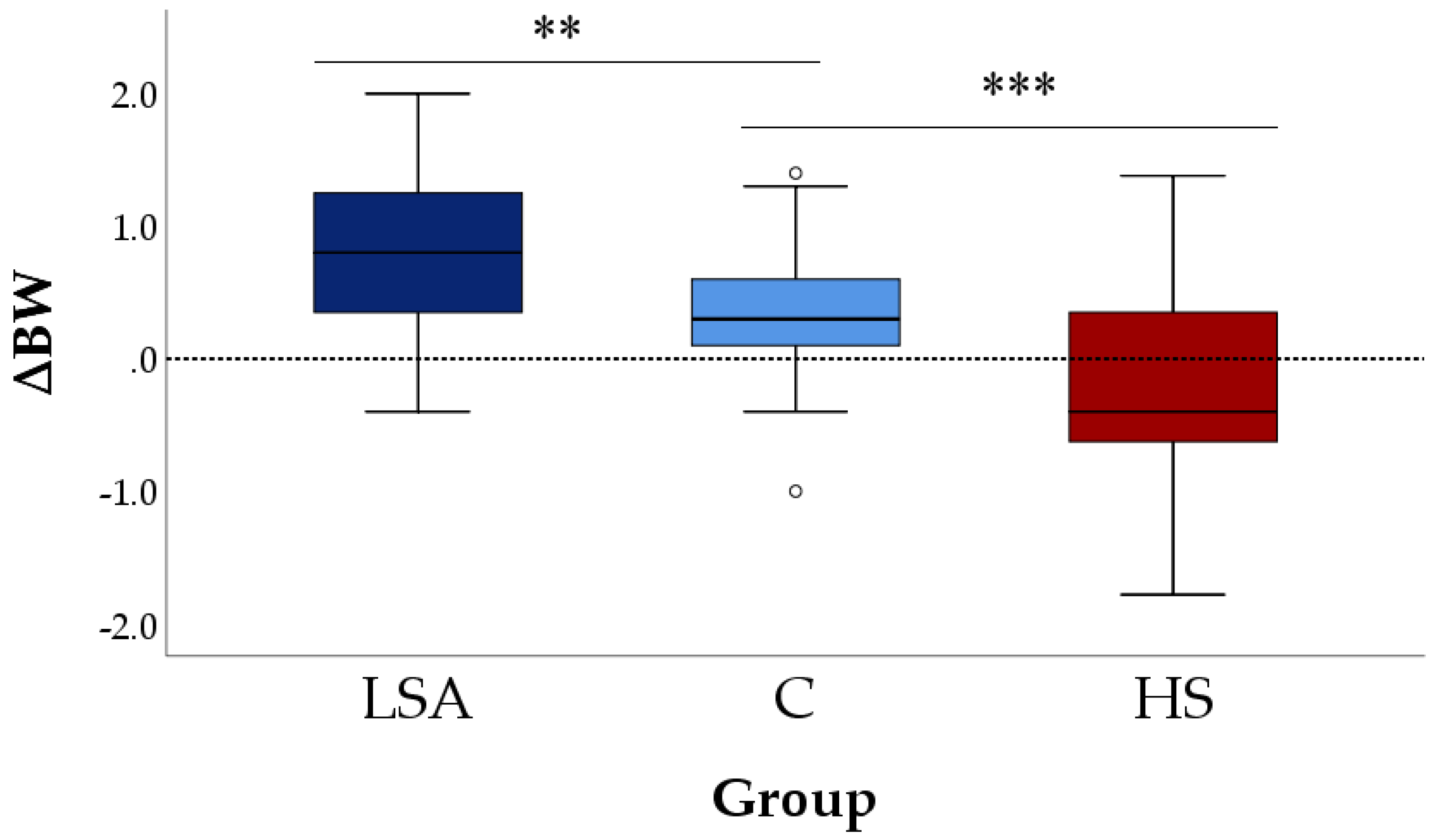
3.1. Body Weight Changes
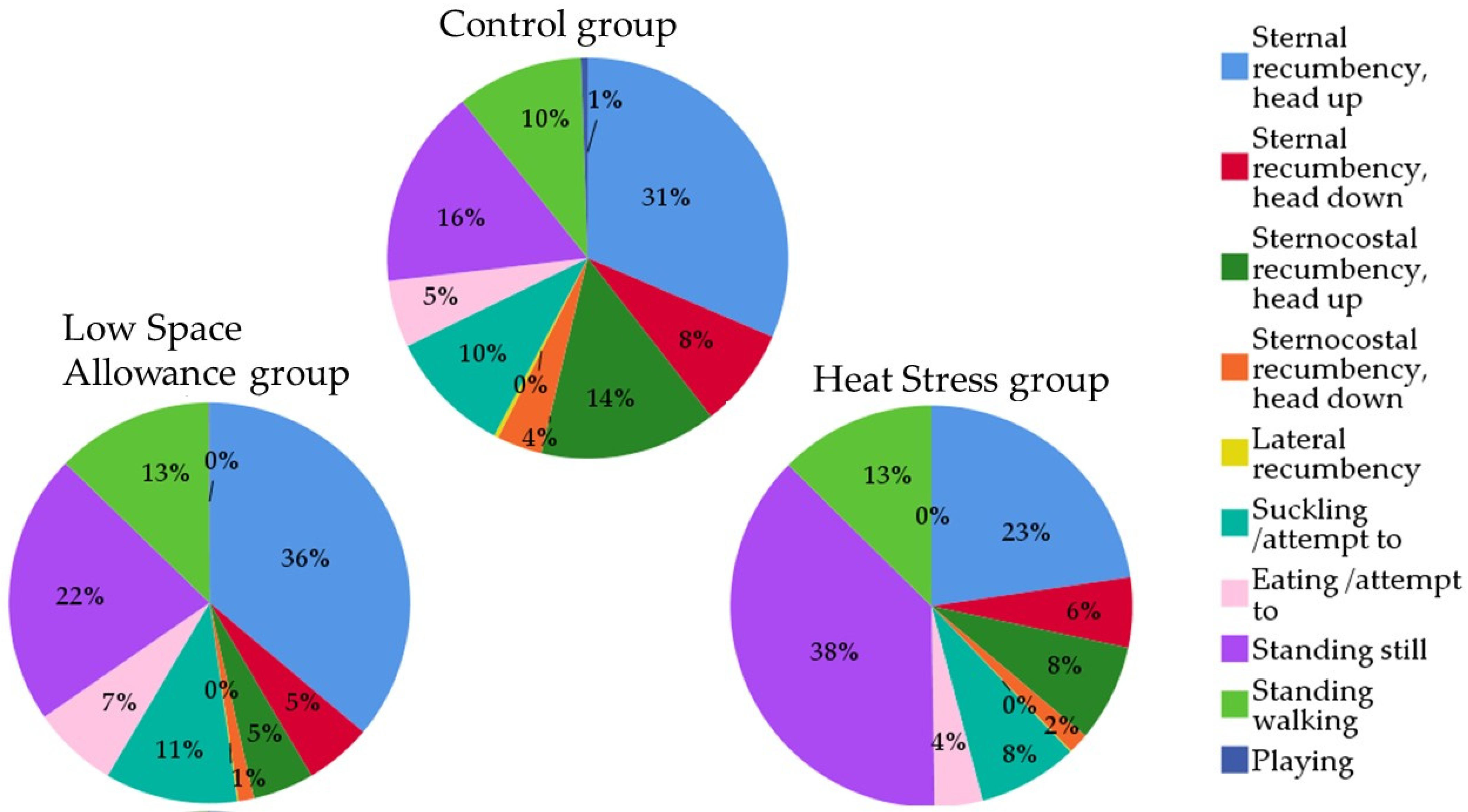
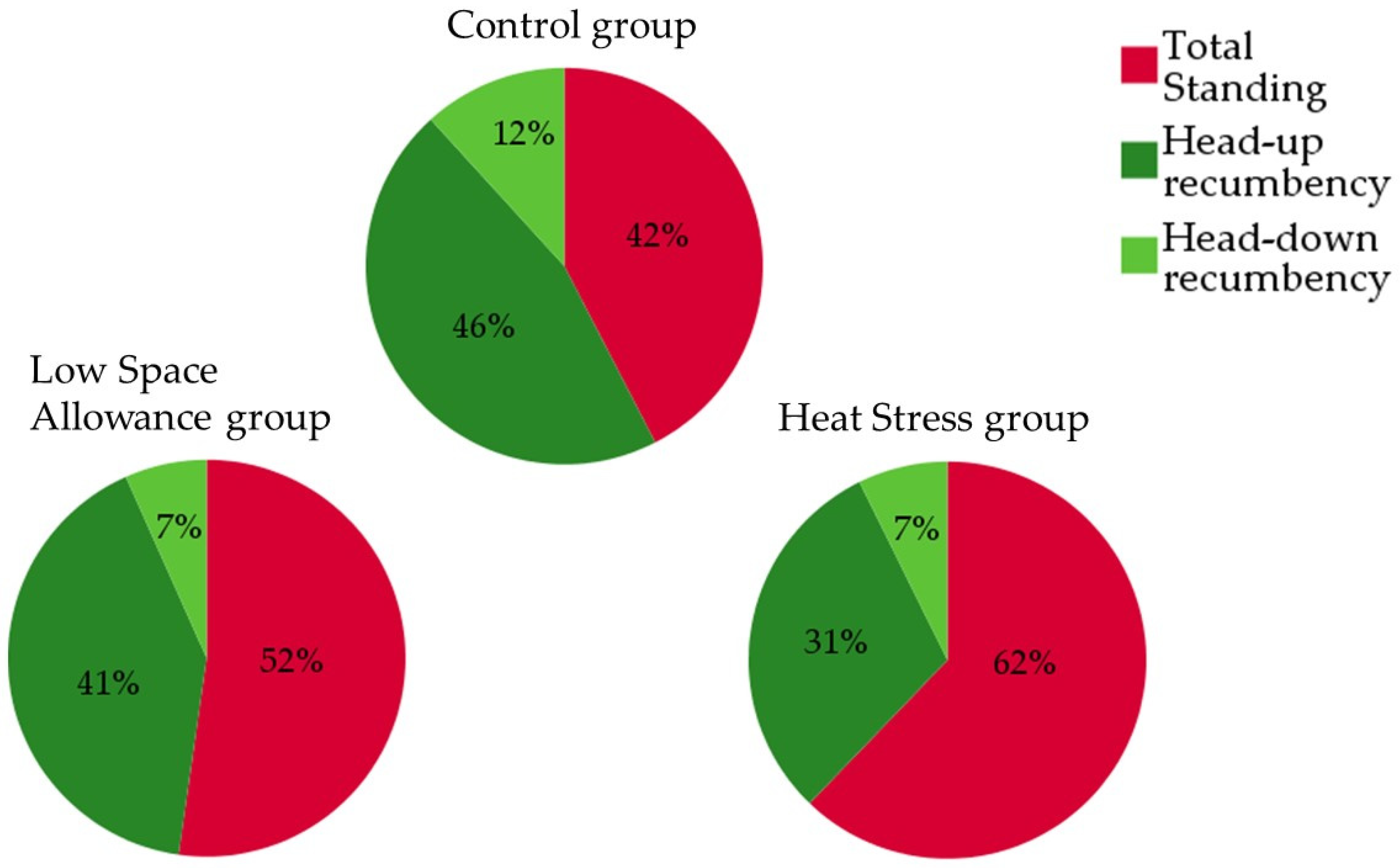
3.2. Activity and Lying Position
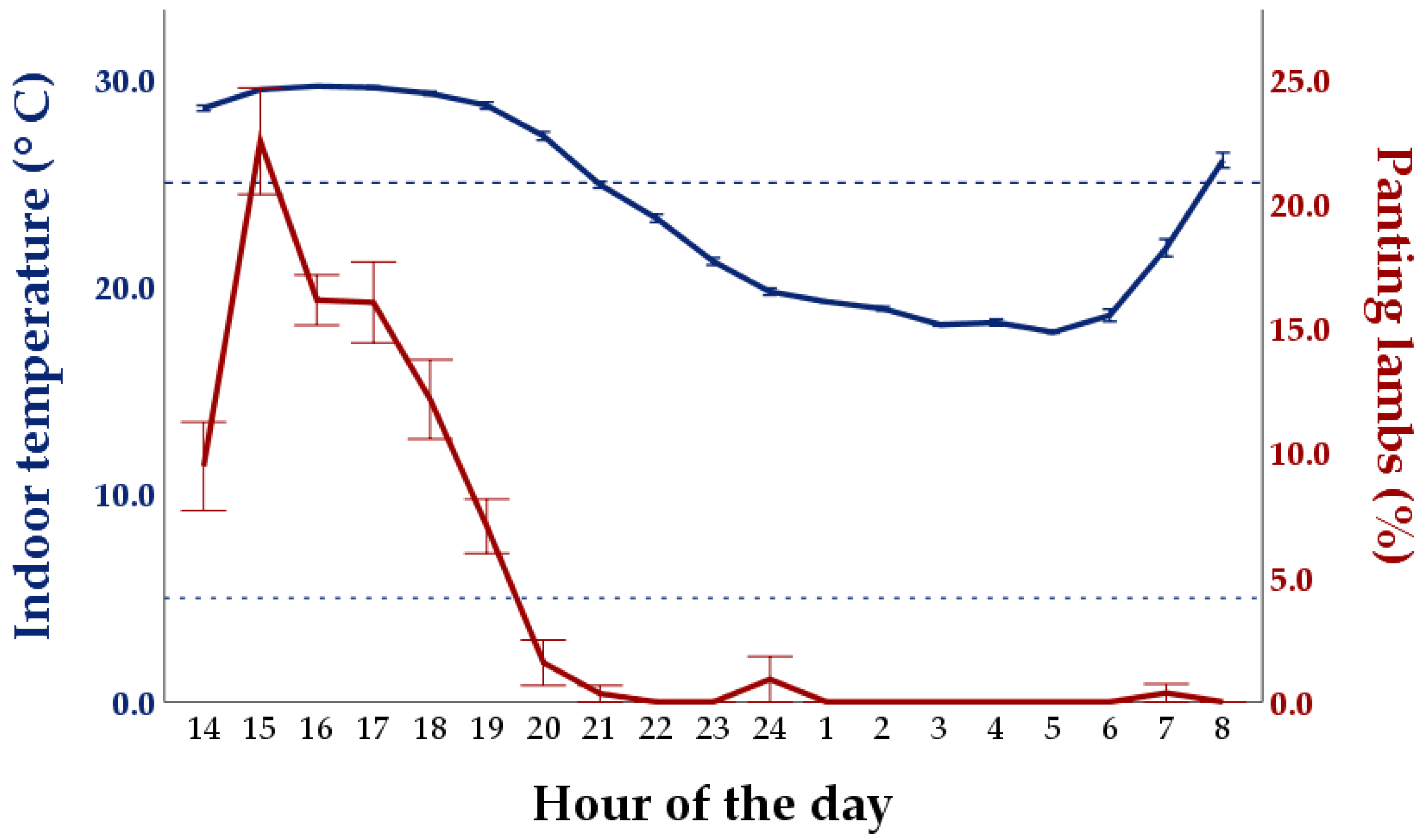
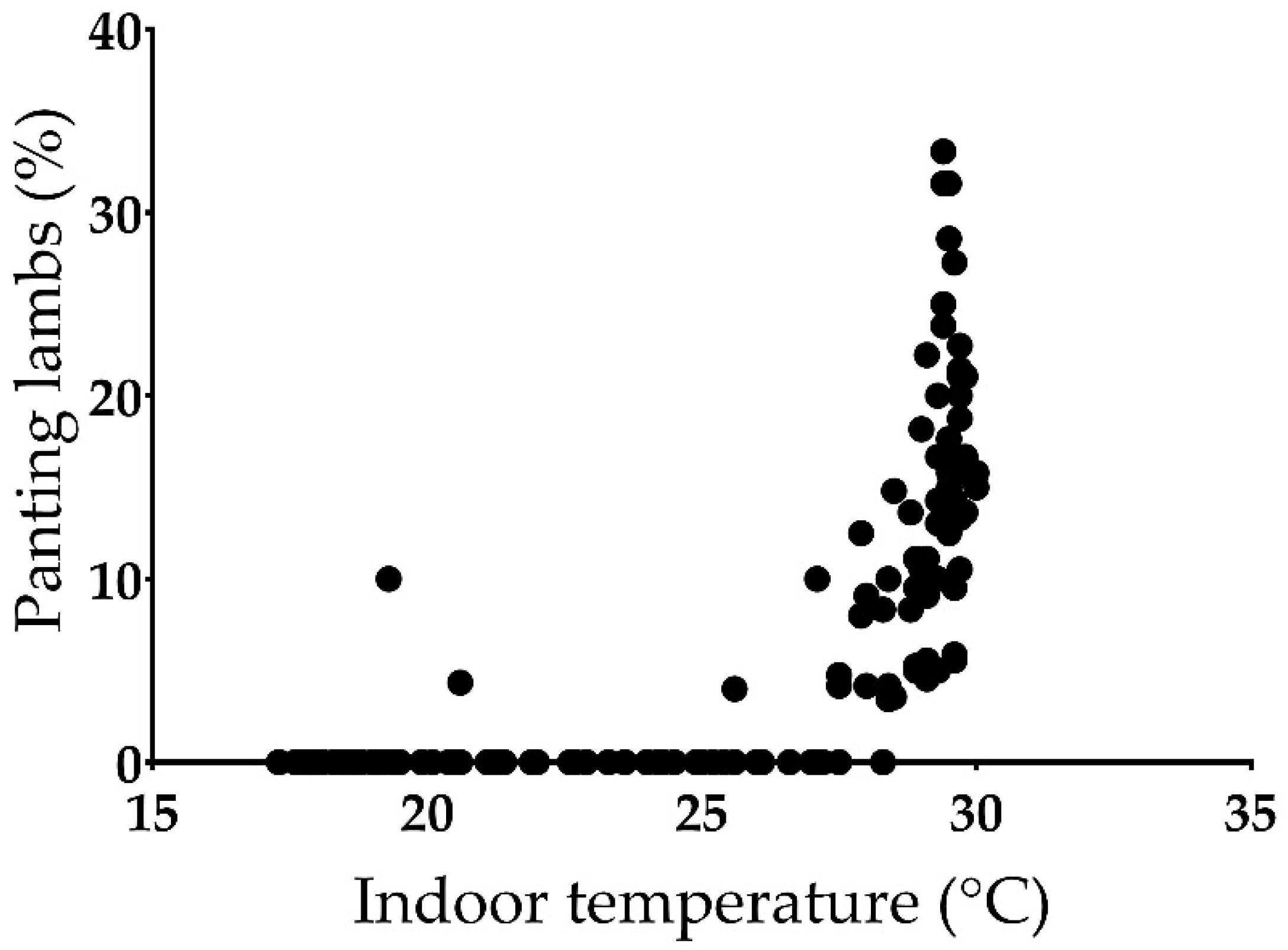
3.3. Other Behaviors and Panting
3.4. Eye Temperature (ET)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubeuf, J.P.; Aw-Hassan, A.; Chentouf, M.; Mena, Y.; Pacheco, F.; Boutonnet, J.P. The Mediterranean Sheep and Goat Sectors between Constants and Changes over the Last Decade Future Challenges and Prospects. Options Méditerr. Ser. Mediterr. Semin. 2016, 115, 43–52. [Google Scholar]
- Skapetas, B.; Kalaitzidou, M. Current Status and Perspectives of Sheep Sector in the World. Livest. Res. Rural Dev. 2017, 29, 21. [Google Scholar]
- Joy, A.; Dunshea, F.R.; Leury, B.J.; Clarke, I.J.; DiGiacomo, K.; Chauhan, S.S. Resilience of Small Ruminants to Climate Change and Increased Environmental Temperature: A Review. Anim. Open Access J. 2020, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- ISMEA Settore Ovicaprino. Scheda Di Settore. 2021. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/3518. (accessed on 4 November 2021).
- European Parliament. European Parliament Regulation (EC) No 1/2005 on the Protection of Animals during Transport and Related Operations: European Implementation Assessment; European Parliament: Brussels, Belgium, 2005. [Google Scholar]
- Padalino, B.; Raidal, S.L.; Knight, P.; Celi, P.; Jeffcott, L.; Muscatello, G. Behaviour during Transportation Predicts Stress Response and Lower Airway Contamination in Horses. PLoS ONE 2018, 13, e0194272. [Google Scholar] [CrossRef]
- Farm Animal Welfare Council. What is Animal Welfare? Farm Animal Welfare Council: London, UK, 1992; p. 357. [Google Scholar]
- Petherick, J.C.; Phillips, C.J.C. Space Allowances for Confined Livestock and Their Determination from Allometric Principles. Appl. Anim. Behav. Sci. 2009, 117, 1–12. [Google Scholar] [CrossRef]
- Consortium of the Animal Transport Guides Project. Guide to Good Practices for the Transport of Sheep; Animal Transport Guides Consortium: Wageningen, The Netherlands, 2018. [Google Scholar]
- Randall, J.M. Environmental Parameters Necessary to Define Comfort for Pigs, Cattle and Sheep in Livestock Transporters. Anim. Sci. 1993, 57, 299–307. [Google Scholar] [CrossRef]
- Zhang, Y.; Phillips, C.J.C. Climatic Influences on the Mortality of Sheep during Long-Distance Sea Transport. Animals 2019, 13, 1054–1062. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Cambridge, H.; Foster, S.F.; McGreevy, P.D. Heat Stress: A Major Contributor to Poor Animal Welfare Associated with Long-Haul Live Export Voyages. Vet. J. Lond. Engl. 1997 2014, 199, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to Hot Climate and Strategies to Alleviate Heat Stress in Livestock Production. Anim. Int. J. Anim. Biosci. 2012, 6, 707–728. [Google Scholar] [CrossRef]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological Traits as Affected by Heat Stress in Sheep—A Review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Stockman, C.A.; Barnes, A.L.; Maloney, S.; Taylor, E.; Mccarthy, M.; Pethick, D. Effect of Prolonged Exposure to Continuous Heat and Humidity Similar to Long Haul Live Export Voyages in Merino Wethers. Aust. J. Exp. Agric. 2011, 51, 135–143. [Google Scholar] [CrossRef]
- Sevi, A.; Annicchiarico, G.; Albenzio, M.; Taibi, L.; Muscio, A.; Dell’Aquila, S. Effects of Solar Radiation and Feeding Time on Behavior, Immune Response and Production of Lactating Ewes under High Ambient Temperature. J. Dairy Sci. 2001, 84, 629–640. [Google Scholar] [CrossRef]
- Hahn, G.L. Management and Housing of Farm Animals in Hot Environments; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a Request from the Commission Related to the Welfare of Animals during Transport. EFSA J. 2004, 2, 44. [Google Scholar] [CrossRef]
- Battini, M.; Barbieri, S.; Fioni, L.; Mattiello, S. Feasibility and Validity of Animal-Based Indicators for on-Farm Welfare Assessment of Thermal Stress in Dairy Goats. Int. J. Biometeorol. 2016, 60, 289–296. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of Heat Stress on the Welfare of Extensively Managed Domestic Ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.; Malik, P.K.; Naqvi, S.M.K.; Lal, R. Adapting Sheep Production to Climate Change. In Sheep Production Adapting to Climate Change; Sejian, V., Bhatta, R., Gaughan, J., Malik, P.K., Naqvi, S.M.K., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–29. ISBN 9789811047145. [Google Scholar]
- U.S. Department of Agriculture (USDA). Sheep 2001: Part II: Reference of Sheep Health in the United States, 2001; U.S. Department of Agriculture: Washington, DC, USA, 2003. [Google Scholar]
- Schuijer, M.E. (Marleen) Transport Guide Extreme Temperatures. Available online: https://ec.europa.eu/food/system/files/2019-11/aw_platform_plat-conc_extreme-temp-factsh-cattle.pdf (accessed on 12 November 2021).
- European Food Safety Authority (EFSA). EFSA Assesses Welfare Risks to Animals during Transport. Available online: https://www.efsa.europa.eu/en/press/news/110112 (accessed on 12 November 2021).
- Pucora, E.; Schiffmann, C.; Clauss, M. Resting Postures in Terrestrial Mammalian Herbivores. J. Mammal. 2019, 100, 552–563. [Google Scholar] [CrossRef]
- Aguayo-Ulloa, L.A.; Pascual-Alonso, M.; González-Lagos, C.; Miranda-de la Lama, G.C.; Villarroel, M.; Asenjo, B.; Resconi, V.; María, G.A. Behaviour and Welfare of Fattening Lambs Supplemented with Varying Sizes and Types of Straw. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.M.; Poulin, A. Equid Play Ethogram. Appl. Anim. Behav. Sci. 2002, 78, 263–290. [Google Scholar] [CrossRef]
- McCarthy, M. Pilot Monitoring of Shipboard Environmental Conditions and Animal Performance. Meat Livest. Aust. 2005, 223, 1–31. [Google Scholar]
- Hales, J.R.S.; Webster, M.E.D. Respiratory Function during Thermal Tachypnoea in Sheep. J. Physiol. 1967, 190, 241–260. [Google Scholar] [CrossRef]
- Vik, S.G.; Øyrehagen, O.; Bøe, K.E. Effect of Space Allowance and Flooring on the Behavior of Pregnant Ewes1. J. Anim. Sci. 2017, 95, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D. Positive Animal Welfare States and Encouraging Environment-Focused and Animal-to-Animal Interactive Behaviours. N. Z. Vet. J. 2015, 63, 9–16. [Google Scholar] [CrossRef]
- Redaelli, V.; Luzi, F.; Mazzola, S.; Bariffi, G.D.; Zappaterra, M.; Costa, L.N.; Padalino, B. The Use of Infrared Thermography (IRT) as Stress Indicator in Horses Trained for Endurance: A Pilot Study. Animals 2019, 9, 84. [Google Scholar] [CrossRef]
- George, W.D.; Godfrey, R.W.; Ketring, R.C.; Vinson, M.C.; Willard, S.T. Relationship among Eye and Muzzle Temperatures Measured Using Digital Infrared Thermal Imaging and Vaginal and Rectal Temperatures in Hair Sheep and Cattle. J. Anim. Sci. 2014, 92, 4949–4955. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Dalla Costa, E.; Minero, M.; Padalino, B. Development and Validation of a Test for the Classification of Horses as Broken or Unbroken. Animals 2021, 11, 2303. [Google Scholar] [CrossRef] [PubMed]
- Laurentie, M.P.; Barenton, B.; Charrier, J.; Garcia-Villar, R.; Marnet, P.G.; BLANCHARD, M.; Toutain, P.L. Instantaneous Secretion Rate of Growth Hormone in Lambs: Relationships with Sleep, Food Intake, and Posture. Endocrinology 1989, 125, 642–651. [Google Scholar] [CrossRef]
- Bertolucci, L.F. Pandiculation: Nature’s Way of Maintaining the Functional Integrity of the Myofascial System? J. Bodyw. Mov. Ther. 2011, 15, 268–280. [Google Scholar] [CrossRef]
- Fraser, A.F. Pandiculation: The Comparative Phenomenon of Systematic Stretching. Appl. Anim. Behav. Sci. 1989, 23, 263–268. [Google Scholar] [CrossRef]
- Fraser, A.F. The Phenomenon of Pandiculation in the Kinetic Behaviour of the Sheep Fetus. Appl. Anim. Behav. Sci. 1989, 24, 169–182. [Google Scholar] [CrossRef]
- Schütz, K.E.; Rogers, A.R.; Poulouin, Y.A.; Cox, N.R.; Tucker, C.B. The Amount of Shade Influences the Behavior and Physiology of Dairy Cattle. J. Dairy Sci. 2010, 93, 125–133. [Google Scholar] [CrossRef]
- Camp Montoro, J.; Boyle, L.A.; Solà-Oriol, D.; Muns, R.; Gasa, J.; Garcia Manzanilla, E. Effect of Space Allowance and Mixing on Growth Performance and Body Lesions of Grower-Finisher Pigs in Pens with a Single Wet-Dry Feeder. Porc. Health Manage. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; mohamed, R.; osman, A. Effects of Stocking Density on Some Behavioral and Some Blood Biochemical Parameters in Camel during the Rut Period. Egypt. J. Vet. Sci. 2020, 51, 253–262. [Google Scholar] [CrossRef]
- Zappaterra, M.; Menchetti, L.; Nanni Costa, L.; Padalino, B. Do Camels (Camelus Dromedarius) Need Shaded Areas? A Case Study of the Camel Market in Doha. Animals 2021, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Arnold, G.W.; Dudzinski, M.L. Ethology of Free-Ranging Domestic Animals; Elsevier Scientific Pub. Co.: New York, NY, USA, 1978. [Google Scholar]
- Lynch, J.J.; Wood-Gush, D.G.M.; Davies, H.I. Aggression and Nearest Neighbours in a Flock of Scottish Blackface Ewes. Aggress. Nearest Neighb. Flock Scott. Blackface Ewes 1985, 10, 215–225. [Google Scholar]
- Vasseur, S.; Paull, D.R.; Atkinson, S.J.; Colditz, I.G.; Fisher, A.D. Effects of dietary fibre and feeding frequency on wool biting and aggressive behaviours in housed Merino Sheep. Aust. J. Exp. Agr. 2006, 46, 777–782. [Google Scholar] [CrossRef]
- Xing, T.; Gao, F.; Tume, R.K.; Zhou, G.; Xu, X. Stress Effects on Meat Quality: A Mechanistic Perspective. Compr. Rev. Food Sci. Food Saf. 2019, 18, 380–401. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Sánchez, M.; Pérez, C.; Lauzurica, S.; Vieira, C.; González de Chávarri, E.; Díaz, M.T. Physiological Response and Carcass and Meat Quality of Suckling Lambs in Relation to Transport Time and Stocking Density during Transport by Road. Anim. Int. J. Anim. Biosci. 2010, 4, 250–258. [Google Scholar] [CrossRef]
- Georgiev, A.V.; Klimczuk, A.C.E.; Traficonte, D.M.; Maestripieri, D. When Violence Pays: A Cost-Benefit Analysis of Aggressive Behavior in Animals and Humans. Evol. Psychol. Int. J. Evol. Approaches Psychol. Behav. 2013, 11, 678–699. [Google Scholar] [CrossRef]
- Marsden, M.D.; Wood-Gush, D.G.M. The Use of Space by Group-Housed Sheep. Appl. Anim. Behav. Sci. 1986, 15, 178. [Google Scholar] [CrossRef]
- Færevik, G.; Andersen, I.L.; Bøe, K.E. Preferences of Sheep for Different Types of Pen Flooring. Appl. Anim. Behav. Sci. 2005, 90, 265–276. [Google Scholar] [CrossRef]
- Rook, A.J.; Penning, P.D. Synchronisation of Eating, Ruminating and Idling Activity by Grazing Sheep. Appl. Anim. Behav. Sci. 1991, 32, 157–166. [Google Scholar] [CrossRef]
- Jørgensen, G.H.M.; Andersen, I.L.; Berg, S.; Bøe, K.E. Feeding, Resting and Social Behaviour in Ewes Housed in Two Different Group Sizes. Appl. Anim. Behav. Sci. 2009, 116, 198–203. [Google Scholar] [CrossRef]
- Averós, X.; Lorea, A.; de Heredia, I.B.; Arranz, J.; Ruiz, R.; Estevez, I. Space Availability in Confined Sheep during Pregnancy, Effects in Movement Patterns and Use of Space. PLoS ONE 2014, 9, e94767. [Google Scholar] [CrossRef]
- Andersen, I.L.; Bøe, K.E. Resting Pattern and Social Interactions in Goats—The Impact of Size and Organisation of Lying Space. Appl. Anim. Behav. Sci. 2007, 108, 89–103. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and How Should We Use Environmental Enrichment to Tackle Stereotypic Behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Done-Currie, J.R.; Hecker, J.F.; Wodzicka-Tomaszewska, M. Behaviour of Sheep Transferred from Pasture to an Animal House. Appl. Anim. Behav. Sci. 1984, 12, 121–130. [Google Scholar] [CrossRef]
- Cook, N.B.; Mentink, R.L.; Bennett, T.B.; Burgi, K. The Effect of Heat Stress and Lameness on Time Budgets of Lactating Dairy Cows. J. Dairy Sci. 2007, 90, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Hall, L.W.; Collier, R.J.; Smith, J.F. Effect of Core Body Temperature, Time of Day, and Climate Conditions on Behavioral Patterns of Lactating Dairy Cows Experiencing Mild to Moderate Heat Stress. J. Dairy Sci. 2015, 98, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.C.; Lindberg, A.C.; Main, D.C.J.; Whay, H.R. Assessment of the Welfare of Working Horses, Mules and Donkeys, Using Health and Behaviour Parameters. Prev. Vet. Med. 2005, 69, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Schrøder-Petersen, D.L.; Simonsen, H.B. Tail Biting in Pigs. Vet. J. 2001, 162, 196–210. [Google Scholar] [CrossRef]
- Olsson, K.; Josäter-Hermelin, M.; Hossaini-Hilali, J.; Hydbring, E.; Dahlborn, K. Heat Stress Causes Excessive Drinking in Fed and Food Deprived Pregnant Goats. Comp. Biochem. Physiol. A Physiol. 1995, 110, 309–317. [Google Scholar] [CrossRef]
- Phillips, C. The Welfare Risks and Impacts of Heat Stress on Sheep Shipped from Australia to the Middle East. Vet. J. 2016, 218, 78–85. [Google Scholar] [CrossRef]
- Emeash, H.H.; El-Bably, M.A. Stress Resulting of Management Practices on Sheep Herds. Egypt. J. Sheep Goat Sci. 2012, 65, 1–19. [Google Scholar] [CrossRef]
- Kogan, L.R.; Schoenfeld-Tacher, R.; Simon, A.A. Behavioral Effects of Auditory Stimulation on Kenneled Dogs. J. Vet. Behav. 2012, 7, 268–275. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Olivier, B.; Paylor, R.E. The Stress-Induced Hyperthermia Paradigm as a Physiological Animal Model for Anxiety: A Review of Pharmacological and Genetic Studies in the Mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Nagashima, K.; Nakai, S.; Tanaka, M.; Kanosue, K. Neuronal Circuitries Involved in Thermoregulation. Auton. Neurosci. Basic Clin. 2000, 85, 18–25. [Google Scholar] [CrossRef]
- McKinley, M.; Trevaks, D.; Weissenborn, F.; McAllen, R. Interaction between Thermoregulation and Osmoregulation in Domestic Animals. Rev. Bras. Zootec. 2017, 46, 783–790. [Google Scholar] [CrossRef][Green Version]
- Nääs, I.A.; Garcia, R.G.; Caldara, F.R. Infrared Thermal Image for Assessing Animal Health and Welfare. J. Anim. Behav. Biometeorol. 2014, 2, 66–72. [Google Scholar] [CrossRef]
- Cook, N.J.; Schaefer, A.L. Infrared Thermography and Disease Surveillance. In Thermography: Current Status and Advances in Livestock Animals and in Veterinary Medicine; Luzi, F., Mitchell, M., Nanni Costa, L., Redaelli, V., Eds.; Fondazione Iniziative Zooprofilattiche: Brescia, Italy, 2013; pp. 41–46. [Google Scholar]
- Wijffels, G.; Sullivan, M.; Gaughan, J. Methods to Quantify Heat Stress in Ruminants: Current Status and Future Prospects. Methods 2021, 186, 3–13. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Scientific Opinion on the Use of Animal-Based Measures to Assess Welfare in Pigs. EFSA J. 2012, 10, 2512. [CrossRef]
- Oliveira, A.F.S.; Rossi, A.O.; Silva, L.F.R.; Lau, M.C.; Barreto, R.E. Play Behaviour in Nonhuman Animals and the Animal Welfare Issue. J. Ethol. 2009, 28, 1. [Google Scholar] [CrossRef]
- Held, S.D.E.; Špinka, M. Animal Play and Animal Welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Broom, D.M. Animal Welfare: Concepts and Measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- Krohn, C.C. Behaviour of Dairy Cows Kept in Extensive (Loose Housing/Pasture) or Intensive (Tie Stall) Environments. III. Grooming, Exploration and Abnormal Behaviour. Appl. Anim. Behav. Sci. 1994, 42, 73–86. [Google Scholar] [CrossRef]
- Hart, B.L.; Pryor, P.A. Developmental and Hair-Coat Determinants of Grooming Behaviour in Goats and Sheep. Anim. Behav. 2004, 67, 11–19. [Google Scholar] [CrossRef]
- Mellor, D. Enhancing Animal Welfare by Creating Opportunities for Positive Affective Engagement. N. Z. Vet. J. 2015, 63, 3–8. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Vigors, B.; Sandøe, P. What Is so Positive about Positive Animal Welfare?—A Critical Review of the Literature. Animals 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]



| Group | Temperature (°C), Mean and Range | Humidity (%), Mean and Range | N° Lamb/Pen | Space Allowance (m2/Lamb) | Mean BW ± SD (kg) | Animal Density (kg/m2) | Coefficient k |
|---|---|---|---|---|---|---|---|
| C | 15 (12–18) | 51(40–60) | 39 | 0.27 | 22.79 ± 3.73 | 85.49 | 0.033 |
| LSA | 15 (12–18) | 51(40–60) | 52 | 0.20 | 21.18 ± 3.31 | 105.9 | 0.026 |
| HS | 25 (19–30) | 65 (44–80) | 39 | 0.27 | 23.46 ± 4.08 | 88.00 | 0.033 |
| Category | Behavior | Description |
|---|---|---|
| Activity and lying position1 | Sternal recumbency, head up | Lamb is lying with the majority of body weight on the sternum; all limbs are under the body [25] and the head is up (above the height at the withers) |
| Sternal recumbency, head down | Lamb is lying with the majority of body weight on the sternum; all limbs are under the body [25] and the head is down (at or below the height at the withers) | |
| Sternocostal recumbency, head up | Lamb is lying with the majority of body weight on the sternum but at least one hind limb is on one side, bent but visible and the head is up (above the height at the withers) | |
| Sternocostal recumbency, head down | Lamb is lying with the majority of body weight on the sternum but at least one hind limb is on one side, bent but visible and the head is down (at or below the height at the withers) | |
| Lateral recumbency, head down | Lamb is lying with the majority of body weight on the left or right side with all limbs outstretched to respective side [25], head and neck are extended on the floor | |
| Standing, suckling/attempt to | Lamb standing on all four legs, while milk feeding or trying to reach a teat | |
| Standing, eating/attempt to | Lamb standing on all four legs, searching for forage straw in the bedding or in the fodder rake (when it was offered), chewing and eating it [26] | |
| Standing, still | Lamb standing on all four legs, without moving [26], far from the milk station | |
| Standing, walking | Lamb stands on four legs but it moves around [26] | |
| Standing, Playing | Lamb shows any locomotory play or play fighting [27] | |
| Panting1 | No | No panting, normal respiration (panting score = 0) [28] |
| Yes | Lamb shows a high respiration rate, it appears to “breath” from its flanks; the mouth could be closed (first phase, panting score = 1–2) or open (second phase, panting score > 2) [28,29] | |
| Interactions | Aggressive interaction when lying | Lamb tramples with its front legs or pushes with its head another animal which is resting [30] |
| Aggressive interactions when being active | Lamb pushes another animal with its head or other parts of its body or mount another active ewe (which is standing, eating, or moving) [30], often to reach the milk station | |
| Affiliative behavior [31] | Lamb interacts with another in a nonaggressive way (sniffing, licking, or allogrooming) | |
| Other behaviors | Trampling | Lamb climbs over another lying animal trampling it, in a nonaggressive way, often having no alternative ways |
| Drinking | Lamb drinks water from the drinker [26] | |
| Self-grooming | Lamb licks itself or lamb scratches itself against pen equipment | |
| Stretching | Lamb extends/stretches part or all of the body | |
| Shaking | Lamb performs quick sudden movements of the head or whole body | |
| Stereotypies | Lamb performing a frequent and non-functional oral manipulation (licking) of object such as pen bars, walls, fodder rake [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menchetti, L.; Nanni Costa, L.; Zappaterra, M.; Padalino, B. Effects of Reduced Space Allowance and Heat Stress on Behavior and Eye Temperature in Unweaned Lambs: A Pilot Study. Animals 2021, 11, 3464. https://doi.org/10.3390/ani11123464
Menchetti L, Nanni Costa L, Zappaterra M, Padalino B. Effects of Reduced Space Allowance and Heat Stress on Behavior and Eye Temperature in Unweaned Lambs: A Pilot Study. Animals. 2021; 11(12):3464. https://doi.org/10.3390/ani11123464
Chicago/Turabian StyleMenchetti, Laura, Leonardo Nanni Costa, Martina Zappaterra, and Barbara Padalino. 2021. "Effects of Reduced Space Allowance and Heat Stress on Behavior and Eye Temperature in Unweaned Lambs: A Pilot Study" Animals 11, no. 12: 3464. https://doi.org/10.3390/ani11123464
APA StyleMenchetti, L., Nanni Costa, L., Zappaterra, M., & Padalino, B. (2021). Effects of Reduced Space Allowance and Heat Stress on Behavior and Eye Temperature in Unweaned Lambs: A Pilot Study. Animals, 11(12), 3464. https://doi.org/10.3390/ani11123464








