Anatomical Evaluation of Rat and Mouse Simulators for Laboratory Animal Science Courses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
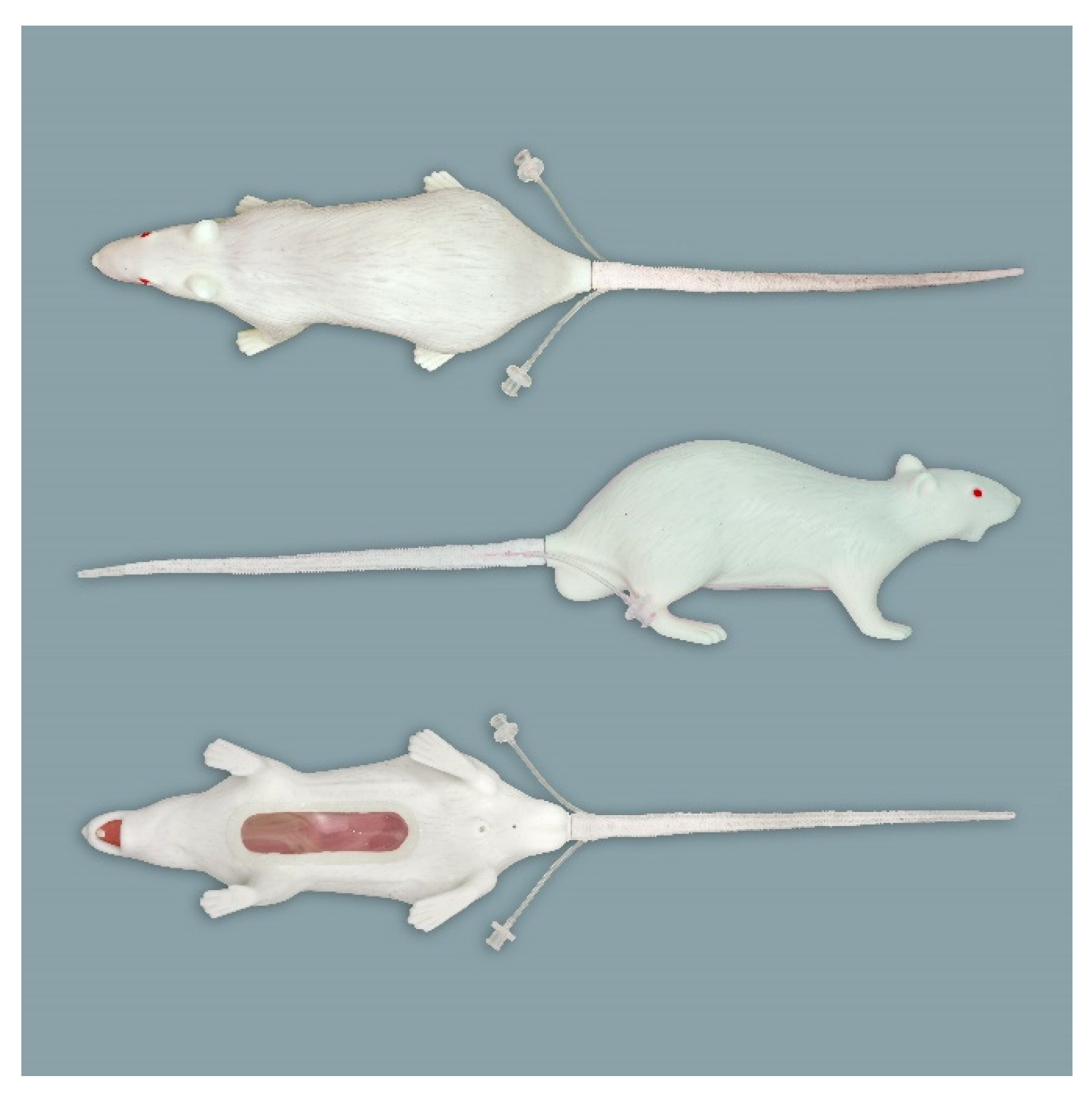
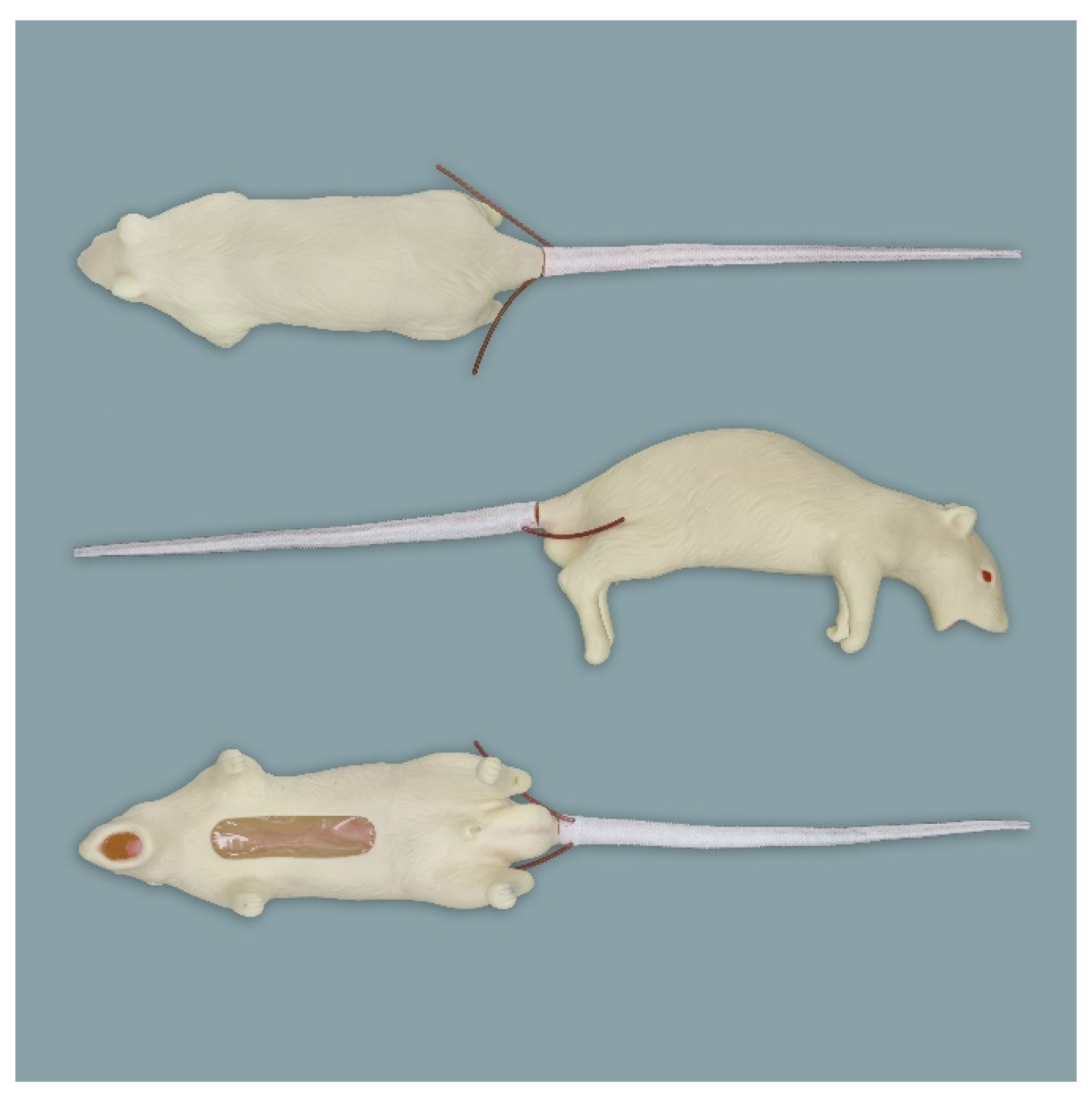
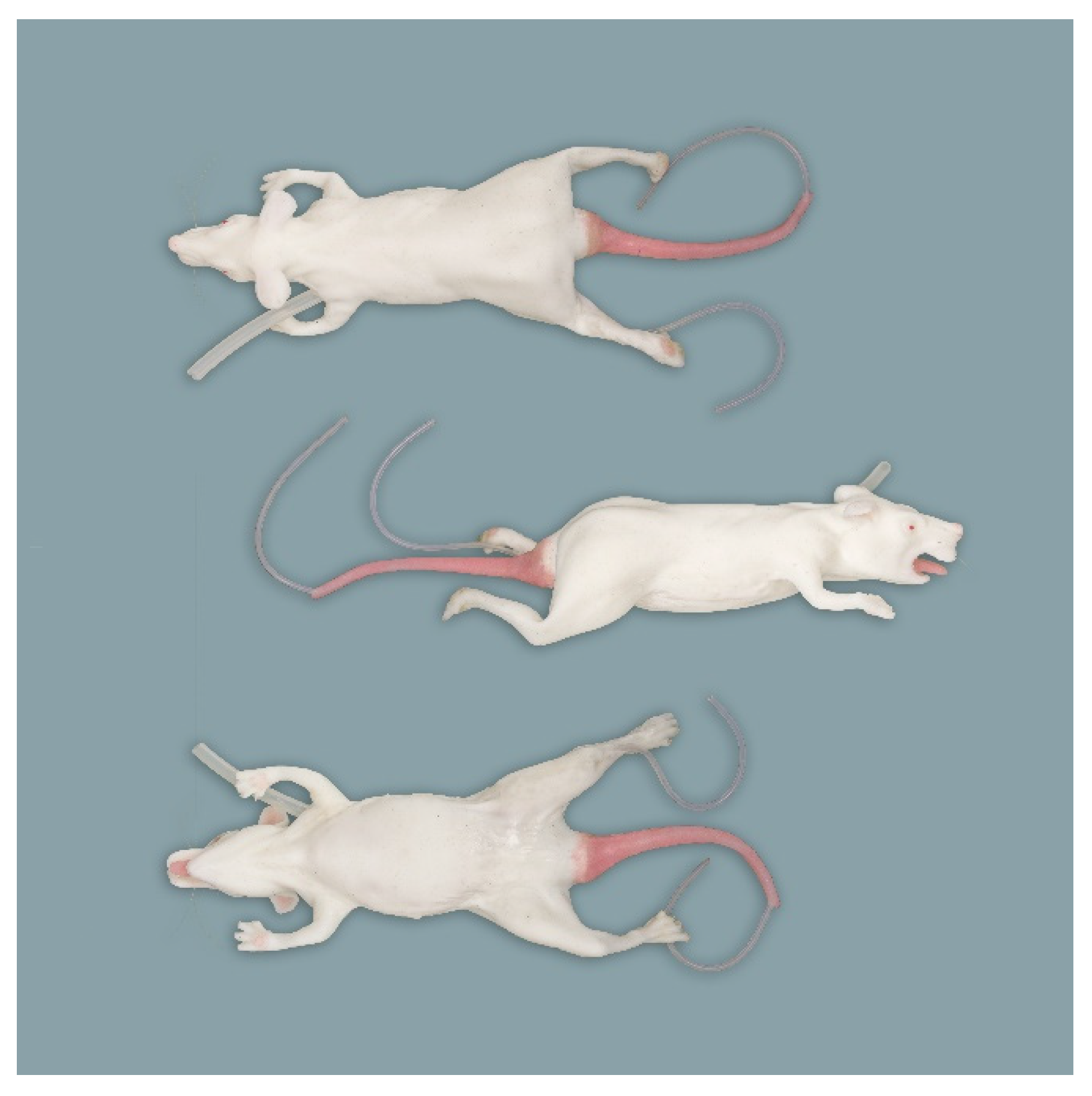
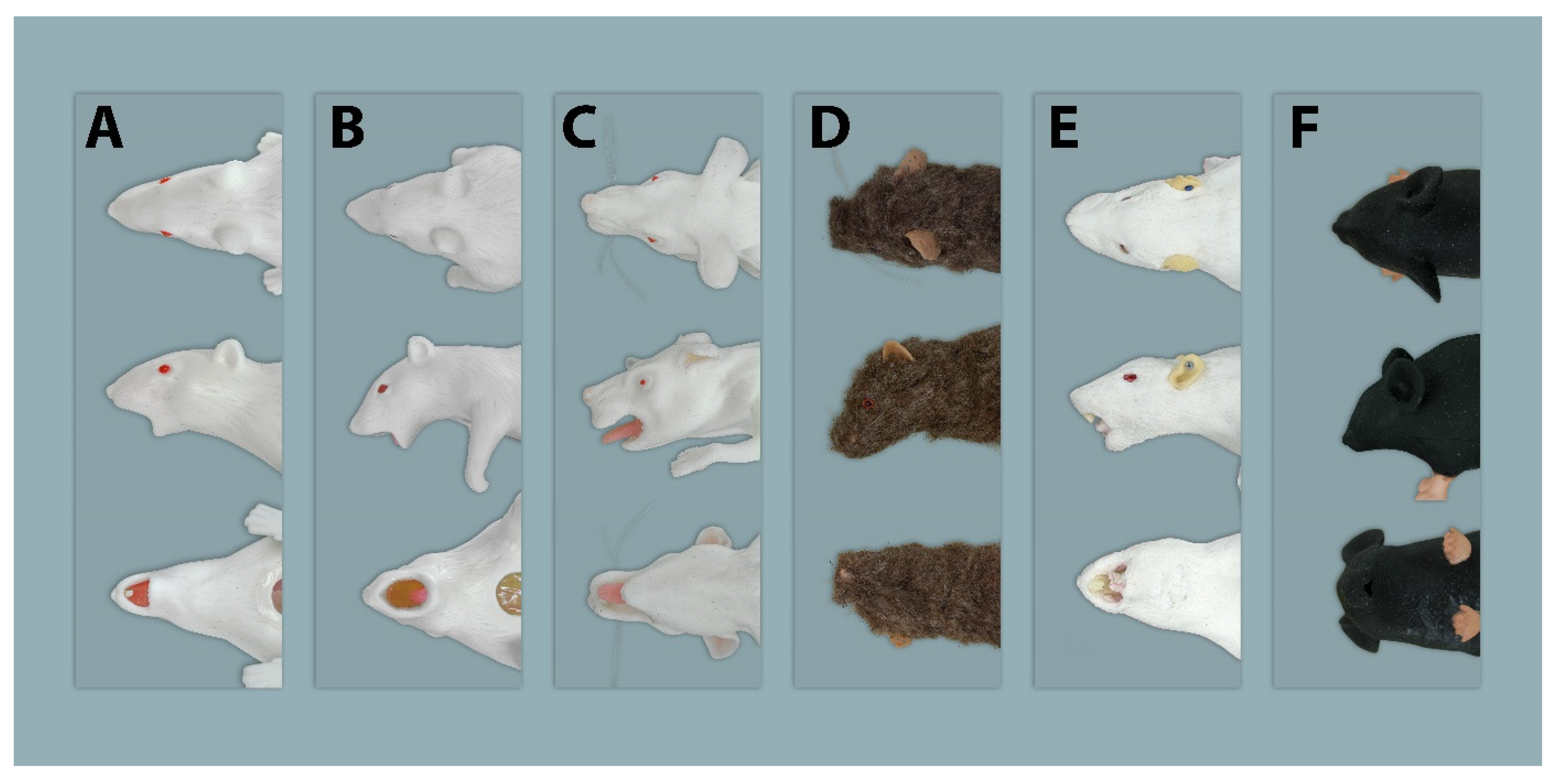
2.1. Simulators
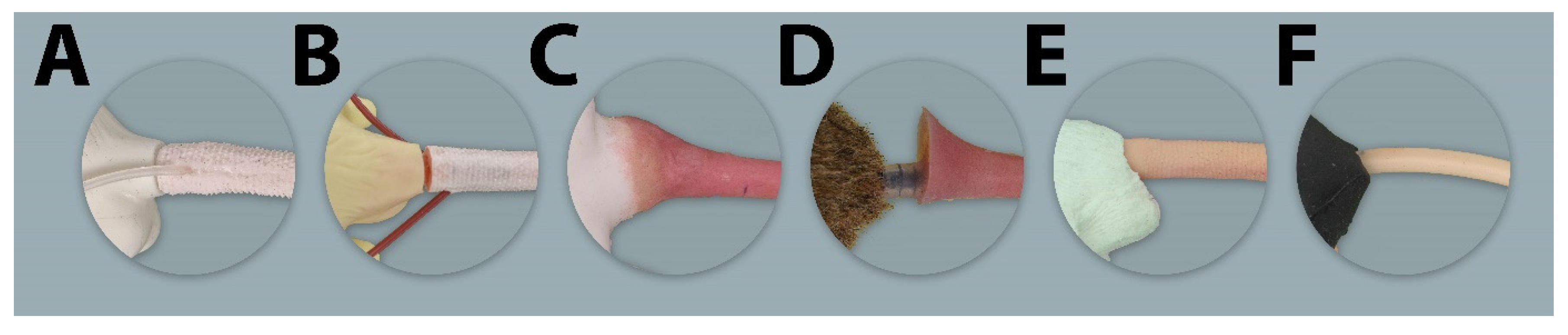
2.1.1. Rat Simulators
Rat Simulator A
Rat Simulator B
Rat Simulator C
Rat Simulator D
Rat Simulator E
2.1.2. Mouse Simulator
2.2. Evaluators and Questionnaires
- From an anatomical point of view, what did you particularly like about rat simulator X?
- From an anatomical point of view, what did you particularly dislike about rat simulator X?
- What would you improve from an anatomical point of view in rat simulator X?
2.3. Statistics
3. Results
3.1. Rat Simulator A
3.2. Rat Simulator B
3.3. Rat Simulator C
3.4. Rat Simulator D
3.5. Rat Simulator E
3.6. Mouse Simulator
3.7. Analysis of Variance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Crossan, A.; Brewster, S.; Reid, S.; Mellor, D. A horse ovary palpation simulator for veterinary training. In International Workshop on Haptic Human-Computer Interaction; Springer: Berlin/Heidelberg, Germany, 2001; Volume 2058, pp. 157–164. [Google Scholar]
- Walshaw, S. Incorporating animal alternatives in a training programme in laboratory animal care and use. Altern. Lab. Anim. 2004, 32 (Suppl. S1), 549–551. [Google Scholar] [CrossRef]
- Conarello, S.L.; Shepherd, M.J. Training strategies for research investigators and technicians. ILAR J. 2007, 48, 120–130. [Google Scholar] [CrossRef][Green Version]
- European Commission. Summary Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union and Norway in 2018. 2018. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/SWD_%20part_A_and_B.pdf (accessed on 21 October 2021).
- Bundesministerium für Ernährung und Ladnwirtschaft. Verwendung von Versuchstieren im Jahr 2019. 2019. Available online: https://www.bmel.de/DE/themen/tiere/tierschutz/versuchstierzahlen2019.html (accessed on 21 October 2021).
- European Union 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79.
- Akaike, M.; Fukutomi, M.; Nagamune, M.; Fujimoto, A.; Tsuji, A.; Ishida, K.; Iwata, T. Simulation-based medical education in clinical skills laboratory. J. Med. Investig. JMI 2012, 59, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Duff, J.; Grant, E.; Kissoon, N.; Grant, V.J. Simulation in paediatrics: An educational revolution. Paediatr. Child Health 2007, 12, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Scalese, R.J.; Obeso, V.T.; Issenberg, S.B. Simulation technology for skills training and competency assessment in medical education. J. Gen. Intern. Med. 2008, 23 (Suppl. S1), 46–49. [Google Scholar] [CrossRef]
- Baillie, S. Utilisation of simulators in veterinary training. J. Cattle Pract. 2007, 15, 224. [Google Scholar]
- Baillie, S.; Mellor, D.J.; Brewster, S.A.; Reid, S.W. Integrating a bovine rectal palpation simulator into an undergraduate veterinary curriculum. J. Vet. Med. Educ. 2005, 32, 79–85. [Google Scholar] [CrossRef]
- Parkes, R.; Forrest, N.; Baillie, S. A mixed reality simulator for feline abdominal palpation training in veterinary medicine. Stud. Health Technol. Inform. 2009, 142, 244–246. [Google Scholar]
- Uson-Gargallo, J.; Tapia-Araya, A.E.; Diaz-Guemes Martin-Portugues, I.; Sanchez-Margallo, F.M. Development and evaluation of a canine laparoscopic simulator for veterinary clinical training. J. Vet. Med. Educ. 2014, 41, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Valliyate, M.; Robinson, N.; Goodman, J. Current concepts in simulation and other alternatives for veterinary education: A review. J. Vet. Med. 2012, 57, 325. [Google Scholar] [CrossRef]
- Remie, R. The PVC-rat and other alternatives in microsurgical training. Lab. Anim. 2001, 30, 48–52. [Google Scholar] [CrossRef]
- Humpenöder, M.; Corte, G.M.; Pfützner, M.; Wiegard, M.; Merle, R.; Hohlbaum, K.; Erickson, N.A.; Plendl, J.; Thöne-Reineke, C. Alternatives in Education-Rat and Mouse Simulators Evaluated from Course Trainers’ and Supervisors’ Perspective. Animals 2021, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- NORINA Database. Available online: https://norecopa.no/databases-guidelines/norina-database (accessed on 21 October 2021).
- InterNICHE. Available online: http://www.interniche.org/ (accessed on 21 October 2021).
- Chang, L. A psychometric evaluation of 4-point and 6-point Likert-type scales in relation to reliability and validity. Appl. Psychol. Meas. 1994, 18, 205–215. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Heer, B. Additive manufacturing of multi-material structures. Mater. Sci. Eng. R Rep. 2018, 129, 1–16. [Google Scholar] [CrossRef]
- Beeton, C.; Garcia, A.; Chandy, K.G. Drawing blood from rats through the saphenous vein and by cardiac puncture. JoVE 2007, 7, 266. [Google Scholar] [CrossRef]
- Sharp, P.; Villano, J.S. The Laboratory Rat; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Arantes-Rodrigues, R.; Henriques, A.; Pinto-Leite, R.; Faustino-Rocha, A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Seixas, F.; Gama, A.; Colaco, B.; Colaco, A.; et al. The effects of repeated oral gavage on the health of male CD-1 mice. Lab. Anim. 2012, 41, 129–134. [Google Scholar] [CrossRef]
- Jones, C.P.; Boyd, K.L.; Wallace, J.M. Evaluation of Mice Undergoing Serial Oral Gavage While Awake or Anesthetized. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 805–810. [Google Scholar] [PubMed]
- Yang, J.H.; Kim, Y.M.; Chung, H.S.; Cho, J.; Lee, H.M.; Kang, G.H.; Kim, E.C.; Lim, T.; Cho, Y.S. Comparison of four manikins and fresh frozen cadaver models for direct laryngoscopic orotracheal intubation training. Emerg. Med. J. EMJ 2010, 27, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Scott, S.; Mihai, R. Litigation related to airway and respiratory complications of anaesthesia: An analysis of claims against the NHS in England 1995–2007. Anaesthesia 2010, 65, 556–563. [Google Scholar] [CrossRef]
- Schebesta, K.; Hupfl, M.; Rossler, B.; Ringl, H.; Muller, M.P.; Kimberger, O. Degrees of reality: Airway anatomy of high-fidelity human patient simulators and airway trainers. Anesthesiology 2012, 116, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yang, Y.; Gu, Y.; Zhu, P.; Zhang, M.; Cheng, Z.; Liu, X.; Yu, Y.; Peng, X. Repeated Blood Collection from Tail Vein of Non-Anesthetized Rats with a Vacuum Blood Collection System. J. Vis. Exp. 2017, 130, 55852. [Google Scholar] [CrossRef]
- Morton, D.B.; Jennings, M.; Buckwell, A.; Ewbank, R.; Godfrey, C.; Holgate, B.; Inglis, I.; James, R.; Page, C.; Sharman, I.; et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 2001, 35, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hsu, H.H.; Lai, I.R.; Tai, H.C.; Lai, H.S.; Lee, Y.C.; Shaw, J.S.; Hung, Y.P.; Lee, P.H.; Chang, K.J. Validation of a computer-based bronchoscopy simulator developed in Taiwan. J. Formos. Med. Assoc. Taiwan Yi Zhi 2006, 105, 569–576. [Google Scholar] [CrossRef]
- Farmer, E.; Van Rooij, J.; Riemersma, J.; Jorna, P. Handbook of Simulator-Based Training; Routledge: London, UK, 2017. [Google Scholar]
- Maran, N.J.; Glavin, R.J. Low-to high-fidelity simulation–a continuum of medical education? J. Med. Educ. 2003, 37, 22–28. [Google Scholar] [CrossRef]
- Munshi, F.; Lababidi, H.; Alyousef, S. Low-versus high-fidelity simulations in teaching and assessing clinical skills. J. Taibah Univ. Med. Sci. 2015, 10, 12–15. [Google Scholar] [CrossRef]
- Higgins, G.A.; Merrill, G.L.; Hettinger, L.J.; Kaufmann, C.R.; Champion, H.R.; Satava, R.M.J.P.T.; Environments, V. New simulation technologies for surgical training and certification: Current status and future projections. Presence Teleoper. Virtual Environ. 1997, 6, 160–172. [Google Scholar] [CrossRef]
- Aulmann, M.; Marz, M.; Burgener, I.A.; Alef, M.; Otto, S.; Mulling, C.K. Development and Evaluation of Two Canine Low-Fidelity Simulation Models. J. Vet. Med. Educ. 2015, 42, 151–160. [Google Scholar] [CrossRef]
- Fransson, B.A.; Ragle, C.A. Assessment of laparoscopic skills before and after simulation training with a canine abdominal model. J. Am. Vet. Med. Assoc. 2010, 236, 1079–1084. [Google Scholar] [CrossRef]
- Nagel, C.; Ille, N.; Aurich, J.; Aurich, C. Teaching of diagnostic skills in equine gynecology: Simulator-based training versus schooling on live horses. Theriogenology 2015, 84, 1088–1095. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, C.; Li, T.; Gan, T.J. Airway management education: Simulation based training versus non-simulation based training-A systematic review and meta-analyses. BMC Anesthesiol. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Youngquist, S.T.; Henderson, D.P.; Gausche-Hill, M.; Goodrich, S.M.; Poore, P.D.; Lewis, R.J. Paramedic self-efficacy and skill retention in pediatric airway management. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2008, 15, 1295–1303. [Google Scholar] [CrossRef] [PubMed]




| Simulators | Rat Simulator A | Rat Simulator B | Rat Simulator C | Rat Simulator D | Rat Simulator E | Mouse Simulator |
|---|---|---|---|---|---|---|
| General exterior |  |  |  |  |  |  |
| Body weight [gram] 1 | 220 | 150 | 550 | 410 | 350 | 35 |
| Body length, width 2, height 2,3 (L × W × H) [cm] | 16.5 × 4.5 × 6.5 | 17.0 × 4.5 × 7.0 | 21.5 × 6.0 × 5.0 | 21.0 × 4.5 × 6.5 | 25.0 × 6.5 × 8.0 | 7.5 × 2.5 × 3.0 |
| Tail length [cm] | 18 | 18.5 | 16.0 | 16.5 | 17.5 | 8.5 |
| Limb length 4 (forelimb /hindlimb) [cm] | 3.5/2.5 | 4.0/5.0 | 6.5/10.5 | 6.5/9.5 | 5.0/7.0 | 1.5/1.0 |
| Materials | silicon | silicon; soft vinyl chloride | silicon | hard plastic (inner body and eyes); fur-like material | hard plastic (head and eyes); foam (inner body); silicon (outer body layer); plastic wire (inner limb structures) | silicon; hard plastic (paws) |
| Training options | A; B; E; F; I | A; B; E; F; I | A; B; C; D; F; I | A; B; F; J | A; B; E; F; G; H; K; L | A; E; F |
| General Exterior | Simulators | |||||
|---|---|---|---|---|---|---|
| Simulator | Rat A | Rat B | Rat C | Rat D | Rat E | Mouse |
| Parameter | ||||||
| Overall appearance | 2.93 | 3.21 | 5.21 | 4.93 | 3.64 | 3.14 |
| Haptic | 3.57 | 4.50 | 5.57 | 4.71 | 4.00 | 3.07 |
| Mobility of the skin on the neck | 3.79 | 4.29 | 5.93 | 4.36 | 3.14 | 2.57 |
| Mobility of the skin on the flank | 3.86 | 4.43 | 6.07 | 5.00 | 3.71 | 2.57 |
| Consistency of the skin surface | 4.07 | 4.93 | 5.36 | 4.50 | 4.00 | 3.29 |
| Body size | 2.14 | 2.71 | 3.85 | 2.86 | 3.21 | 2.43 |
| Body weight | 2.21 | 3.29 | 4.29 | 3.86 | 3.57 | 2.29 |
| Body shape | 2.07 | 2.93 | 4.93 | 4.57 | 3.86 | 2.71 |
| Proportions | 2.14 | 2.79 | 4.64 | 4.71 | 3.71 | 3.00 |
| Gender-specific characteristics | 3.21 | 3.29 | 7.00 | 7.00 | 3.92 | 7.00 |
| Blood vessels | 3.86 | 3.71 | 4.57 | 5.62 | 4.62 | 4.08 |
| Position of the blood vessels | 3.29 | 2.86 | 4.64 | 5.62 | 4.07 | 3.93 |
| Course of the blood vessels | 3.29 | 2.86 | 5.14 | 5.85 | 4.14 | 3.57 |
| Consistency of the blood vessels | 4.07 | 3.21 | 5.07 | 5.69 | 4.93 | 4.29 |
| Mobility of joints | 5.79 | 6.07 | 5.93 | 5.07 | 5.36 | 6.50 |
| Mean score (Ø) | 3.35 | 3.67 | 5.21 | 4.96 | 3.99 | 3.63 |
| Overall mean score (Rats) | 4.24 | |||||
| HEAD | Simulators | |||||
|---|---|---|---|---|---|---|
| Simulator | Rat A | Rat B | Rat C | Rat D | Rat E | Mouse |
| Parameter | ||||||
| Overall appearance of the head | 2.86 | 3.43 | 5.43 | 4.36 | 3.36 | 3.43 |
| Head shape | 2.29 | 3.14 | 5.21 | 4.50 | 3.29 | 3.07 |
| Head proportions | 2.36 | 3.15 | 5.38 | 4.50 | 3.43 | 3.29 |
| Mobility of the head | 3.86 | 5.50 | 4.86 | 3.86 | 4.00 | 4.21 |
| Mobility of the lower jaw | 4.64 | 6.07 | 5.21 | 7.00 | 3.29 | 6.43 |
| Degree of mouth opening | 3.50 | 4.43 | 4.71 | 7.00 | 3.21 | 5.00 |
| Appearance of the mouth opening | 3.57 | 4.93 | 5.14 | 7.00 | 3.57 | 5.00 |
| Pharynx and larynx region | 3.93 | 5.36 | 5.71 | 7.00 | 4.07 | 6.79 |
| Teeth | 3.00 | 7.00 | 4.79 | 7.00 | 3.36 | 7.00 |
| Tongue | 4.36 | 5.50 | 4.64 | 7.00 | 3.86 | 7.00 |
| Eyes | 4.07 | 4.29 | 5.21 | 4.71 | 3.86 | 6.07 |
| Ears | 4.93 | 5.21 | 5.36 | 3.29 | 2.86 | 4.14 |
| Mean score (Ø) | 3.61 | 4.83 | 5.14 | 5.60 | 3.51 | 5.12 |
| Overall mean score (Rats) | 4.54 | |||||
| TAIL | Simulators | |||||
|---|---|---|---|---|---|---|
| Simulator | Rat A | Rat B | Rat C | Rat D | Rat E | Mouse |
| Parameter | ||||||
| Overall appearance of the tail | 2.57 | 2.93 | 4.54 | 5.07 | 2.50 | 3.07 |
| Haptic of the tail | 2.79 | 2.93 | 4.36 | 4.57 | 2.43 | 3.21 |
| Mobility of the tail | 2.71 | 2.64 | 3.64 | 3.79 | 2.36 | 2.86 |
| Skin texture | 2.93 | 3.36 | 4.86 | 4.93 | 2.71 | 3.36 |
| Length of the tail | 2.00 | 2.21 | 4.00 | 3.29 | 2.14 | 2.36 |
| Connection to the trunk/torso | 2.92 | 3.86 | 5.36 | 5.93 | 3.14 | 3.00 |
| Position of the tail’s blood vessels | 2.64 | 2.29 | 4.93 | 5.07 | 3.50 | 3.29 |
| Course of the tail’s blood vessels | 2.50 | 2.21 | 4.86 | 5.21 | 3.71 | 3.00 |
| Consistency of the tail’s blood vessels | 3.29 | 3.00 | 5.00 | 5.36 | 3.93 | 3.71 |
| Size of the tail’s blood vessels | 3.21 | 2.43 | 5.14 | 5.21 | 3.71 | 3.21 |
| Visibility of the tail’s blood vessels through the skin | 3.64 | 2.71 | 4.79 | 4.93 | 3.71 | 3.21 |
| Mean score (Ø) | 2.84 | 2.78 | 4.68 | 4.85 | 3.08 | 3.12 |
| Overall mean score (Rats) | 3.65 | |||||
| LIMBS | Simulator | |||||
|---|---|---|---|---|---|---|
| Simulator | Rat A | Rat B | Rat C | Rat D | Rat E | Mouse |
| Parameter | ||||||
| Overall appearance of the limbs | 3.86 | 4.36 | 5.50 | 4.43 | 4.07 | 4.50 |
| Haptic of the limbs | 4.79 | 4.93 | 5.50 | 4.43 | 4.50 | 5.36 |
| Proportions of the limbs | 3.36 | 3.71 | 5.14 | 4.14 | 3.64 | 4.29 |
| Posture of the limbs | 3.43 | 4.07 | 5.64 | 4.43 | 4.07 | 4.21 |
| Mobility of the limbs | 4.86 | 5.07 | 5.29 | 4.21 | 4.79 | 5.50 |
| Length of the limbs | 3.57 | 3.50 | 5.14 | 3.86 | 3.57 | 4.14 |
| Toes of the limbs | 4.93 | 5.79 | 5.29 | 4.79 | 3.86 | 3.79 |
| Mean score (Ø) | 4.11 | 4.49 | 5.36 | 4.33 | 4.07 | 4.54 |
| Overall mean score | 4.47 | |||||
| Ranking | Simulators | ||||
|---|---|---|---|---|---|
| Rat A | Rat B | Rat C | Rat D | Rat E | |
| Mean rank | 1.36 | 2.29 | 4.43 | 4.43 | 2.50 |
| Overall rank | 1. | 2. | 4. | 4. | 3. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corte, G.M.; Humpenöder, M.; Pfützner, M.; Merle, R.; Wiegard, M.; Hohlbaum, K.; Richardson, K.; Thöne-Reineke, C.; Plendl, J. Anatomical Evaluation of Rat and Mouse Simulators for Laboratory Animal Science Courses. Animals 2021, 11, 3432. https://doi.org/10.3390/ani11123432
Corte GM, Humpenöder M, Pfützner M, Merle R, Wiegard M, Hohlbaum K, Richardson K, Thöne-Reineke C, Plendl J. Anatomical Evaluation of Rat and Mouse Simulators for Laboratory Animal Science Courses. Animals. 2021; 11(12):3432. https://doi.org/10.3390/ani11123432
Chicago/Turabian StyleCorte, Giuliano M., Melanie Humpenöder, Marcel Pfützner, Roswitha Merle, Mechthild Wiegard, Katharina Hohlbaum, Ken Richardson, Christa Thöne-Reineke, and Johanna Plendl. 2021. "Anatomical Evaluation of Rat and Mouse Simulators for Laboratory Animal Science Courses" Animals 11, no. 12: 3432. https://doi.org/10.3390/ani11123432
APA StyleCorte, G. M., Humpenöder, M., Pfützner, M., Merle, R., Wiegard, M., Hohlbaum, K., Richardson, K., Thöne-Reineke, C., & Plendl, J. (2021). Anatomical Evaluation of Rat and Mouse Simulators for Laboratory Animal Science Courses. Animals, 11(12), 3432. https://doi.org/10.3390/ani11123432






