Age at First Gestation in Beef Heifers Affects Fetal and Postnatal Growth, Glucose Metabolism and IGF1 Concentration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Milk Production and Composition
2.3. Calf Measurement
2.4. Blood Collection and Assays
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Patterson, D.J.; Perry, R.C.; Kiracofe, G.H.; Bellows, R.A.; Staigmiller, R.B.; Corah, L.R. Management considerations in heifer development and puberty. J. Anim. Sci. 1992, 70, 4018–4035. [Google Scholar] [CrossRef]
- Brooks, A.; Morrow, R.; Youngquist, R. Body composition of beef heifers at puberty. Theriogenology 1985, 24, 235–250. [Google Scholar] [CrossRef]
- Ferrell, C.L.; Jenkins, T.G. Cow Type and the Nutritional Environment: Nutritional Aspects. J. Anim. Sci. 1985, 61, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, J.N.; Kasson, C.W.; Ingalls, J.E. Puberty in Crossbred and Straightbred Beef Heifers on two Levels of Feed. J. Anim. Sci. 1969, 29, 602–605. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Robinson, D.L.; Cafe, L.M.; Greenwood, P.L. Meat Science and Muscle Biology Symposium: Developmental programming in cattle: Consequences for growth, efficiency, carcass, muscle, and beef quality characteristics1,2. J. Anim. Sci. 2013, 91, 1428–1442. [Google Scholar] [CrossRef]
- Bellows, R.A.; Short, R.E.; Richardson, G.V. Effects of Sire, Age of Dam and Gestation Feed Level on Dystocia and Postpartum Reproduction. J. Anim. Sci. 1982, 55, 18–27. [Google Scholar] [CrossRef]
- LeMaster, C.; Taylor, R.; Ricks, R.; Long, N. The effects of late gestation maternal nutrient restriction with or without protein supplementation on endocrine regulation of newborn and postnatal beef calves. Theriogenology 2017, 87, 64–71. [Google Scholar] [CrossRef]
- Bauer, M.K.; Breier, B.H.; Harding, J.E.; Veldhuis, J.D.; Gluckman, P.D. The fetal somatotropic axis during long term maternal undernutrition in sheep: Evidence for nutritional regulation in utero. Endocrinology 1995, 136, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Brameld, J.; Mostyn, A.; Dandrea, J.; Stephenson, T.; Dawson, J.; Buttery, P.; Symonds, M.; Symonds, M. Maternal nutrition alters the expression of insulin-like growth factors in fetal sheep liver and skeletal muscle. J. Endocrinol. 2000, 167, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Long, N.; Pavan, E.; Quintans, G. Effect of protein restriction of bovine dams during late gestation on offspring postnatal growth, glucose-insulin metabolism and IGF-1 concentration. Livest. Sci. 2018, 212, 120–126. [Google Scholar] [CrossRef]
- López Valiente, S.; Maresca, S.; Rodríguez, A.M.; Long, M.M.; Quintans, G.; Palladino, R.A. Effect of Protein Restriction During Mid-to Late Gestation of Beef Cows on Female Offspring Fertility, Lactation Performance and Calves Development. EC Vet. Sci. 2019, 4, 1–12. [Google Scholar]
- Wagner, J.J.; Lusby, K.S.; Oltjen, J.W.; Rakestraw, J.; Wettemann, R.P.; Walters, L.E. Carcass Composition in Mature Hereford Cows: Estimation and Effect on Daily Metabolizable Energy Requirement during Winter. J. Anim. Sci. 1988, 66, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Quintans, G.; Banchero, G.; Carriquiry, M.; López-Mazz, C.; Baldi, F. Effect of body condition and suckling restriction with and without presence of the calf on cow and calf performance. Anim. Prod. Sci. 2010, 50, 931–938. [Google Scholar] [CrossRef]
- Restle, J.; Pacheco, P.S.; Pascoal, L.L.; Pádua, J.T.; Moletta, J.L.; Freitas, A.K.D.; Leite, D.T. Efeito da Pastagem, da Produção e da Composição do Leite no Desempenho de Bezerros de Diferentes Grupos Genéticos. Rev. Bras. Zootec. 2004, 33, 691–703. [Google Scholar] [CrossRef]
- Sharma, R.K.; Blair, H.T.; Jenkinson, C.M.C.; Kenyon, P.R.; Cockrem, J.; Parkinson, T.J. Uterine environment as a regulator of birth weight and body dimensions of newborn lambs1. J. Anim. Sci. 2012, 90, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Wittrock, J.; Duffield, T.; LeBlanc, S. Short communication: Validation of a point-of-care glucometer for use in dairy cows. J. Dairy Sci. 2013, 96, 4514–4518. [Google Scholar] [CrossRef] [PubMed]
- Lacau-Mengido, I.M.; Mejía, M.E.; Díaz-Torga, G.S.; Iglesias, A.G.; Formía, N.; Libertun, C.; Becú-Villalobos, D. Endocrine studies in ivermectin-treated heifers from birth to puberty. J. Anim. Sci. 2000, 78, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-K.; Wen, S.W.; Fleming, N.; Demissie, K.; Rhoads, G.G.; Walker, M. Teenage pregnancy and adverse birth outcomes: A large population based retrospective cohort study. Int. J. Epidemiology 2007, 36, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Bourke, D.A.; Aitken, R.P.; Leitch, N.; Hay, W.W. Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am. J. Physiol. Integr. Comp. Physiol. 2002, 282, R1027–R1036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luther, J.S.; Redmer, D.A.; Reynolds, L.P.; Wallace, J.M. Nutritional paradigms of ovine fetal growth restriction: Implications for human pregnancy. Hum. Fertil. 2005, 8, 179–187. [Google Scholar] [CrossRef]
- Kamal, M.; Van Eetvelde, M.; Depreester, E.; Hostens, M.; Vandaele, L.; Opsomer, G. Age at calving in heifers and level of milk production during gestation in cows are associated with the birth size of Holstein calves. J. Dairy Sci. 2014, 97, 5448–5458. [Google Scholar] [CrossRef] [PubMed]
- Winks, L.; O’Rourke, P.; Venamore, P.; Tyler, R. Factors affecting birth weight and performance to weaning of beef calves in the dry tropics of north Queensland. Aust. J. Exp. Agric. 1978, 18, 494–499. [Google Scholar] [CrossRef]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Wilsher, S.; Allen, W.R. The influences of maternal size, age and parity on placental and fetal development in the horse. Theriogenology 2002, 58, 833–835. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Means, W.J.; Hess, B.W.; Nathanielsz, P.; Du, M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J. Physiol. 2006, 575, 241–250. [Google Scholar] [CrossRef]
- Platz, E.; Newman, R. Diagnosis of IUGR: Traditional Biometry. Semin. Perinatol. 2008, 32, 140–147. [Google Scholar] [CrossRef]
- McMillen, I.C.; Adams, M.B.; Ross, J.; Coulter, C.L.; Simonetta, G.; Owens, J.; Robinson, J.S.; Edwards, L.J. Fetal growth restriction: Adaptations and consequences. Reproduction 2001, 122, 195–204. [Google Scholar] [CrossRef]
- Barker, D. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115. [Google Scholar] [CrossRef]
- López Valiente, S.; Maresca, S.; Rodriguez, A.; Palladino, R.; Lacau-Mengido, I.; Long, N.; Quintans, G. Effect of protein restriction of Angus cows during late gestation: Subsequent reproductive performance and milk yield. Prof. Anim. Sci. 2018, 34, 261–268. [Google Scholar] [CrossRef]
- Rodrigues, P.F.; Menezes, L.M.; Azambuja, R.C.C.; Suñé, R.W.; Silveira, I.D.B.; Cardoso, F.F. Milk yield and composition from Angus and Angus-cross beef cows raised in southern Brazil1. J. Anim. Sci. 2014, 92, 2668–2676. [Google Scholar] [CrossRef]
- López Valiente, S.; Rodriguez, A.M.; Long, N.M.; Lacau-Mengido, I.M.; Maresca, S. The degree of maternal nutrient restriction during late gestation influences the growth and endocrine profiles of offspring from beef cows. Anim. Prod. Sci. 2021. [Google Scholar] [CrossRef]
- Hansen, P.J.; Baik, D.H.; Rutledge, J.J.; Hauser, E.R. Genotype × Environmental Interactions on Reproductive Traits of Bovine Females. II. Postpartum Reproduction as Influenced by Genotype, Dietary Regimen, Level of Milk Production and Parity3. J. Anim. Sci. 1982, 55, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.L.; Smith, E.F.; Schalles, R.R.; Brent, B.E.; Corah, L.R.; Pruitt, R.J. Effects of Milk and Forage Intake on Calf Performance. J. Anim. Sci. 1980, 51, 550–553. [Google Scholar] [CrossRef]
- Clutter, A.C.; Nielsen, M.K. Effect of Level of Beef Cow Milk Production on Pre- and Postweaning Calf Growth. J. Anim. Sci. 1987, 64, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. NRC—Nutrient Requirements of Beef Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Fox, D.G.; Sniffen, C.J.; Connor, J.D.O. Adjusting nutrient requirements of beff cattle for animal and environmental variations. J. Anim. Sci. 1988, 66, 1475–1495. [Google Scholar] [CrossRef]
- Johnson, C.R.; Lalman, D.L.; Brown, M.A.; Appeddu, L.A.; Buchanan, D.S.; Wettemann, R.P. Influence of milk production potential on forage dry matter intake by multiparous and primiparous Brangus females. J. Anim. Sci. 2003, 81, 1837–1846. [Google Scholar] [CrossRef]
- Miller, S.P.; Wilton, J.W.; Pfeiffer, W.C. Effects of milk yield on biological efficiency and profit of beef production from birth to slaughter. J. Anim. Sci. 1999, 77, 344–352. [Google Scholar] [CrossRef]
- MacNeil, M.D.; Mott, T.B. Genetic analysis of gain from birth to weaning, milk production, and udder conformation in Line 1 Hereford cattle1,2. J. Anim. Sci. 2006, 84, 1639–1645. [Google Scholar] [CrossRef]
- Liu, T.; Mays, A.R.; Turner, K.E.; Wu, J.P.; Brown, M.A. Relationships of milk yield and quality from six breed groups of beef cows to preweaning average daily gain of their calves1. J. Anim. Sci. 2015, 93, 1859–1864. [Google Scholar] [CrossRef]
- Brown, M.; Brown, A. Relationship of milk yield and quality to preweaning gain of calves from Angus, Brahman and reciprocal-cross cows on different forage systems. J. Anim. Sci. 2002, 80, 2522–2527. [Google Scholar]
- Beal, W.E.; Notter, D.R.; Akers, R.M. Techniques for estimation of milk yield in beef cows and relationships of milk yield to calf weight gain and postpartum reproduction. J. Anim. Sci. 1990, 68, 937–943. [Google Scholar] [CrossRef]
- Marston, T.T.; Lusby, K.S.; Wettemann, R.P.; Purvis, H.T. Performance of spring-calving cows grazing native range Effects of Feeding Energy or Protein Supplements Before or After Calving on Performance of Cows Grazing Native Range. J. Anim. Sci. 1995, 73, 657–664. [Google Scholar] [CrossRef]
- Fowden, A.L.; Forsling, M.L.; Kelestimur, H.; Windle, R. Effects of Arginine and Glucose on the Release of Insulin in the Sheep Fetus. J. Endocrinol. 1980, 85, 121–129. [Google Scholar] [CrossRef]
- Mccarty, K.; Washburn, J.; Taylor, R.; Long, N. The effects of early- or mid-gestation nutrient restriction on bovine fetal pancreatic development. Domest. Anim. Endocrinol. 2020, 70, 106377. [Google Scholar] [CrossRef] [PubMed]
- Haldre, K.; Rahu, K.; Karro, H.; Rahu, M. Is a poor pregnancy outcome related to young maternal age? A study of teenagers in Estonia during the period of major socio-economic changes (from 1992 to 2002). Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 45–51. [Google Scholar] [CrossRef]
- Mericq, V.; Ong, K.K.; Bazaes, R.; Peña, V.; Avila, A.; Salazar, T.; Soto, N.; Iñiguez, G.; Dunger, D.B. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 2005, 48, 2609–2614. [Google Scholar] [CrossRef]
- Gardner, D.S.; Tingey, K.; Van Bon, B.W.M.; Ozanne, S.E.; Wilson, V.; Dandrea, J.; Keisler, D.H.; Stephenson, T.; Symonds, M.E. Programming of glucose-insulin metabolism in adult sheep after maternal undernutrition. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R947–R954. [Google Scholar] [CrossRef]
- Ford, S.P.; Hess, B.W.; Schwope, M.M.; Nijland, M.; Gilbert, J.S.; Vonnahme, K.A.; Means, W.J.; Han, H.; Nathanielsz, P. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 2007, 85, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Pavan, E.; Quintans, G.; Long, N. Late-gestation protein restriction negatively impacts muscle growth and glucose regulation in steer progeny. Domest. Anim. Endocrinol. 2019, 69, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Long, N.M.; Prado-Cooper, M.J.; Krehbiel, C.R.; Wettemann, R.P. Effects of nutrient restriction of bovine dams during early gestation on postnatal growth and regulation of plasma glucose1. J. Anim. Sci. 2010, 88, 3262–3268. [Google Scholar] [CrossRef]
- Brameld, J.; Buttery, P.J.; Dawson, J.M.; Harper, J.M.M. Nutritional and hormonal control of skeletal-muscle cell growth and differentiation. Proc. Nutr. Soc. 1998, 57, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.D.; Hossner, K.L.; Williams, S.E.; Wallace, C.R.; Niswender, G.D.; Odde, K.G. Serum concentrations of insul-like growth factors and placental lactogen during gestation in cattle. Domest. Anim. Endocrinol. 1997, 14, 231–239. [Google Scholar] [CrossRef]
- Owens, J.; Kind, K.L.; Carbone, F.; Robinson, J.S.; Owens, P.C. Circulating insulin-like growth factors-I and -II and substrates in fetal sheep following restriction of placental growth. J. Endocrinol. 1994, 140, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Oksbjerg, N.; Gondret, F.; Vestergaard, M. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest. Anim. Endocrinol. 2004, 27, 219–240. [Google Scholar] [CrossRef]
- Gicquel, C.; Le Bouc, Y. Hormonal Regulation of Fetal Growth. Horm. Res. Paediatr. 2006, 65, 28–33. [Google Scholar] [CrossRef]
- Wallace, J.M.; Aitken, R.P.; Milne, J.S.; Hay, J.W.W. Nutritionally Mediated Placental Growth Restriction in the Growing Adolescent: Consequences for the Fetus1. Biol. Reprod. 2004, 71, 1055–1062. [Google Scholar] [CrossRef]
- Wade, G.N.; Schneider, J.E. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 1992, 16, 235–272. [Google Scholar] [CrossRef]
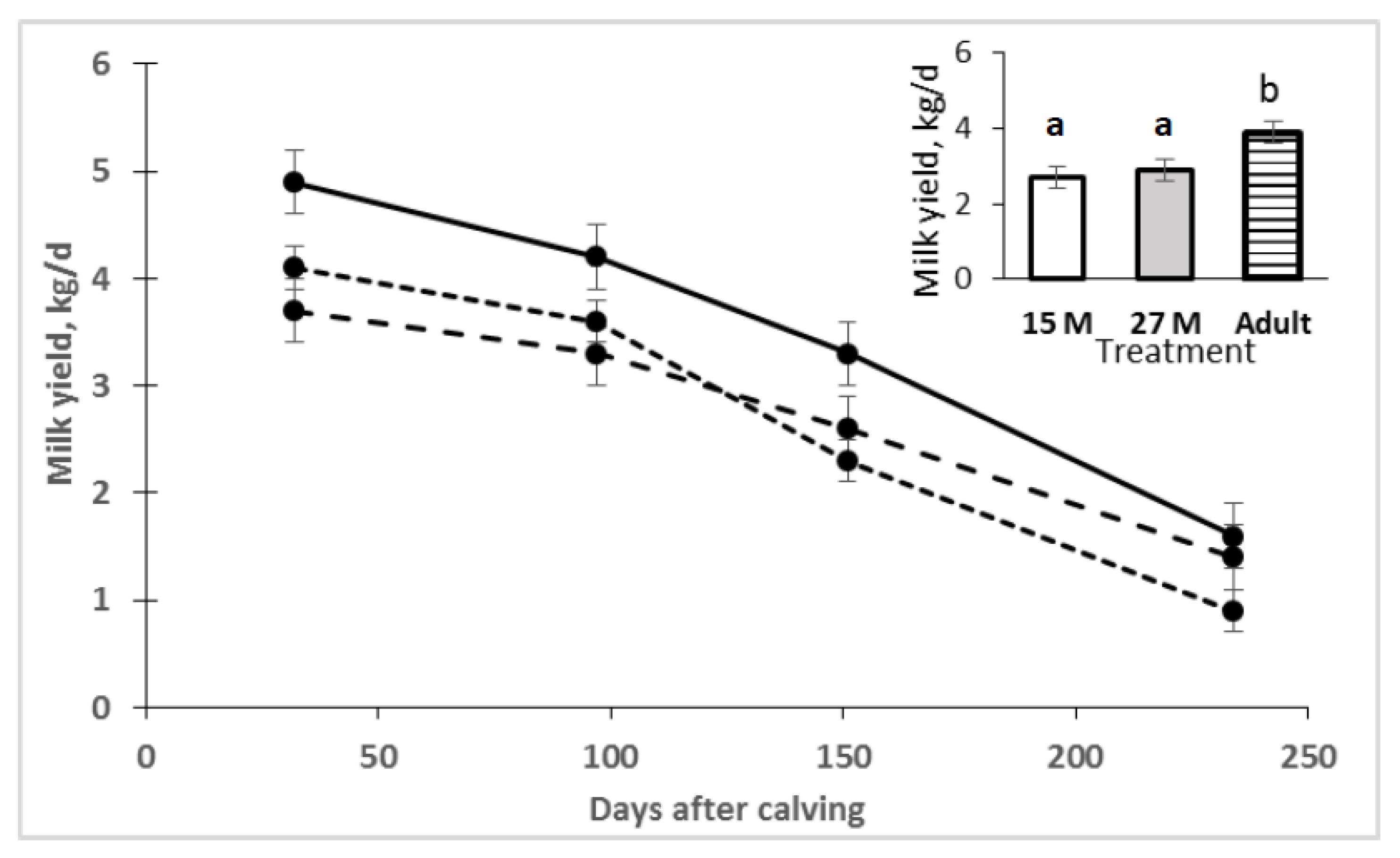
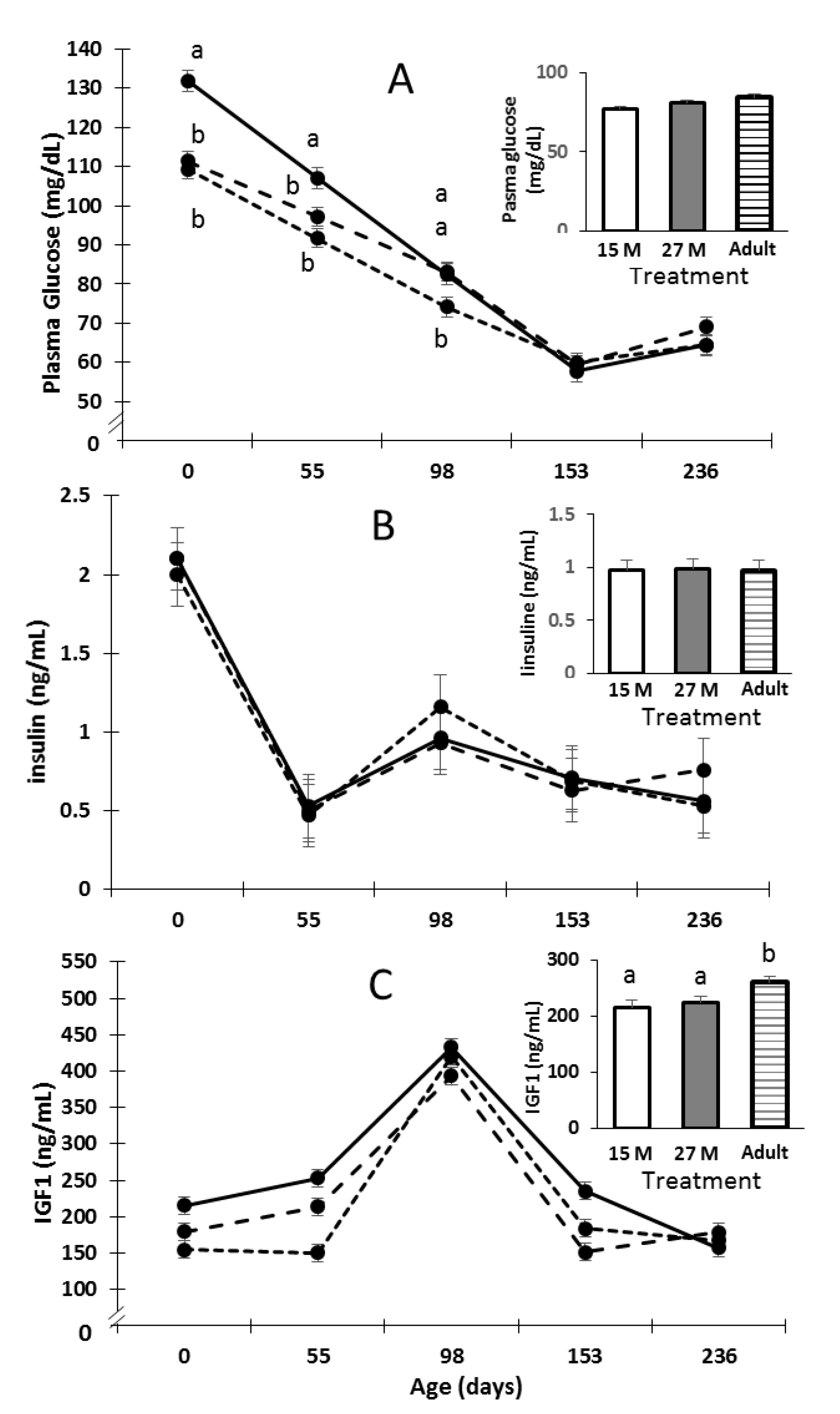
| Treatments 1 | |||||
|---|---|---|---|---|---|
| Item | 15 M | 27 M | Adult | SEM | p-Value |
| BW, kg | |||||
| Initial 2 | 320 a | 394 b | 437 c | 8.6 | <0.001 |
| at calving | 343 a | 423 b | 504 c | 7.7 | <0.001 |
| at weaning | 321 a | 383 b | 450 c | 8.5 | <0.001 |
| BCS 3 | |||||
| Initial | 5.3 a | 5.6 a | 4.8 b | 0.07 | <0.001 |
| at calving | 5.4 a | 5.4 a | 5.0 b | 0.04 | 0.03 |
| at weaning | 4.0 a | 4.2 a,b | 4.4 b | 0.06 | 0.02 |
| Treatments 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 15 M | 27 M | Adult | SEM | Treat 2 | Sex 3 | Treat x Sex |
| Gestation length, d | 282.8 a | 282.1 a | 279.6 b | 0.75 | 0.009 | 0.30 | 0.38 |
| BW at birth, kg | 28.0 a | 30.3 b | 33.0 c | 0.96 | 0.002 | <0.001 | 0.60 |
| BW at weaning, kg | 158.1 a | 181.1 b | 206.4 c | 5.2 | <0.001 | 0.08 | 0.78 |
| ADG, kg/d | 0.590 a | 0.680 b | 0.790 c | 0.02 | <0.001 | 0.32 | 0.62 |
| Head circ. 4, cm | 45.7 a | 45.9 a | 47.2 b | 0.44 | 0.03 | 0.15 | 0.27 |
| Heart girth, cm | 71.8 a | 74.2 b | 75.3 b | 0.7 | 0.009 | 0.01 | 0.39 |
| Cannon circ. 4, cm | 11.7 | 11.5 | 12.0 | 0.2 | 0.15 | 0.004 | 0.84 |
| Body length, cm | 72.1 a | 70.3 a | 76.2 b | 1.4 | 0.01 | 0.90 | 0.13 |
| Height, cm | 70.4 a | 70.7 a | 73.9 b | 1.1 | 0.03 | 0.54 | 0.85 |
| Treatments 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 15 M | 27 M | Adult | SEM | Treat 2 | Sex 3 | Treat x Sex |
| Head cir. 4/birth BW, cm/kg | 1.6 a | 1.5 b | 1.4 c | 0.04 | 0.005 | 0.0008 | 0.92 |
| Heart girth/birth BW, cm/kg | 2.6 a | 2.5 a | 2.3 b | 0.06 | 0.01 | 0.001 | 0.84 |
| Cannon cir. 4 /birth BW, cm/kg | 0.42 a | 0.39 b | 0.37 b | 0.01 | 0.002 | 0.03 | 0.41 |
| Body length/birth BW, cm/kg | 2.6 a | 2.3 b | 2.4 b | 0.07 | 0.04 | 0.0009 | 0.69 |
| Height/birth BW, cm/kg | 2.6 a | 2.4 a | 2.3 b | 0.05 | 0.001 | <0.001 | 0.41 |
| Body mass index | 3.3 a | 3.5 a | 3.8 b | 0.09 | 0.002 | 0.0002 | 0.66 |
| Treatments 1 | |||||||
|---|---|---|---|---|---|---|---|
| Item | 15 M | 27 M | Adult | SEM | Treat 2 | Period | Treat × Period |
| Fat, g/100 mL | 3.7 a | 2.9 b | 2.9 b | 0.1 | <0.0001 | <0.0001 | 0.11 |
| Protein, g/100 mL | 3.28 a | 3.45 b | 3.34 a | 0.06 | 0.007 | <0.0001 | 0.11 |
| Urea, mg/dL | 12.5 a | 12.7 a | 11.6 b | 0.3 | 0.04 | <0.0001 | 0.36 |
| Lactose, g/100 mL | 4.4 a | 4.3 a | 4.6 b | 0.06 | 0.03 | <0.0001 | 0.30 |
| Total solids, g/100 mL | 12.4 a | 11.8 b | 11.8 b | 0.2 | <0.0001 | 0.0002 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Valiente, S.; Rodríguez, A.M.; Long, N.M.; Quintans, G.; Miccoli, F.E.; Lacau-Mengido, I.M.; Maresca, S. Age at First Gestation in Beef Heifers Affects Fetal and Postnatal Growth, Glucose Metabolism and IGF1 Concentration. Animals 2021, 11, 3393. https://doi.org/10.3390/ani11123393
López Valiente S, Rodríguez AM, Long NM, Quintans G, Miccoli FE, Lacau-Mengido IM, Maresca S. Age at First Gestation in Beef Heifers Affects Fetal and Postnatal Growth, Glucose Metabolism and IGF1 Concentration. Animals. 2021; 11(12):3393. https://doi.org/10.3390/ani11123393
Chicago/Turabian StyleLópez Valiente, Sebastian, Alejandro M. Rodríguez, Nathan M. Long, Graciela Quintans, Florencia E. Miccoli, Isabel M. Lacau-Mengido, and Sebastian Maresca. 2021. "Age at First Gestation in Beef Heifers Affects Fetal and Postnatal Growth, Glucose Metabolism and IGF1 Concentration" Animals 11, no. 12: 3393. https://doi.org/10.3390/ani11123393
APA StyleLópez Valiente, S., Rodríguez, A. M., Long, N. M., Quintans, G., Miccoli, F. E., Lacau-Mengido, I. M., & Maresca, S. (2021). Age at First Gestation in Beef Heifers Affects Fetal and Postnatal Growth, Glucose Metabolism and IGF1 Concentration. Animals, 11(12), 3393. https://doi.org/10.3390/ani11123393







