Progesterone Concentrations during Canine Pregnancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
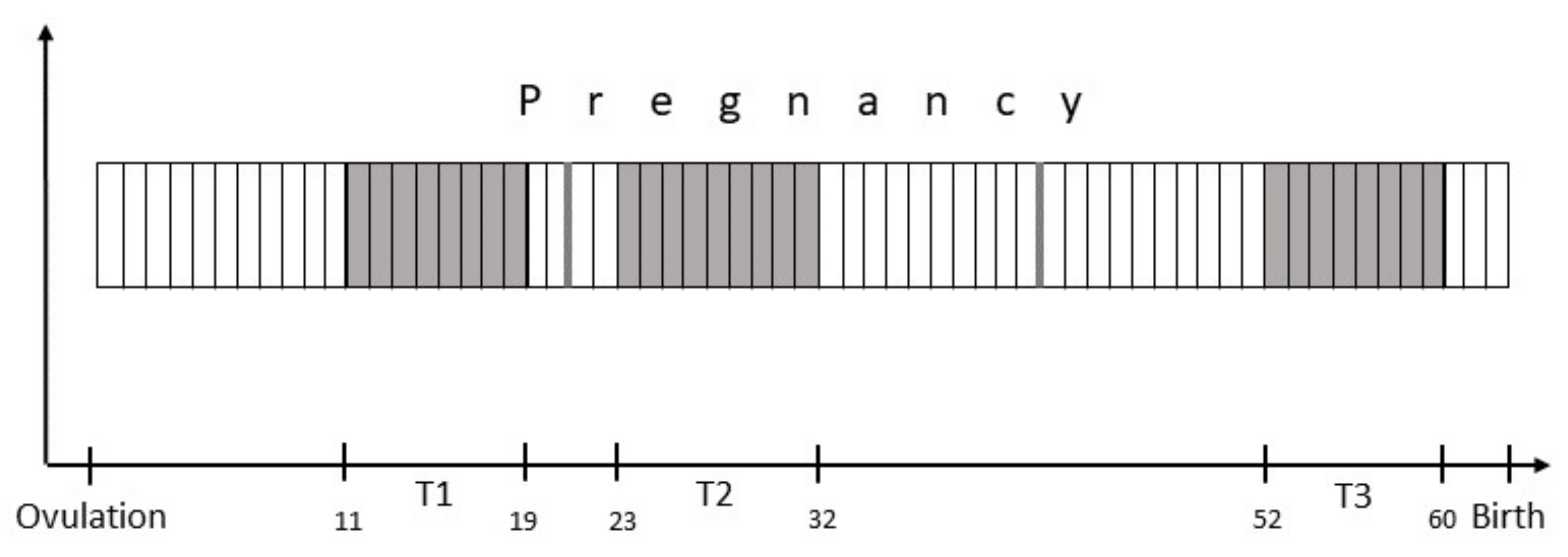
2.2. Study Design
2.3. Blood Sampling and Sample Processing
2.4. Statistical Analysis
3. Results
3.1. General Information
3.2. Delivery and Litter Size
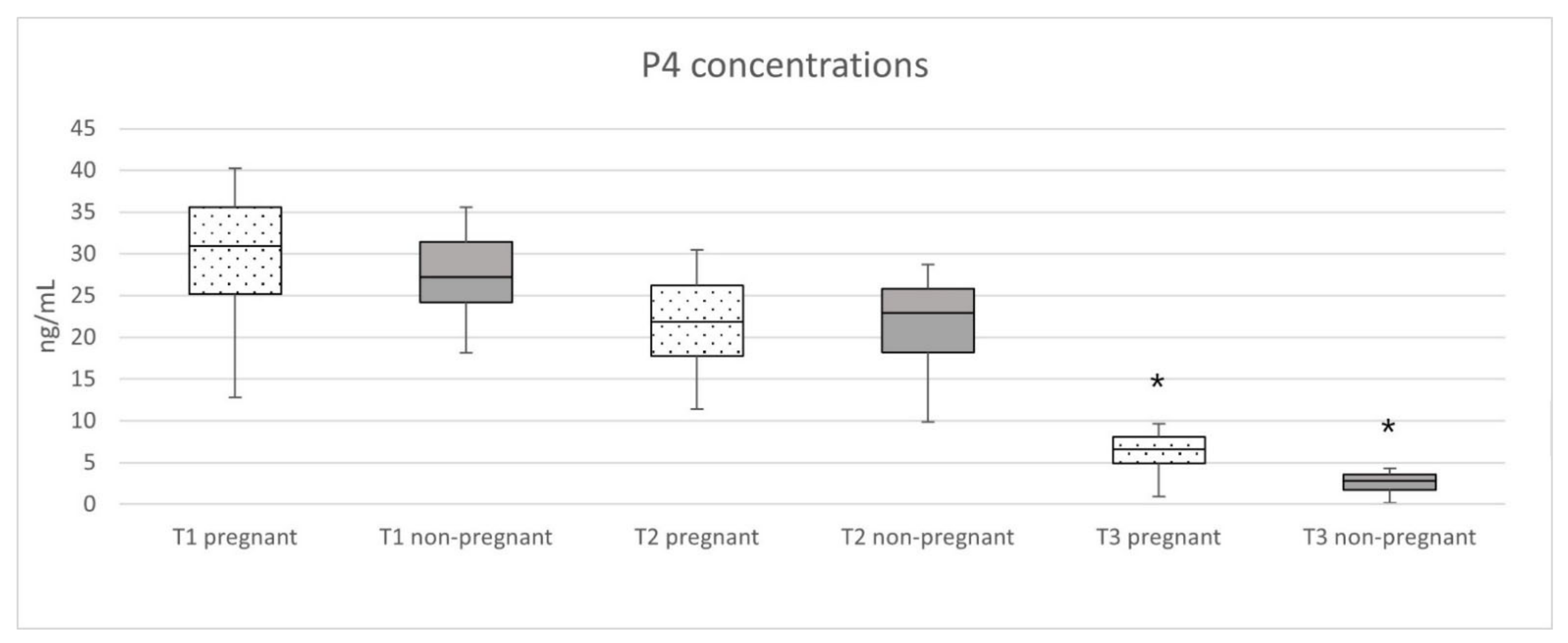
3.3. Progesterone Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nr | Breed | Breed Group | Age (y) | Weight (kg) | Previous Litters | Previous Litter Sizes | Pregnant/Non- Pregnant | C- Section | Litter Size (incl. Stillborn) | ♂ | ♀ | Stillborn or | Deceased |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | Miniature Schnauzer | Terrier | 2.5 | 6.3 | NP | ||||||||
| 002 | Gordon Setter | Sporting | 3.8 | 25.0 | P | 9 | 2 | 7 | |||||
| 003 | Beagle | Hound | 6.8 | 14.6 | 2 | 7/7 | P | 7 | 3 | 4 | |||
| 004 | English Cocker Spaniel | Sporting | 5.9 | 13.2 | 4 | 8/5/2/5 | P | 3 | 3 | 0 | |||
| 005 | English Springer Spaniel | Sporting | 2.9 | 20.4 | 1 | 6 | NP | ||||||
| 006 | Dogues des Bordeaux | Working | 7.0 | 51.3 | 4 | 11/12/12/13 | P | 2 | 1 | 1 | |||
| 007 | Rottweiler | Working | 3.1 | 43.6 | P | CS | 7 | 0 | 3 | 4 | |||
| 008 | Petit Brabançon | Toy | 3.6 | 3.6 | 1 | 1 | P | 5 | 2 | 3 | 1 | ||
| 009 | Standard Wire-Haired Dachshund | Hound | 2.2 | 7.8 | P | 5 | 3 | 2 | |||||
| 010 | Standard Wire-Haired Dachshund | Hound | 5.0 | 5.6 | 2 | 5/5 | P | 4 | 2 | 2 | |||
| 011 | Dobermann | Working | 4.2 | 38.8 | 1 | 3 | NP | ||||||
| 012 | Miniature Smooth Haired Dachshund | Hound | 4.3 | 5.8 | 2 | 5/4 | P | 6 | 1 | 5 | |||
| 013 | Beagle | Hound | 2.6 | 11.4 | P | 5 | 3 | 2 | |||||
| 014 | Rhodesian Ridgeback | Hound | 4.9 | 38.0 | 2 | 8/10 | P | 12 | 8 | 3 | 1 | ||
| 015 | Chihuahua | Toy | 2.3 | 2.4 | 1 | 2 | P | 3 | 1 | 2 | |||
| 016 | Boxer | Working | 2.9 | 28.8 | P | CS | 3 | 2 | 0 | 1 | |||
| 017 | Irish Terrier | Terrier | 5.7 | 12.8 | 1 | 16 | P | 7 | 2 | 1 | 4 | ||
| 018 | Smooth Collie | Herding | 1.3 | 19.6 | P | 5 | 3 | 2 | 1 | ||||
| 019 | Rhodesian Ridgeback | Hound | 4.9 | 32.2 | P | 13 | 6 | 6 | 1 | ||||
| 020 | Picardy Shepherd | Herding | 2.2 | 23.5 | NP | ||||||||
| 021 | Saarloos Wolfdog | Hound | 6.2 | 28.8 | 2 | 5/6 | P | 5 | 2 | 3 | |||
| 022 | Standard Smooth Haired Dachshund | Hound | 3.7 | 7.2 | 1 | 5 | P | 6 | 2 | 4 | |||
| 023 | Miniature Bull Terrier | Terrier | 5.8 | 17.0 | 1 | 7 | NP | ||||||
| 024 | Miniature Australian Shepherd | Herding | 3.3 | 12.2 | NP | ||||||||
| 025 | Collie | Herding | 6.3 | 24.0 | 2 | 7/1 | P | 5 | 2 | 3 | |||
| 026 | Cavalier King Charles Spaniel | Toy | 3.7 | 7.0 | 1 | 3 | P | 4 | 2 | 2 | |||
| 027 | Dutch Sheepdog | Herding | 5.0 | 14.4 | 1 | 7 | P | 6 | 4 | 2 | |||
| 028 | Borzoi | Hound | 8.1 | 36.4 | P | CS | 8 | 4 | 4 | ||||
| 029 | Miniature Wire-Haired Dachshund | Hound | 1.6 | 4.2 | P | 4 | 3 | 1 | |||||
| 030 | Miniature Poodle | Non-Sporting | 1.9 | 7.6 | P | 4 | 2 | 2 | |||||
| 031 | Hovawart | Working | 3.4 | 35.8 | 1 | 10 | P | 12 | 4 | 8 | |||
| 032 | Miniature Wire-Haired Dachshund | Hound | 6.9 | 5.5 | 4 | 6/6/6/6 | P | 6 | 5 | 1 | |||
| 033 | Jack Russell Terrier | Terrier | 1.6 | 5.6 | P | 4 | 3 | 1 | |||||
| 034 | Afghan Hound | Hound | 4.1 | 24.4 | NP | ||||||||
| 035 | Labrador Retriever | Sporting | 2.6 | 22.4 | P | 9 | 7 | 2 | |||||
| 036 | Vizsla | Sporting | 3.6 | 24.0 | 1 | 9 | NP | ||||||
| 037 | Hovawart | Working | 1.9 | 32.4 | P | 9 | 4 | 5 | |||||
| 038 | German Shepherd | Herding | 7.2 | 32.4 | 4 | 5/7/7/6 | P | 8 | 3 | 4 | 1 | ||
| 039 | Staffordshire Bull Terrier | Terrier | 1.8 | 14.2 | P | 1 | 1 | ||||||
| 040 | French Bulldog | Non-Sporting | 2.7 | 13.8 | P | 6 | 2 | 3 | 1 | ||||
| 041 | Pyrenean Shepherd | Herding | 5.5 | 13.0 | 2 | 5/1 | P | 1 | 1 | ||||
| 042 | Standard Smooth Haired Dachshund | Hound | 3.3 | 10.2 | 1 | 4 | P | 6 | 2 | 4 | |||
| 043 | French Bulldog | Non-Sporting | 1.9 | 12.4 | unclear | P | 7 | 2 | 5 | ||||
| 044 | Kromfohrlander | Non-Sporting | 5.2 | 10.4 | 1 | 8 | P | 7 | 3 | 4 | |||
| 045 | French Bulldog | Non-Sporting | 1.4 | 10.8 | P | 10 | 8 | 1 | 1 | ||||
| 046 | French Bulldog | Non-Sporting | 2.6 | 11.8 | 1 | 3 | P | 3 | 0 | 2 | 1 | ||
| 047 | Newfoundland Dog | Working | 3.5 | 45.0 | NP | ||||||||
| 048 | Standard Schnauzer | Working | 7.8 | 18.2 | 1 | 5 | P | 6 | 3 | 3 | |||
| 049 | Miniature Wire-Haired Dachshund | Hound | 6.1 | 5.5 | 3 | 5/5/6 | P | 3 | 2 | 1 | |||
| 050 | Old English Bulldog | Non-Sporting | 3.0 | 28.4 | 1 | 11 | P | 9 | 5 | 3 | 1 | ||
| 051 | Parson Russell Terrier | Terrier | 7.2 | 7.0 | 3 | 4/3/3 | P | 2 | 1 | 1 | |||
| 052 | German Shepherd | Herding | 7.9 | 33.6 | 4 | 6/9/8/6 | NP | ||||||
| 053 | Old English Bulldog | Non-Sporting | 2.0 | 32.2 | P | 12 | 6 | 5 | 1 | ||||
| 054 | Miniature Bull Terrier | Terrier | 4.7 | 19.0 | 1 | 8 | P | CS | 7 | 6 | 1 | ||
| 055 | Collie | Herding | 6.1 | 29.6 | 1 | 8 | NP | ||||||
| 056 | Giant Schnauzer | Working | 2.4 | 37.6 | P | 6 | 4 | 1 | 1 | 1 | |||
| 057 | Tervuren | Herding | 5.8 | 19.6 | 1 | 5 | P | 6 | 4 | 2 | |||
| 058 | Miniature Bull Terrier | Terrier | 4.8 | 16.4 | 1 | 7 | P | 6 | 1 | 3 | 2 | ||
| 059 | Scottish Terrier | Terrier | 5.5 | 8.8 | 1 | 1 | P | CS | 3 | 0 | 3 | ||
| 060 | Weimaraner | Sporting | 3.4 | 30.8 | P | 8 | 3 | 5 | |||||
| 061 | Cairn Terrier | Terrier | 1.9 | 8.0 | P | 2 | 1 | 1 | |||||
| 062 | Australian Shepherd | Herding | 6.4 | 26.8 | NP | ||||||||
| 063 | Affenpinscher | Toy | 6.1 | 7.8 | 2 | 2/7 | P | CS | 6 | 1 | 4 | 1 | |
| 064 | Standard Wire-Haired Dachshund | Hound | 4.9 | 9.0 | 2 | 7/6 | P | 7 | 3 | 4 | |||
| 065 | Collie | Herding | 1.6 | 22.0 | P | 2 | 1 | 1 | |||||
| 066 | Vizsla | Sporting | 4.0 | 26.4 | 1 | 8 | P | 10 | 5 | 5 | 1 | ||
| 067 | Boxer | Working | 6.8 | 27.8 | 1 | 6 | P | 4 | 1 | 3 | 2 | ||
| 068 | Pug | Toy | 2.9 | 7.6 | NP | ||||||||
| 069 | Dobermann | Working | 4.9 | 38.6 | 1 | 3 | P | 9 | 4 | 2 | 3 | 3 | |
| 070 | Jack Russell Terrier | Terrier | 4.1 | 5.5 | 1 | 4 | P | 3 | 2 | 1 | 1 | ||
| 071 | Kromfohrlander | Non-Sporting | 2.3 | 9.2 | P | 9 | 7 | 2 | |||||
| 072 | Hovawart | Working | 6.7 | 41.6 | 2 | 10/12 | P | 8 | 3 | 4 | 1 | ||
| 073 | Malinois | Herding | 4.1 | 21.6 | P | 4 | 0 | 4 | |||||
| 074 | Nova Scotia Duck Tolling Retriever | Sporting | 3.8 | 21.0 | 1 | 9 | P | CS | 8 | 4 | 3 | 1 | |
| 075 | Old German Shepherd Dog | Herding | 1.8 | 33.2 | P | 10 | 5 | 5 | 1 | ||||
| 076 | Irish Wolfhound | Hound | 2.9 | 56.4 | P | 13 | 8 | 4 | 1 | ||||
| 077 | Black Russian Terrier | Terrier | 3.4 | 46.4 | 1 | 13 | P | 12 | 9 | 2 | 1 | ||
| 078 | Newfoundland Dog | Working | 4.0 | 46.0 | NP | ||||||||
| 079 | Golden Retriever | Sporting | 4.4 | 22.8 | P | 5 | 2 | 3 | |||||
| 080 | Golden Retriever | Sporting | 3.8 | 33.6 | 1 | 13 | P | 8 | 5 | 3 | |||
| 081 | Entlebucher Mountain Dog | Herding | 2.6 | 23.6 | P | 7 | 3 | 4 | |||||
| 082 | Cairn Terrier | Terrier | 1.9 | 8.2 | P | 7 | 3 | 4 | |||||
| 083 | Boerboel | Working | 4.0 | 71.4 | NP | ||||||||
| 084 | Pug | Toy | 2.1 | 8.8 | P | 5 | 2 | 3 | |||||
| 085 | Cairn Terrier | Terrier | 2.7 | 8.0 | P | 5 | 1 | 4 | |||||
| 086 | Beagle | Hound | 3.5 | 11.0 | 1 | 5 | NP | ||||||
| 087 | Miniature Bull Terrier | Terrier | 1.8 | 14.6 | P | 3 | 2 | 1 | |||||
| 088 | French Bulldog | Non-Sporting | 3.1 | 13.6 | 1 | 8 | P | CS | 7 | 3 | 4 | ||
| 089 | Greater Swiss Mountain Dog | Working | 2.0 | 48.0 | P | CS | 13 | 3 | 7 | 3 | 4 | ||
| 090 | English Setter | Sporting | 2.4 | 25.0 | P | 7 | 4 | 3 | 1 | ||||
| 091 | Polish Lowland Sheepdog | Herding | 3.8 | 15.0 | 2 | 3/2 | P | 3 | 0 | 3 | |||
| 092 | Miniature Poodle | Non-Sporting | 1.8 | 7.8 | P | 5 | 3 | 2 | |||||
| 093 | German Shepherd | Herding | 6.9 | 27.0 | P | CS | 1 | 1 | |||||
| 094 | Dogues des Bordeaux | Working | 3.3 | 49.0 | P | 13 | 7 | 6 | |||||
| 095 | Golden Retriever | Sporting | 4.6 | 33.0 | P | 6 | 2 | 4 | |||||
| 096 | Miniature Bull Terrier | Terrier | 5.4 | 16.0 | 2 | 6/5 | P | CS | 6 | 2 | 3 | 1 | 1 |
| 097 | Eurasian | Non-Sporting | 3.8 | 20.8 | 1 | 5 | P | 5 | 3 | 2 | |||
| 098 | Azawakh | Hound | 7.2 | 19.0 | 1 | 8 | NP | ||||||
| 099 | Standard Wire-Haired Dachshund | Hound | 3.4 | 9.2 | P | 6 | 2 | 4 | |||||
| 100 | Nova Scotia Duck Tolling Retriever | Sporting | 5.8 | 15.8 | 1 | 4 | P | 5 | 3 | 2 | |||
| 101 | Kromfohrlander | Non-Sporting | 2.9 | 10.4 | P | 7 | 3 | 3 | 1 | ||||
| 102 | English Cocker Spaniel | Sporting | 2.9 | 11.2 | 1 | 8 | P | 8 | 6 | 2 | |||
| 103 | Golden Retriever | Sporting | 2.6 | 32.8 | P | 8 | 4 | 4 | |||||
| 104 | Labrador Retriever | Sporting | 5.3 | 28.2 | 1 | 7 | P | 6 | 2 | 4 | |||
| 105 | English Cocker Spaniel | Sporting | 2.9 | 11.2 | 1 | 8 | P | CS | 6 | 4 | 2 | ||
| 106 | Dutch Shepherd | Herding | 3.4 | 38.8 | P | 13 | 5 | 8 | |||||
| 107 | Spinone Italiano | Sporting | 6.3 | 32.0 | NP | ||||||||
| 108 | English Cocker Spaniel | Sporting | 8.2 | 12.6 | 2 | 4/3 | NP | ||||||
| 109 | Smooth Collie | Herding | 2.2 | 21.6 | NP | ||||||||
| 110 | Black Russian Terrier | Terrier | 7.1 | 54.0 | 2 | 5/5 | P | CS | 3 | 1 | 1 | 1 | 1 |
| 111 | Entlebucher Mountain Dog | Herding | 5.0 | 23.2 | 2 | 2/3 | P | 4 | 2 | 2 | |||
| 112 | Dogues des Bordeaux | Working | 8.0 | 53.2 | NP | ||||||||
| 113 | Azawakh | Hound | 7.2 | 19.4 | NP | ||||||||
| 114 | Miniature Bull Terrier | Terrier | 2.3 | 12.0 | NP | ||||||||
| 115 | English Setter | Sporting | 3.0 | 26.4 | NP | ||||||||
| 116 | Miniature Bull Terrier | Terrier | 1.3 | 14.6 | P | 3 | 1 | 2 | 1 | ||||
| 117 | Azawakh | Hound | 7.9 | 18.8 | 1 | 8 | P | 7 | 2 | 5 | |||
| 118 | Saarloos Wolfdog | Hound | 4.5 | 30.4 | P | 6 | 2 | 4 | 3 | ||||
| 119 | French Bulldog | Non-Sporting | 1.4 | 12.6 | P | 8 | 3 | 5 | |||||
| 120 | French Bulldog | Non-Sporting | 2.1 | 12.2 | P | 6 | 3 | 2 | 1 | 2 | |||
| 121 | Greater Swiss Mountain Dog | Working | 3.7 | 39.0 | 1 | 8 | P | CS | 10 | 2 | 5 | 3 | |
| 122 | French Bulldog | Non-Sporting | 1.8 | 10.4 | NP | ||||||||
| 123 | Miniature Bull Terrier | Terrier | 2.9 | 16.8 | P | CS | 6 | 1 | 2 | 3 |
| Number | Pregnant/ Non-Pregnant | T1 P4 | T2 P4 | T3 P4 |
|---|---|---|---|---|
| 001 | NP | 23.00 | 24.00 | 4.44 |
| 002 | P | 35.30 | 30.90 | 9.99 |
| 003 | P | 35.50 | 23.60 | 4.95 |
| 004 | P | 28.2 | 20.4 | 4.17 |
| 005 | NP | 25.00 | 17.20 | 3.37 |
| 006 | P | 13.10 | 16.6 | 4.49 |
| 007 | P | 29.00 | 26.10 | 8.40 |
| 008 | P | 40.00 | 32.20 | 6.58 |
| 009 | P | 36.90 | 21.40 | 7.30 |
| 010 | P | 38.6 | 23.70 | 7.35 |
| 011 | NP | 25.1 | 19.50 | 3.40 |
| 012 | P | 19.90 | 21.00 | 4.15 |
| 013 | P | 24.80 | 16.40 | 3.58 |
| 014 | P | 12.8 | 11.40 | 3.93 |
| 015 | P | 32.50 | 28.20 | 9.64 |
| 016 | P | 21.10 | 13.2 | 4.78 |
| 017 | P | 36.10 | 22.50 | 6.48 |
| 018 | P | 31.90 | 21.10 | 4.15 |
| 019 | P | 32.10 | 17.80 | 5.78 |
| 020 | NP | 26.20 | 23.80 | 1.61 |
| 021 | P | 21.00 | 13.30 | 3.72 |
| 022 | P | 31.3 | 16.7 | 6.66 |
| 023 | NP | 31.40 | 28.90 | 6.02 |
| 024 | NP | 22.60 | 22.1 | / |
| 025 | P | 25.10 | 15.30 | 4.50 |
| 026 | P | 33.5 | 17.6 | 6.72 |
| 027 | P | 21.20 | 12.40 | 5.32 |
| 028 | P | 22.3 | 18.60 | 4.62 |
| 029 | P | 20.70 | 15.70 | 5.61 |
| 030 | P | 27.20 | 17.50 | 4.89 |
| 031 | P | 34.20 | 26.30 | 5.69 |
| 032 | P | 27.80 | 21.50 | 6.06 |
| 033 | P | 31.80 | 25.90 | 7.22 |
| 034 | NP | 35.50 | 30.00 | 4.87 |
| 035 | P | 19.50 | 14.40 | 6.43 |
| 036 | NP | 29.60 | 30.70 | 2.49 |
| 037 | P | 35.10 | 31.00 | 4.91 |
| 038 | P | 20.40 | 15.30 | 4.83 |
| 039 | P | 29.30 | 28.90 | 10.6 |
| 040 | P | 32.90 | 29.40 | 10.8 |
| 041 | P | 28.20 | 26.00 | 9.2 |
| 042 | P | 32.50 | 22.60 | 6.6 |
| 043 | P | 29.60 | 26.70 | 10.60 |
| 044 | P | 34.70 | 14.50 | 5.24 |
| 045 | P | 35.6 | 37.10 | 6.51 |
| 046 | P | 26.70 | 21.50 | 9.94 |
| 047 | NP | 27.00 | 28.40 | 3.56 |
| 048 | P | 40.00 | 20.4 | 8.48 |
| 049 | P | 40.00 | 25.10 | 0.90 |
| 050 | P | 39.0 | 24.20 | 9.75 |
| 051 | P | 27.8 | 16.60 | 5.58 |
| 052 | NP | 18.1 | 9.88 | 0.30 |
| 053 | P | 33.20 | 27.30 | 6.92 |
| 054 | P | 36.90 | 32.20 | 7.81 |
| 055 | NP | 29.10 | 21.80 | 3.54 |
| 056 | P | 40.00 | 27.00 | 8.10 |
| 057 | P | 29.00 | 22.80 | 5.14 |
| 058 | P | 27.70 | 21.40 | 7.49 |
| 059 | P | 33.20 | 29.00 | 6.91 |
| 060 | P | 19.10 | 20.70 | 4.15 |
| 061 | P | 30.60 | 21.70 | 8.11 |
| 062 | NP | 39.00 | 22.20 | 2.67 |
| 063 | P | 25.80 | 21.20 | 7.02 |
| 064 | P | 40.00 | 37.70 | 6.30 |
| 065 | P | 31.80 | 22.90 | 8.22 |
| 066 | P | 40.00 | 31.30 | 7.21 |
| 067 | P | 27.10 | 22.50 | 7.37 |
| 068 | NP | 32.50 | 24.90 | / |
| 069 | P | 35.70 | 20.30 | 5.62 |
| 070 | P | 32.40 | 23.10 | 7.51 |
| 071 | P | 40.00 | 33.10 | 7.16 |
| 072 | P | 40.00 | 40.00 | 11.80 |
| 073 | P | 25.90 | 23.80 | 4.93 |
| 074 | P | 35.90 | 19.30 | 5.36 |
| 075 | P | 34.40 | 17.40 | 3.49 |
| 076 | P | 24.40 | 24.10 | 7.77 |
| 077 | P | 38.00 | 20.90 | 5.06 |
| 078 | NP | 24.20 | 18.20 | 2.93 |
| 079 | P | 21.00 | 15.30 | 7.57 |
| 080 | P | 32.20 | 22.60 | 8.10 |
| 081 | P | 25.00 | 17.50 | 4.05 |
| 082 | P | 35.80 | 34.90 | 7.00 |
| 083 | NP | 38.60 | 20.20 | 0.2 |
| 084 | P | 29.00 | 18.50 | 5.27 |
| 085 | P | 37.50 | 40.00 | 9.85 |
| 086 | NP | 31.30 | 25.60 | 1.05 |
| 087 | P | 28.10 | 29.2 | 10.10 |
| 088 | P | 34.30 | 25.70 | 7.11 |
| 089 | P | 21.50 | 15.90 | 3.77 |
| 090 | P | 21.30 | 22.00 | 10.30 |
| 091 | P | 37.6 | 20.10 | 6.33 |
| 092 | P | 20.80 | 16.30 | 5.91 |
| 093 | P | 21.50 | 14.40 | 4.04 |
| 094 | P | 36.50 | 32.60 | 8.33 |
| 095 | P | 33.90 | 17.80 | 6.47 |
| 096 | P | 28.60 | 25.40 | 7.76 |
| 097 | P | 29.20 | 27.40 | 9.89 |
| 098 | NP | 24.40 | 14.50 | 0.40 |
| 099 | P | 19.80 | 19.60 | 5.81 |
| 100 | P | 29.90 | 31.00 | 10.70 |
| 101 | P | 40.00 | 20.80 | 5.52 |
| 102 | P | 40.00 | 24.20 | 2.83 |
| 103 | P | 25.40 | 26.20 | 8.56 |
| 104 | P | 24.90 | 20.80 | 4.74 |
| 105 | P | 32.4 | 14.40 | 2.39 |
| 106 | P | 29.60 | 15.1 | 4.87 |
| 107 | NP | 28.60 | 16.90 | 1.42 |
| 108 | NP | 20.90 | 13.10 | 2.45 |
| 109 | NP | 23.90 | 22.90 | 2.35 |
| 110 | P | 39.40 | 25.00 | 8.72 |
| 111 | P | 24.90 | 15.20 | 4.59 |
| 112 | NP | 18.60 | 23.10 | / |
| 113 | NP | 29.50 | 17.80 | 3.02 |
| 114 | NP | 40.00 | 33.80 | 8.57 |
| 115 | NP | 27.20 | 25.80 | 2.11 |
| 116 | P | 23.40 | 22.20 | 7.23 |
| 117 | P | 23.10 | 19.70 | 9.85 |
| 118 | P | 36.9 | 18.70 | 7.96 |
| 119 | P | 29.00 | 24.30 | 10.10 |
| 120 | P | 33.40 | 31.50 | 9.41 |
| 121 | P | 34.70 | 23.40 | 5.60 |
| 122 | NP | 40.00 | 29.50 | 8.90 |
| 123 | P | 25.60 | 18.80 | 7.63 |
References
- Tsutsui, T. Effects of ovariectomy and progesterone treatment on the maintenance of pregnancy in bitches. Jpn. J. Vet. Sci. 1983, 45, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Okkens, A.C.; Dieleman, S.J.; Bevers, M.M.; Willemse, A.H. Evidence for the non-involvement of the uterus in the lifespan of the corpus luteum in the cyclic dog. Vet. Q. 1985, 7, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Verstegen-Onclin, K.; Verstegen, J. Endocrinology of pregnancy in the dog: A review. Theriogenology 2008, 70, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W.; Hansel, W.; Visek, W.J. The ovarian cycle of the bitch: Plasma estrogen, LH and progesterone. Biol. Reprod. 1975, 13, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Steinetz, B.G.; Goldsmith, L.T.; Hasan, S.H.; Lust, G. Diurnal variation of serum progesterone, but not relaxin, prolactin, or estradiol-17β in the pregnant bitch. Endocrinology 1990, 127, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Tibold, A.; Thuróczy, J. Progesterone, oestradiol, FSH and LH concentrations in serum of progesterone-treated pregnant bitches with suspected luteal insufficiency. Reprod. Domest. Anim. 2009, 44 (Suppl. S2), 129–132. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W.; Hansel, W. Prostaglandin F2α induced luteolysis, hypothermia, and abortions in beagle bitches. Prostaglandins 1977, 13, 533–542. [Google Scholar] [CrossRef]
- Kowalewski, M.P. Endocrine and molecular control of luteal and placental function in dogs: A review. Reprod. Domest. Anim. 2012, 47 (Suppl. S6), 19–24. [Google Scholar] [CrossRef]
- Görlinger, S.; Galac, S.; Kooistra, H.S.; Okkens, A.C. Hypoluteoidism in a bitch. Theriogenology 2005, 64, 213–219. [Google Scholar] [CrossRef]
- Günzel-Apel, A.R.; Urhausen, C.; Wolf, K.; Einspanier, A.; Oei, C.; Piechotta, M. Serum progesterone in pregnant bitches supplemented with progestin because of expected or suspected luteal insufficiency. Reprod. Domest. Anim. 2012, 47 (Suppl. S6), 55–60. [Google Scholar] [CrossRef]
- Johnson, C.A. High-risk pregnancy and hypoluteoidism in the bitch. Theriogenology 2008, 70, 1424–1430. [Google Scholar] [CrossRef]
- Johnson, C.A. Pregnancy management in the bitch. Theriogenology 2008, 70, 1412–1417. [Google Scholar] [CrossRef]
- Günzel-Apel, A.R.; Zabel, S.; Bunck, C.F.; Dieleman, S.J.; Einspanier, A.; Hoppen, H.-O. Concentrations of progesterone, prolactin and relaxin in the luteal phase and pregnancy in normal and short-cycling German Shepherd dogs. Theriogenology 2006, 66, 1431–1435. [Google Scholar] [CrossRef]
- Krachudel, J.; Bondzio, A.; Einspanier, R.; Einspanier, A.; Gottschalk, J.; Kuechenmeister, U.; Muennich, A. Luteal insufficiency in bitches as a consequence of an autoimmune response against progesterone? Theriogenology 2013, 79, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; Wehrend, A.; Goericke-Pesch, S. Luteal insufficiency in the bitch—Symptoms, diagnosis, consequences and therapy. A review of the literature. Tierarztl. Praxis Ausg. K Kleintiere Heimtiere 2010, 38, 389–396. [Google Scholar] [CrossRef]
- Rosset, E.; Mazereaux, C.; Buff, S. Retrospective study of hypoluteoidism cases in the bitch. In Proceedings of the 2010 EVSSAR Congress, Luvain La Neuve, Belgium, 14–15 May 2010. [Google Scholar]
- Verstegen, J.; Dhaliwal, G.; Verstegen-Onclin, K. Canine and feline pregnancy loss due to viral and non-infectious causes: A review. Theriogenology 2008, 70, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; Taylor, D.J. Bacterial reproductive pathogens of cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Root Kustritz, M.V. Pregnancy diagnosis and abnormalities of pregnancy in the dog. Theriogenology 2005, 64, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Günzel-Apel, A.R.; Zabel, S.; Einspanier, A.; Hoppen, H.O. „Significance, diagnostics and handling of luteal insufficiency in the dog: Breeders‘ wishes and the veterinarians‘ responsibility. In ” In Proceedings of the 49th yearly conference of the section small animal diseases of the German Veterinary Society DVG, Leipzig, Germany, 6–9 November 2003. [Google Scholar]
- Root Kustritz, M.V. Use of supplemental progesterone in management of canine pregnancy. Recent Advances in Small Animal Reproduction; Concannon, P.W., England, G., Verstegen, J., III, Linde Forsberg, C., Eds.; International Veterinary Information Service: Ithaca, NY, USA, 2001. [Google Scholar]
- Parkes, M.F.; Bell, E.T.; Christie, D.W. Plasma progesterone levels during pregnancy in the beagle bitch. Br. Vet. J. 1972, 128, xv. [Google Scholar]
- Jones, G.E.; Boyns, A.R.; Cameron, E.H.D.; Bell, E.T.; Christie, D.W.; Parkes, M.F. Plasma oestradiol, luteinizing hormone and progesterone during pregnancy in the Beagle bitch. Reproduction 1973, 35, 187–189. [Google Scholar] [CrossRef][Green Version]
- Smith, M.S.; McDonald, L.E. Serum levels of luteinizing hormone and progesterone during the estrous cycle, pseudopregnancy and pregnancy in the dog. Endocrinology 1974, 94, 404–412. [Google Scholar] [CrossRef]
- Hadley, J.C. Total unconjugated oestrogen and progesterone concentrations in peripheral blood during pregnancy in the dog. Reproduction 1975, 44, 450–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edqvist, L.-E.; Johansson, E.D.B.; Kasström, H.; Olsson, S.-E.; Richkind, M. Blood plasma levels of progesterone and oestradiol in the dog during the oestrous cycle and pregnancy. J. Endocrinol. 1975, 78, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Austad, R.; Lunde, A.; Sjaastad, O.V. Peripheral plasma levels of oestradiol-17 β and progesterone in the bitch during the oestrous cycle, in normal pregnancy and after dexamethasone treatment. J. Reprod. Fertil. 1976, 46, 129–136. [Google Scholar] [CrossRef]
- Gräf, K.-J. Serum oestrogen, progesterone and prolactin concentrations in cyclic, pregnant and lactating beagle dogs. Reproduction 1978, 52, 9–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chakraborty, P.K. Reproductive hormone concentrations during estrus, pregnancy and pseudopregnancy in the Labrador bitch. Theriogenology 1987, 27, 827–840. [Google Scholar] [CrossRef]
- Steinetz, B.G.; Goldsmith, L.T.; Lust, G. Plasma relaxin levels in pregnant and lactating dogs. Biol. Reprod. 1987, 37, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Steinetz, B.G.; Goldsmith, L.T.; Harvey, H.J.; Lust, G. Serum relaxin and progesterone concentrations in pregnant, pseudopregnant, and ovariectomized, progestin-treated pregnant bitches: Detection of relaxin as a marker of pregnancy. Am. J. Vet. Res. 1989, 50, 68–71. [Google Scholar]
- Luz, M.R.; Bertan, C.M.; Binelli, M.; Lopes, M.D. Plasma concentrations of 13,14-dihydro-15-keto prostaglandin F2-alpha (PGFM), progesterone and estradiol in pregnant and nonpregnant diestrus cross-bred bitches. Theriogenology 2006, 66, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Günzel-Apel, A.R.; Beste, N.; Nottorf, S.; Eschricht, F.; Hoppen, H.O.; Dieleman, S.; Einspanier, A. Comparison of selected endocrine parameters during luteal phase and pregnancy in German Shepherd dogs and Beagles. Reprod. Domest. Anim. 2009, 44 (Suppl. S2), 59–64. [Google Scholar] [CrossRef]
- Thuróczy, J.; Müller, L.; Kollár, E.; Balogh, L. Thyroxin and progesterone concentrations in pregnant, nonpregnant bitches, and bitches during abortion. Theriogenology 2016, 85, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Thejll Kirchhoff, K.; Goericke-Pesch, S. Changes in serum progesterone concentrations in Bernese mountain dogs and Cavalier King Charles Spaniels during pregnancy. Theriogenology 2016, 86, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Sontas, B.H.; Speroni, M.; Stelletta, C.; Günzel-Apel, A.R.; Romagnoli, S. Serum progesterone patterns in the pregnant bitch: A meta-analysis. In Proceedings of the 7th International Symposium on Canine and Feline Reproduction, Whistler, BC, Canada, 26-29 July 2012. [Google Scholar]
- Hadley, J.C. Total unconjugated oestrogen and progesterone concentrations in peripheral blood during the oestrous cycle of the dog. Reproduction 1975, 44, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Papa, P.C.; Hoffmann, B. The corpus luteum of the dog: Source and target of steroid hormones? Reprod. Domest. Anim. 2011, 46, 750–756. [Google Scholar] [CrossRef]
- Papa, P.C.; Kowalewski, M.P. Factors affecting the fate of the canine corpus luteum: Potentital contributors to pregnancy and non-pregnancy. Theriogenology 2020, 150, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Farstad, W. Bitch fertility after natural mating and after artifical insemination with fresh or frozen semen. J. Small Anim. Pract. 1984, 25, 561–565. [Google Scholar] [CrossRef]
- Baalbergen, T. Ovulation Timing in the Bitch: Conception Rate and Influencing Factors in 1401 Estrus Cycles. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2021; p. 20. [Google Scholar]
- Romagnoli, S.; Concannon, P.W. Clinical use of progestins in bitches and queens: A review. In Recent Advances in Small Animal Reproduction; Concannon, P.W., England, G., Verstegen, J., III, Linde Forsberg, C., Eds.; International Veterinary Information Service: Ithaca, NY, USA, 2003. [Google Scholar]
- Tsutsui, T.; Hori, T.; Kirihara, N.; Kawakami, E.; Concannon, P.W. Relation between mating or ovulation and the duration of gestation in dogs. Theriogenology 2006, 66, 1706–1708. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Mohammed, H.O.; Lamb, S.V.; Meyers-Wallen, V.N. Accuracy of canine parturition date prediciton from the initital rise in preovulatory progesterone concentration. Theriogenology 2003, 60, 1187–1196. [Google Scholar] [CrossRef]
- Mir, F.; Billault, C.; Fontaine, E.; Sendra, J.; Fontbonne, A. Estimated pregnancy length from ovulation to parturition in the bitch and its influencing factors: A retrospective study in 162 pregnancies. Reprod. Domest. Anim. 2011, 46, 994–998. [Google Scholar] [CrossRef]
- Eilts, B.E.; Davidson, A.P.; Hosgood, G.; Paccamonti, D.L.; Baker, D.G. Factors affecting gestation duration in the bitch. Theriogenology 2005, 64, 242–251. [Google Scholar] [CrossRef]
- Gavrilovic, B.B.; Andersson, K.; Linde Forsberg, C. Reproductive patterns in the domestic dog—A retrospective study of the Drever breed. Theriogenology 2008, 70, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Borge, K.S.; Tønnessen, R.; Nødtvedt, A.; Indrebø, A. Litter size at birth in purebred dogs—A retrospective study of 224 breeds. Theriogenology 2011, 75, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W.; McCann, J.P.; Temple, M. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. J. Reprod. Fertil. Suppl. 1989, 39, 3–25. [Google Scholar]
- Okkens, A.C.; Teunissen, J.M.; Van Osch, W.; Van Den Brom, W.E.; Dieleman, S.J.; Kooistra, H.S. Influence of litter size and breed on the duration of gestation in dogs. J. Reprod. Fertil. Suppl. 2001, 57, 193–197. [Google Scholar]
- Okkens, A.C.; Hekerman, T.W.M.; De Vogel, J.W.A.; Van Haaften, B. Influence of litter size and breed on variation in length of gestation in the dog. Vet. Q. 1993, 15, 160–161. [Google Scholar] [CrossRef]
- Athorn, R.Z.; Stott, P.; Bouwman, E.G.; Chen, T.Y.; Kennaway, D.J.; Langendijk, P. Effect of feeding level on luteal function and progesterone concentration in the vena cava during early pregnancy in gilts. Reprod. Fertil. Dev. 2013, 25, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.; Chai, J.-K.; Meguid, M.M.; Laviano, A.; Gleason, J.R.; Yang, Z.-J.; Blaha, V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol. Behav. 1999, 68, 99–107. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef]
- Thuróczy, J.; Wölfling, A.; Tibold, A.; Balogh, L.; Jánoki, G. A; Solti, L Effect of anticoagulants and sampling time on results of progesterone determination in canine blood samples. Reprod. Domest. Anim. 2003, 38, 386–389. [Google Scholar] [CrossRef]
- Marinelli, L.; Rota, A.; Carnier, P.; Da Dalt, L.; Gabai, G. Factors affecting progesterone production in corpora lutea from pregnant and diestrous bitches. Anim. Reprod. Sci. 2009, 114, 289–300. [Google Scholar] [CrossRef] [PubMed]

| Number (Breed) | Study Conclusion | Reference |
|---|---|---|
| 6 (Beagle) | pregnant and non-pregnant P4 values were very similar | [22] |
| 3 (Beagle) | P4 rose during estrus and remained increased for most of metestrus | [23] |
| 9 (Beagle) | P4 peak at 20–25 days, then decreased until parturition | [24] |
| 12 (Beagle) | P4 peak around day 25 after LH peak, declined from day 30 on | [4] |
| 3 (Beagle, Crossbred) | P4 peak 18 days after mating, then declined | [25] |
| 6 (German Shepherd, Greyhound) | no consistent P4 pattern in all bitches until 5th week, then gradually declining | [26] |
| 3 (Labrador Retriever) | P4 peak within few days from estradiol peak, then decline (undetectable in one bitch 10 days before parturition) | [27] |
| 8 (Beagle) | P4 peak day 14 after mating, decreased from day 35 on | [28] |
| 7 (Labrador Retriever) | P4 peak at day 6, remaining elevated for 9 weeks, then declining | [29] |
| 10 (Labrador Retriever, Beagle) | P4 remained high through 5–6 weeks, then declined | [30] |
| 9 (Labrador Retriever) | highest P4 values from 2nd through 5th week, then declined | [31] |
| 5 (Labrador Retriever) | diurnal differences from weeks 3 to 6; decline from week 3–4 on | [5] |
| 8 (Crossbred) | P4 peak at days 18–20, declined from day 30 on | [32] |
| 11 (German Shepherd) | P4 peak at days 5–15, then declined | [13] |
| 5 (Beagle) | P4 peak at days 5–15, then declined | [33] |
| 15 (breed not specified) | P4 peak in 4th week, then declined | [6] |
| 12 (*) | P4 peak at days 12–18, then declined | [14] |
| 14 (*1) | P4 peak in 2nd week, declined from 4th week on | [34] |
| 12 (*2) | P4 19.2 ± 4.3/22.2 ± 3.9 ng/mL at days 23 to 29, 6.0 ± 1.3/8.7 ± 7.1 ng/mL at days 50 to 54, 4. ± 1.2/5.3 ± 2.8 ng/mL at days 55 to 59 in BMD and CKCS respectively | [35] |
| Group | n | Litter Size | p-Value | Gestation Length (days) | p-Value |
|---|---|---|---|---|---|
| Weight | <0.001 | 0.13 | |||
| ≤7 kg | 11 | 4 (2–6) | 62.5 (60–64) | ||
| 7.1–14 kg | 29 | 6 (1–10) | 62.6 (59–65) | ||
| 14.1–32 kg | 35 | 6 (1–10) | 63.3 (60–68) | ||
| >32 kg | 23 | 9 (2–13) | 62.8 (59–65) | ||
| Age | 0.03 | 0.22 | |||
| <2 years | 19 | 5 (1–12) | 63.1 (61–67) | ||
| 2–3 years | 20 | 6 (1–13) | 62.7 (60–68) | ||
| 3–4 years | 19 | 8 (3–13) | 62.4 (59–65) | ||
| >4 years | 40 | 6 (1–13) | 63.2 (59 -65) | ||
| Parity | 0.05 | 0.94 | |||
| 0–2 litters | 91 | 6 (1–13) | 62.9 (59–68) | ||
| 3–4 litters | 6 | 3 (2–8) | 62.9 (61–65) | ||
| Breed group | 0.01 | 0.02 | |||
| Herding | 14 | 5 (1–13) | 63.1 (61–67) | ||
| Hound | 19 | 6 (3–13) | 62.6 (59–65) | ||
| Non-Sporting | 15 | 7 (3–12) | 62.7 (62–65) | ||
| Sporting | 15 | 8 (3–10) | 62.4 (60–64) | ||
| Terrier | 17 | 3 (1–12) | 63.7 (62–65) | ||
| Toy | 5 | 5 (3–6) | 61.6 (60–63) | ||
| Working | 13 | 8 (2–13) | 63.4 (61–68) |
| Weight Group | T1 | n | T2 | n | T3 | n |
|---|---|---|---|---|---|---|
| ≤7 kg | 30.67 ± 7.03 | 12 | 22.88 ± 4.82 | 12 | 6.12 ± 2.22 | 11 |
| 7.1–14 kg | 31.82 ± 5.90 | 35 | 24.42 ± 7.04 | 35 | 6.85 ± 2.24 | 29 |
| 14.1–32 kg | 28.57 ± 5.93 | 47 | 21.80 ± 5.50 | 47 | 6.95 ± 2.25 | 35 |
| 32.1–50 kg | 29.81 ± 7.25 | 24 | 22.04 ± 7.07 | 24 | 6.24 ± 2.09 | 20 |
| >50 kg | 26.82 ± 11.82 | 5 | 21.80 ± 3.42 | 5 | 6.99 ± 2.22 | 3 |
| Breed Groups | T1 (p = 0.10) | n | T2 (p = 0.002) | n | T3 (p ≤ 0.001) | n |
|---|---|---|---|---|---|---|
| Herding | 27.27 ± 5.49 | 20 | 19.10 ± 4.37 | 20 | 5.26 ± 1.62 | 14 |
| Hound | 28.74 ± 7.46 | 23 | 20.72 ± 5.59 | 23 | 5.73 ± 2.01 | 19 |
| Non-Sporting | 32.85 ± 5.35 | 16 | 25.43 ± 6.01 | 16 | 7.98 ± 2.14 | 15 |
| Sporting | 28.52 ± 6.32 | 20 | 21.75 ± 5.82 | 20 | 6.60 ± 2.65 | 15 |
| Terrier | 31.83 ± 5.05 | 20 | 26.22 ± 5.73 | 20 | 7.71 ± 1.47 | 17 |
| Toy | 32.22 ± 4.36 | 6 | 23.77 ± 5.23 | 6 | 7.05 ± 1.60 | 5 |
| Working | 30.08 ± 8.02 | 18 | 23.59 ± 6.4 | 18 | 6.72 ± 2.25 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinderer, J.; Lüdeke, J.; Riege, L.; Haimerl, P.; Bartel, A.; Kohn, B.; Weber, C.; Müller, E.; Arlt, S.P. Progesterone Concentrations during Canine Pregnancy. Animals 2021, 11, 3369. https://doi.org/10.3390/ani11123369
Hinderer J, Lüdeke J, Riege L, Haimerl P, Bartel A, Kohn B, Weber C, Müller E, Arlt SP. Progesterone Concentrations during Canine Pregnancy. Animals. 2021; 11(12):3369. https://doi.org/10.3390/ani11123369
Chicago/Turabian StyleHinderer, Janna, Julia Lüdeke, Lisa Riege, Peggy Haimerl, Alexander Bartel, Barbara Kohn, Corinna Weber, Elisabeth Müller, and Sebastian P. Arlt. 2021. "Progesterone Concentrations during Canine Pregnancy" Animals 11, no. 12: 3369. https://doi.org/10.3390/ani11123369
APA StyleHinderer, J., Lüdeke, J., Riege, L., Haimerl, P., Bartel, A., Kohn, B., Weber, C., Müller, E., & Arlt, S. P. (2021). Progesterone Concentrations during Canine Pregnancy. Animals, 11(12), 3369. https://doi.org/10.3390/ani11123369






