Changes in the Secretion of Anti-Inflammatory Cytokines and Acute-Phase Proteins in the Uterus after Artificial Insemination in the Mare
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Ultrasound Examination
2.3. Experimental Groups
- Group 1 served as a control group. In this group, no fluid was detected in the uterus during estrus or 7 h after AI (n = 9; two maiden and seven multiparous; mares aged from 5 to 10 years; 7.78 ± 1.90);
- Group 2. In this group, no fluid was detected in the uterus during estrus, but, 7 h after AI, more than 2 cm depth of fluid was detected in the uterus (n = 8; one maiden and seven multiparous; mares aged from 4 to19 years; 12.36 ± 5.68);
- Group 3. Mares were enrolled into this group if more than 2 cm depth of IUF was detected in two consecutive examinations during estrus, and also 7h after AI (n = 8; one primiparous and seven multiparous; mares aged from 5 to 20 years; 15.33 ± 4.47).
2.4. Artificial Insemination
2.5. Sample Collection
2.6. Cytology and Microbiology
2.7. Measurements of Cytokines
2.8. Measurement of APPs
2.9. Statistical Analysis
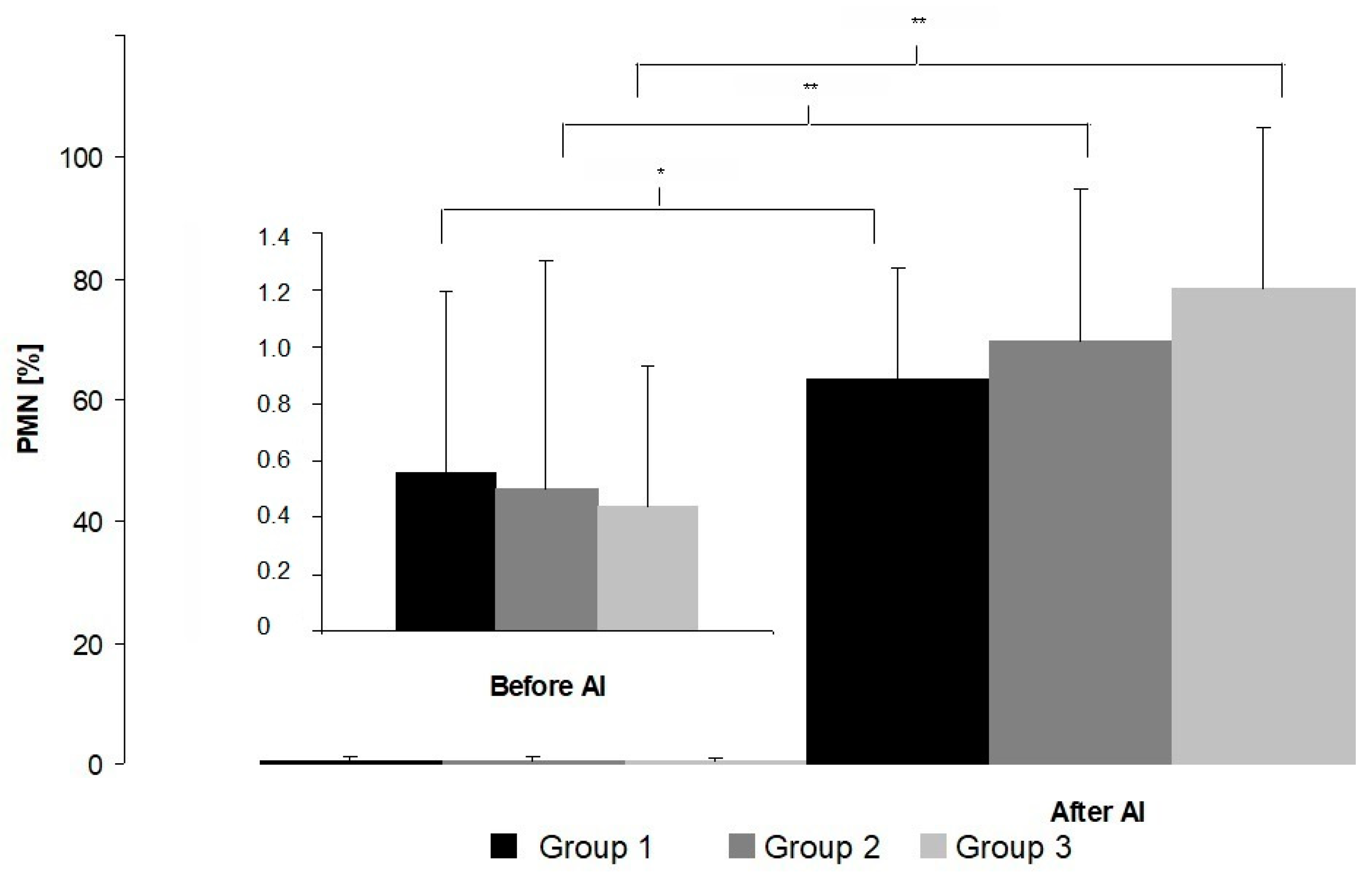
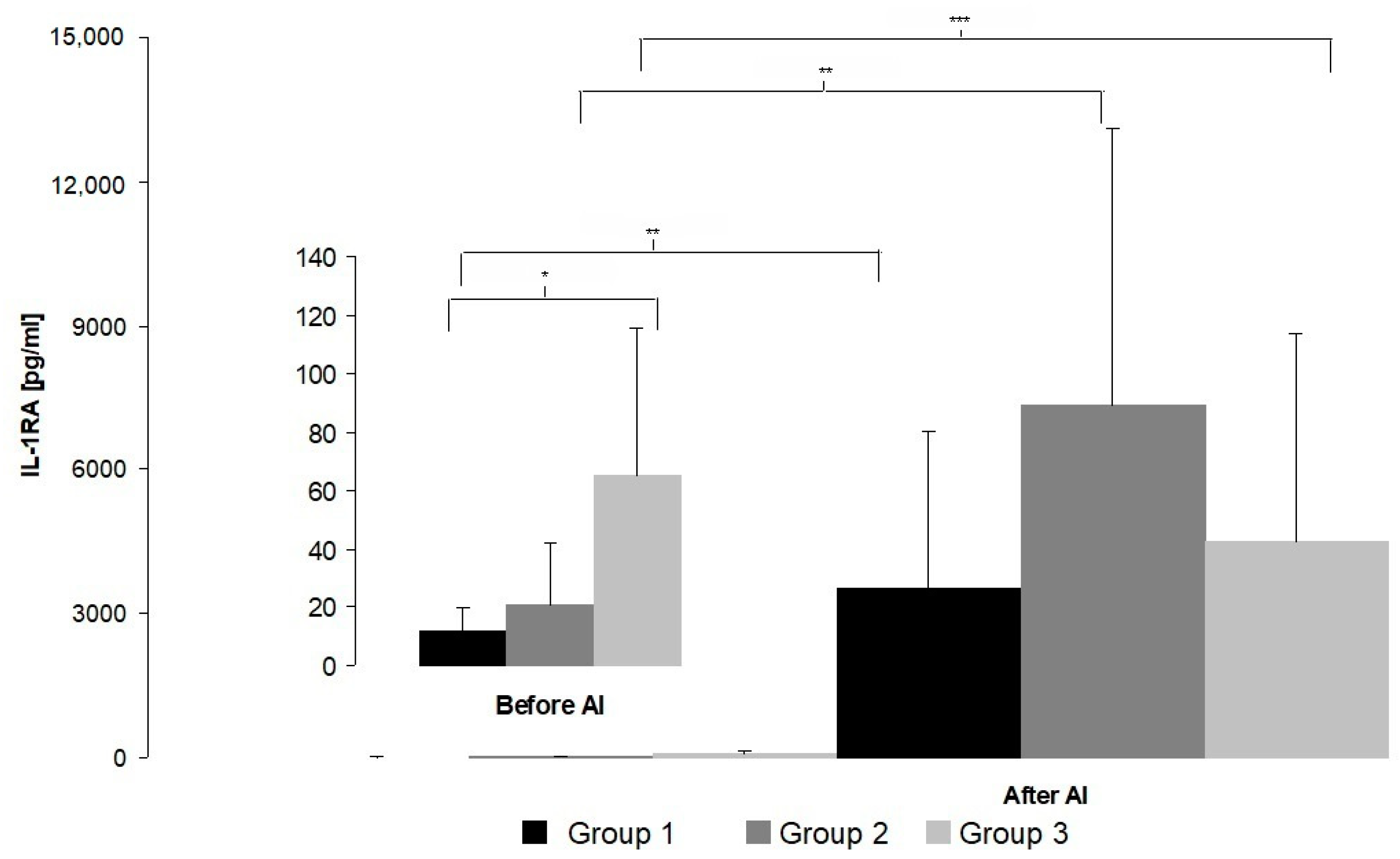
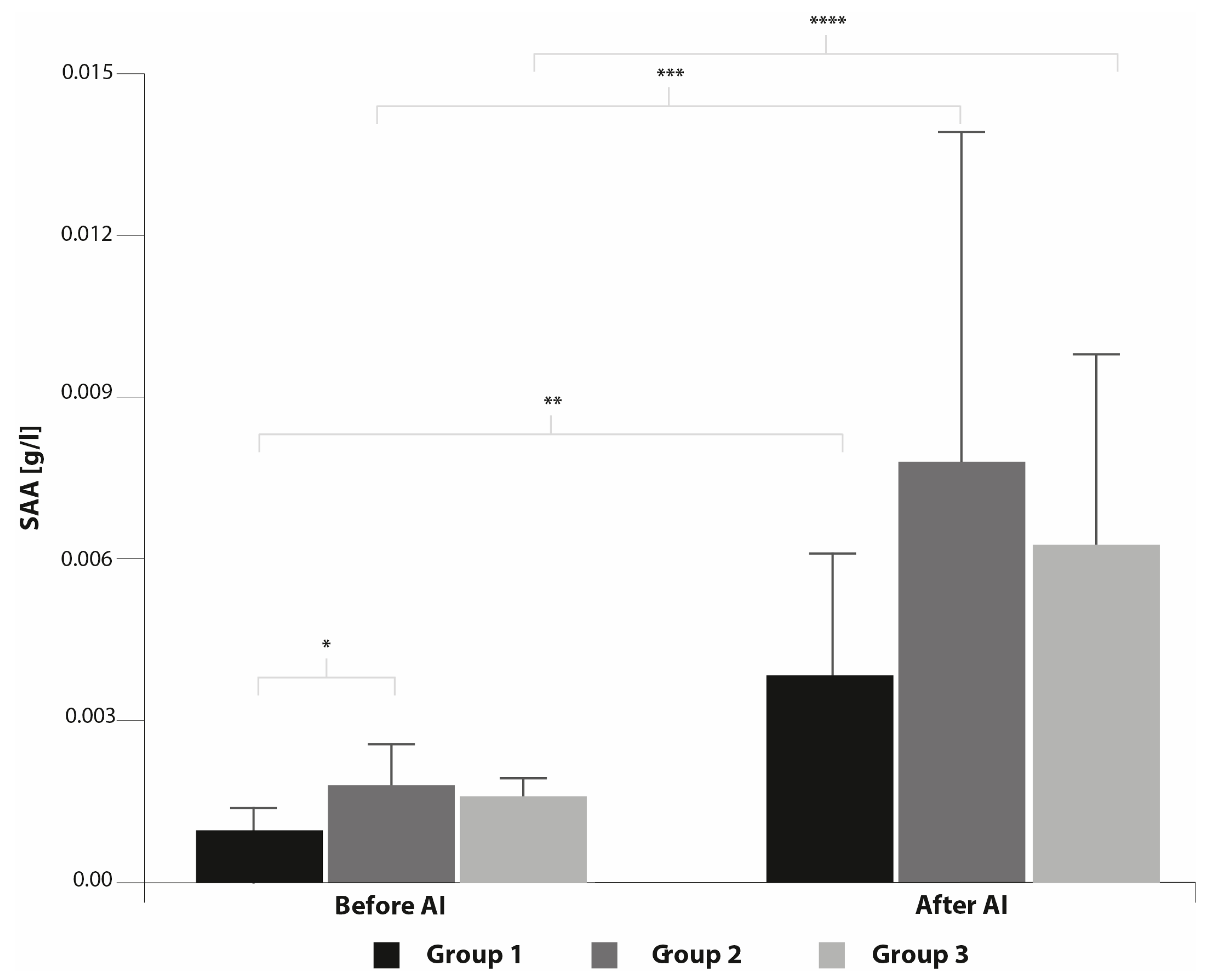
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katila, T. Post-mating inflammatory responses of the uterus. Reprod. Dom. Anim. 2012, 47, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Onset and duration of uterine inflammatory response of mares after insemination with fresh semen. Biol. Reprod. Monogr. 1995, 1, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Uterine clearance and resistance to persistent endometritis in the mare. Theriogenology 1999, 52, 461–471. [Google Scholar] [CrossRef]
- Nash, D.M.; Sheldon, I.M.; Herath, S.; Lane, E.A. Markers of the uterine innate immune response of the mare. Anim. Reprod. Sci. 2010, 119, 31–39. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Neuwirth, L.; Asbury, A.C.; Tran, T.; Mauragis, D.; Klapstein, E. Scintigraphic measurement of uterine clearance in normal mares and mares with recurrent endometritis. Equine Vet. J. 1994, 26, 109–113. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N.; Carlson, C.S.; Troedsson, M.H.T. Nitric oxide levels and nitric oxide synthase expression in uterine samples from mares susceptible and resistant to persistent breeding-induced endometritis. Am. J. Reprod. Immunol. 2005, 53, 230–237. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Horohov, D.W.; Scoggin, K.E.; Squires, E.; Troedsson, M.H.T. An investigation of uterine nitric oxide production in mares susceptible and resistant to persistent breeding-induced endometritis and the effects of immunomodulation. Reprod. Dom. Anim. 2013, 48, 554–561. [Google Scholar] [CrossRef]
- Khan, F.A.; Chenier, T.S.; Murrant, C.L.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Dose-dependent inhibition of uterine contractility by nitric oxide: A potential mechanism underlying persistent breeding-induced endometritis in the mare. Theriogenology 2017, 90, 59–64. [Google Scholar] [CrossRef]
- Khan, F.A.; Chenier, T.S.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Endometrial nitric oxide synthase activity in mares susceptible or resistant to persistent breeding-induced endometritis and the effect of a specific iNOS inhibitor in vitro. Reprod. Dom. Anim. 2018, 53, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Brinsko, S.P.; Rigby, S.L.; Varner, D.D.; Blanchard, T.L. A practical method for recognizing mares susceptible to post-breeding endometritis. AAEP Proc. 2003, 49, 363–365. [Google Scholar]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The use of dexamethasone administered to mares AT breeding time in the modulation of persistent mating induced endometritis. Theriogenology 2008, 70, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Squires, E.L.; Troedsson, M.H.T. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Troedsson, M.H.T. Equine breeding-induced endometritis: A review. J. Equine Vet. Sci. 2013, 33, 673–682. [Google Scholar] [CrossRef]
- Christoffersen, M.; Soderling, M.; Rudefalk, S.R.; Pedersen, H.G.; Allen, J.; Krekeler, N. Risk factors associated with uterine fluid after breeding caused by Streptococcus zooepidemicus. Theriogenology 2015, 84, 1283–1290. [Google Scholar] [CrossRef]
- Reilas, T.; Katila, T.; Mäkelä, O.; Huhtinen, M.; Koskinen, E. Intrauterine fluid accumulation in oestrous mares. Acta Vet. Scand. 1997, 38, 69–78. [Google Scholar]
- de Borba, E.V.C.; Camozzato, G.S.; Malschitzky, E.; Bustamante-Filho, I.C.; Martins, A.A.; Mattos, R.C.; Neves, A.P. Is the presence of uterine fluid a reliable indicator of endometrial inflammation. Pferdeheilkunde 2012, 28, 27–29. [Google Scholar] [CrossRef]
- Pycock, J.F.; Newcombe, J.R. The relationship between intraluminal uterine fluid, endometritis, and pregnancy rate in the mare. Equine Pract. 1996, 18, 19–22. [Google Scholar]
- Burleson, M.D.; LeBlanc, M.M.; Riddle, W.T.; Hendricks, K.E.M. Endometrial microbial isolates are associated with different ultrasonographic and endometrial cytology findings in Thoroughbred mares. AAEP Proc. 2010, 56, 317. [Google Scholar]
- Fumuso, E.; Giguere, S.; Wade, J.; Rogan, D.; Videla-Dorna, I.; Bowden, R.A. Endometrial IL-1β, IL-6 and TNF-α, mRNA expression in mares resistant or susceptible to post-breeding endometritis. Effects of estrous cycle, artificial insemination and immunomodulation. Vet. Immunol. Immunopathol. 2003, 96, 31–41. [Google Scholar] [CrossRef]
- Fumuso, E.; Aguilar, G.; Giguere, S.; David, O.; Wade, J.; Rogan, D. Interleukin-8 (IL-8) and 10 (IL-10) mRNA transcriptions in the endometrium of normal mares and mares susceptible to persistent post-breeding endometritis. Anim. Reprod. Sci. 2006, 94, 282–285. [Google Scholar] [CrossRef]
- Fumuso, E.A.; Aguilar, J.; Giguere, S.; Rivulgo, M.; Wade, J.; Rogan, D. Immune parameters in mares resistant and susceptible to persistent post-breeding endometritis: Effects of immunomodulation. Vet. Immunol. Immunopathol. 2007, 118, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial inflammatory markers of the early immune response in mares susceptible or resistant to persistent breeding-induced endometritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Woodward, E.M.; Bojesen, A.M.; Petersen, M.R.; Squires, E.L.; Lehn-Jensen, H.; Troedsson, M.H.T. Inflammatory responses to induced infectious endometritis in mares resistant or susceptible to persistent endometritis. BMC Vet. Res. 2012, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Belgrave, R.L. Haptoglobin quantitation in serum samples from clinically normal and clinically abnormal horses. J. Equine Vet. Sci. 2014, 34, 337–340. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.D.; Żmigrodzka, M.; Winnicka, A.; Miśkiewicz, A.; Strzelec, K.; Cywińska, A. Serum amyloid A in equine health and disease. Equine Vet. J. 2019, 51, 293–298. [Google Scholar] [CrossRef]
- Christoffersen, M.; Baagoe, C.D.; Jacobsen, S.; Bojesen, A.M.; Petersen, M.R.; Lehn-Jensen, H. Evaluation of the systemic acute phase response and endometrial gene expression of serum amyloid A and pro- and anti-inflammatory cytokines in mares with experimentally induced endometritis. Vet. Immunol. Immunopathol. 2010, 138, 95–105. [Google Scholar] [CrossRef]
- Sikora, M.; Król, J.; Nowak, M.; Stefaniak, T.; Aubertsson, G.; Kozdrowski, R. The usefulness of uterine lavage and acute phase protein levels as a diagnostic tool for subclinical endometritis in Icelandic mares. Acta Vet. Scand. 2016, 58, 50. [Google Scholar] [CrossRef]
- Krakowski, L.; Krawczyk, C.H.; Kostro, K.; Stefaniak, T.; Novotny, F.; Obara, J. Serum levels of acute phase proteins: SAA, Hp and progesterone (P4) in mares with early embryonic death. Reprod. Dom. Anim. 2011, 46, 624–629. [Google Scholar] [CrossRef]
- Tuppits, U.; Orro, T.; Einarsson, S.; Kask, K.; Kavak, A. Influence of the uterine inflammatory response after insemination with frozen–thawed semen on serum concentrations of acute phase proteins in mares. Anim. Reprod. Sci. 2014, 146, 182–186. [Google Scholar] [CrossRef]
- Chapwanya, A.; Meade, K.G.; Doherty, M.L.; Callanan, J.J.; O’Farrelly, C. Endometrial epithelial cells are potent producers of tracheal antimicrobial peptide and serum amyloid A3 gene expression in response to E. coli stimulation. Vet. Immunol. Immunopathol. 2013, 151, 157–162. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Brodzki, A.; Ziętek, J. The concentrations of inflammatory cytokines and acute-phase proteins in the peripheral blood and uterine washings in cows with pyometra. Reprod. Dom. Anim. 2015, 50, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Brodzki, P.; Kostro, K.; Krakowski, L.; Marczuk, J. Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet. Res. Commun. 2015, 39, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Kang, H.G.; Jeong, J.K.; Hur, T.Y.; Jung, Y.H. Inflammatory cytokine concentrations in uterine flush and serum samples from dairy cows with clinical or subclinical endometritis. Theriogenology 2014, 82, 427–432. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M.; Magsig, J.; Stromberg, A.J. Use of a low-volume uterine flush for diagnosing endometritis in chronically infertile mares. Theriogenology 2007, 68, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Cocchia, N.; Paciello, O.; Auletta, L.; Uccello, V.; Silvestro, L.; Mallardo, K.; Paraggio, G.; Pasolini, M.P. Comparison of the cytobrush, cottonswab, and low-volume uterine flush techniques to evaluate endometrial cytology for diagnosing endometritis in chronically infertile mares. Theriogenology 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Kozdrowski, R.; Sikora, M.; Buczkowska, J.; Nowak, M.; Raś, A.; Dzięcioł, M. Effects of cycle stage and sampling procedure on interpretation of endometrial cytology in mares. Anim. Reprod. Sci. 2015, 154, 56–62. [Google Scholar] [CrossRef]
- Jones, G.E.; Mould, D.L. Adaptation of Guaiacol (peroxidase) test for haptoglobins to microtitration plate system. Res. Vet. Sci. 1984, 37, 87–92. [Google Scholar] [CrossRef]
- Kątnik, I.; Pupek, M.; Stefaniak, T. Cross reactivities among some mammalian haptoglobins studied by a monoclonal antibody. Comp. Biochem. Physiol. 1998, 119, 335–340. [Google Scholar]
- Wojtysiak, K.; Ryszka, W.; Stefaniak, K.; Kozdrowski, R. Changes in secretion of anti-inflammatory cytokines and acute-phase proteins in the uterus after artificial insemination in the mare. In Proceedings of the Japanese-Polish Joint Seminar Cutting-Edge Reproductive Physiology-Key Processes for Birth of a New Life, Warsaw, Poland, 8–11 September 2019; p. 17. [Google Scholar]
- MacKay, R.J. Inflammation in horses. Vet. Clin. North Am. Equine Pract. 2000, 16, 15–27. [Google Scholar] [CrossRef]
- Badolato, R.; Wang, J.M.; Stornello, S.L.; Negro Ponzi, A.; Duse, M.; Musso, T. Serum amyloid A is an activator of PMN antimicrobial functions: Induction of degranulation, phagocytosis, and enhancement of anti-Candida activity. J. Leukoc. Biol. 2000, 67, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Zaias, J.; Altman, N.H. Acute phase response in animals: A review. Comp. Med. 2009, 59, 517–526. [Google Scholar] [PubMed]
- Horadagoda, N.U.; Knox, K.M.; Gibbs, H.A.; Reid, S.W.; Horadagoda, A.; Edwards, S.E.; Eckersall, P.D. Acute phase proteins in cattle: Discrimination between acute and chronic inflammation. Vet. Rec. 1999, 144, 437–441. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Güell., M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Siemieniuch, M.J.; Szóstek, A.Z.; Gajos, K.; Kozdrowski, R.; Nowak, M.; Okuda, K. Type of inflammation differentially affects expression of interleukin 1β and 6, tumor necrosis factor-α and Toll-like receptors in subclinical endometritis in mares. PLoS ONE 2016, 11, e0154934. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzyński, D.J. Impairment of the interleukin system in equine endometrium during the course of endometrosis. Biol. Reprod. 2013, 89, 1–13. [Google Scholar] [CrossRef]
- Palm, F.M.; Walter, I.; Budik, S.; Kolodziejek, J.; Nowotny, N.; Aurich, C. Influence of different semen extender and seminal plasma on PMN migration and on expression of IL-1β, IL-6, TNF-α and COX-2 mRNA in the equine endometrium. Theriogenology 2008, 70, 843–851. [Google Scholar] [CrossRef]
- Reilas, T.; Rivera Del Alamo, M.M.; Liepina, E.; Yeste, M.; Katila, T. Effects on the equine endometrium of cervical occlusion after insemination. Theriogenology 2016, 85, 617–624. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtysiak, K.; Ryszka, W.; Stefaniak, T.; Król, J.; Kozdrowski, R. Changes in the Secretion of Anti-Inflammatory Cytokines and Acute-Phase Proteins in the Uterus after Artificial Insemination in the Mare. Animals 2020, 10, 2438. https://doi.org/10.3390/ani10122438
Wojtysiak K, Ryszka W, Stefaniak T, Król J, Kozdrowski R. Changes in the Secretion of Anti-Inflammatory Cytokines and Acute-Phase Proteins in the Uterus after Artificial Insemination in the Mare. Animals. 2020; 10(12):2438. https://doi.org/10.3390/ani10122438
Chicago/Turabian StyleWojtysiak, Katarzyna, Wojciech Ryszka, Tadeusz Stefaniak, Jarosław Król, and Roland Kozdrowski. 2020. "Changes in the Secretion of Anti-Inflammatory Cytokines and Acute-Phase Proteins in the Uterus after Artificial Insemination in the Mare" Animals 10, no. 12: 2438. https://doi.org/10.3390/ani10122438
APA StyleWojtysiak, K., Ryszka, W., Stefaniak, T., Król, J., & Kozdrowski, R. (2020). Changes in the Secretion of Anti-Inflammatory Cytokines and Acute-Phase Proteins in the Uterus after Artificial Insemination in the Mare. Animals, 10(12), 2438. https://doi.org/10.3390/ani10122438




