1. Introduction
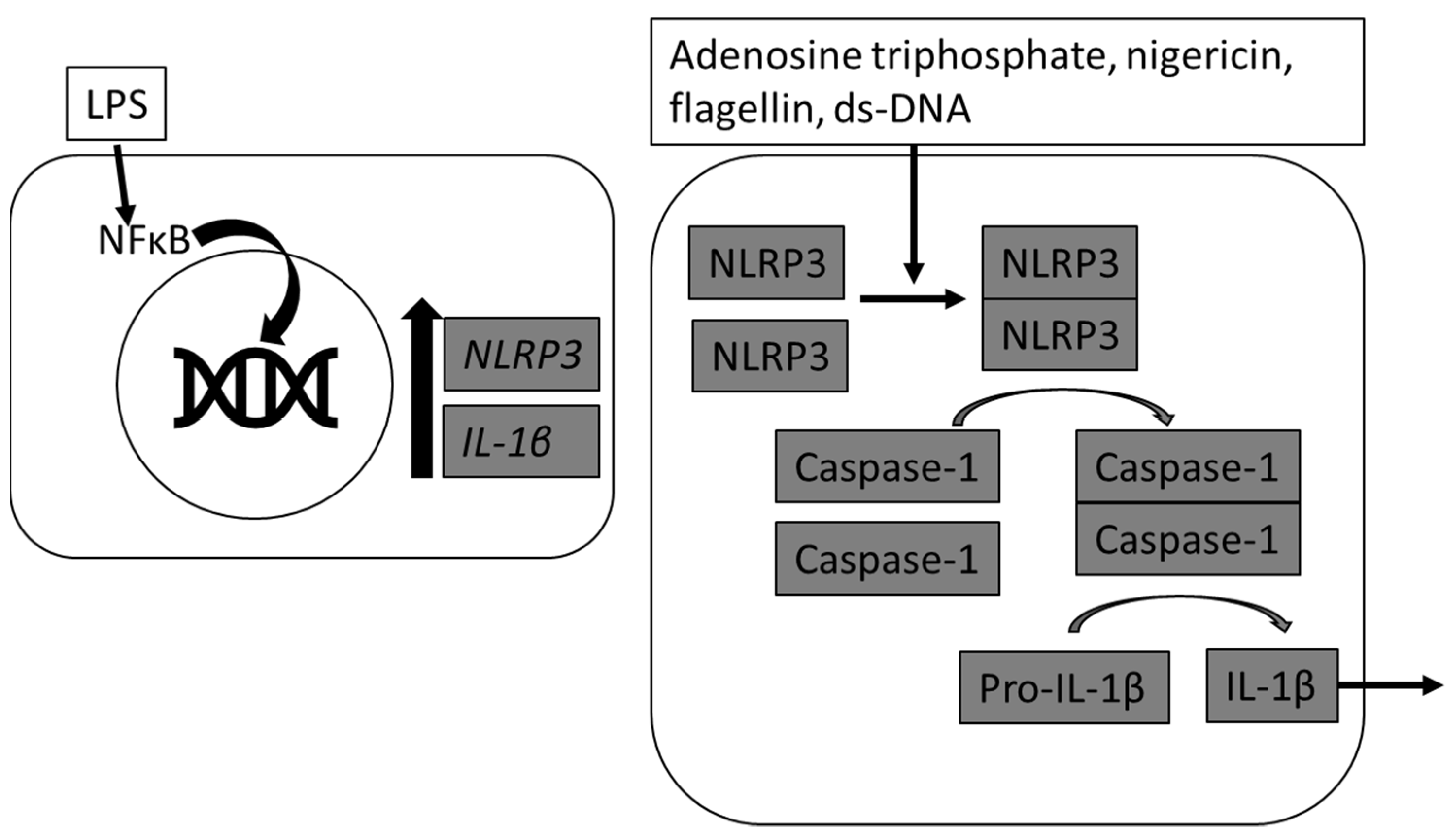
The inflammasome is a multi-protein complex consisting of nucleotide-binding oligomerization domain and leucine-rich repeat-containing receptors (NLRs) and the protease caspase-1 that forms in response to intracellular pathogens and danger signals [
1]. In response to various stimulants and/or danger signals including adenosine triphosphate (ATP), pathogen-associated molecular patterns (PAMPS), damage-associated molecular patterns (DAMPS), or various environmental irritants, the activation of NLRs oligomerize the adaptor protein apoptosis-associated speck-like protein containing caspase activation and recruitment domain (CARD) (ASC), which triggers the proteolytic activity of caspase-1. Activated caspase-1 then mediates cleavage of pro-interleukin (IL)-1β and pro-IL-18 into biologically active IL-1β and IL-18 [
2]. Inflammasome activation is considered to be a two-signal pathway, whereby expression of nucleotide oligomerization domain (NOD)-, leucine rich repeat (LRR)- and Pyrin domain-containing protein 3 (NLRP3) and pro-IL1β are increased following activation of nuclear factor κ B (NFκB) and then a second signal is required to activate the scaffolding of NLRP3.
The first signal step of NFκB activation can be achieved through a variety of microbial products and ligands, including lipopolysaccharide (LPS), which increase mRNA abundance of NLRP3, as shown in rodent and human models [
3] (
Figure 1). The inflammasome is then activated to assemble by a wide array of infectious and non-infectious pathways, including the Gram-positive bacterial toxin nigericin, extracellular ATP, and reactive oxygen species [
4,
5]. In equine peripheral blood monocytes (PBMC) NLRP3 inflammasomes are primed by LPS and triggered to produce mature IL-1β in response to extracellular ATP, nigericin, flagellin, and ds-DNA [
6].
Horses have a mild and transient increase in plasma concentrations of interleukin-1β (IL-1β) in response to consuming a meal that is higher in starch and sugar (non-structural carbohydrates; NSC). Our previous work has demonstrated this finding in Thoroughbred mares consuming 1.14 g NSC/kg bodyweight and in mixed breed geldings consuming 1.2 g NSC/kg bodyweight [
7,
8]. Furthermore, we have also previously shown that blood concentrations of LPS are increased at 2 h post meal consumption in horses [
9], suggesting inflammasome priming could be occurring in the post-prandial state.
Previous research in horses has indicated interacting factors of age and body condition score (BCS) on inflammatory responses. For instance, higher concentrations of inflammatory proteins, even in an unstimulated state, have been documented in horses older than 20 years [
10]. Regarding obesity, Reynolds et al. [
11] demonstrated increased adipose tissue gene expression of
IL-1β in obese horses. For these reasons we planned a preliminary investigation into the effects of age and body condition score on the post-prandial IL-1β response to NSC consumption. While original studies used compounded feeds with higher levels of NSC, these types of feeds are now difficult to source through commercial vendors as they are unpopular in the American marketplace. Therefore, we chose to use steam-rolled barley, a processed grain with high endogenous NSC concentrations that have been shown to alter microbial populations in the equine intestine and increase lactic acid production [
12]. Based on studies in ruminants, these factors are implicated in the production and appearance of LPS in the blood [
13]. Therefore, we hypothesized that horses older than 20 and those with higher BCS would have greater IL-1β responses to NSC consumption than their younger or lean counterparts.
2. Materials and Methods
2.1. Trial 1 Animals and Treatments
The use of animals for this research was approved by The Ohio State University’s Institutional Animal Care and Use Committee. Ten horses were used for this study, consisting of six Quarter Horses, two warmbloods, and two Standardbreds. They ranged in body weight from 468 to 651 kg, 10 to 17 years of age, and included 4 geldings and 6 mares. Horses were assigned to treatment based on BCS, with lean horses having a BCS between 4 and 5 (
n = 5; 468–532 kg) and overweight horses having a BCS between 6.5 and 8 (
n = 5; 500–651 kg). The BCS scale was that of Henneke et al. [
14], which ranks horses from 1 to 9, with 1 indicating emaciation and 9 indicating obesity. Sample size was determined based on a power-analysis of previous data demonstrating an increase in post-prandial IL-1β concentrations in non-obese horses.
Horses were group-housed in an outdoor paddock with access to ad libitum mixed grass hay (
Table 1), salt, and water. Horses received treatment diets for 14 d, with steam-rolled barley (SRB;
Table 1) being fed between 07:00 and 08:00 a.m. and a ration balancer (Essential K, Kalmbach Feeds, Sandusky, OH, USA; 0.45 kg per day,
Table 1) being fed between 15:00 and 16:00 p.m. Horses were fed in individual pans spread at a distance to prevent sharing of feed and were monitored throughout feeding sessions. Steam-rolled barley was fed at a rate to provide 1.2 g of NSC per kg BW.
On days 0 and 13, horses were relocated from paddock to stalls at 16:00 p.m. and provided ad libitum water and 1 kg of their hay. On the following mornings, horses were offered their SRB meal and had blood collected at –30 min (07:30 a.m.), and 1, 4, and 8 h post-feeding. Horses received 2 kg of their grass hay after the 1 h sample collection. Blood was centrifuged at 1500× g for 10 min to separate plasma. Plasma was separated and stored at −20 °C until analysis for concentrations of IL-1β.
2.2. Trial 2 Animals and Treatments
Sam Houston State University’s Institutional Animal Care and Use Committee approved the use of animals for this research. Six horses were used for this study, consisting of two Thoroughbreds, two Tennessee Walking Horses, and two Quarter Horses. Of these horses, there were three geldings and three mares, all with body condition scores between 5 and 6. Horses were grouped by age, where aged horses ranged from 20–23 years (OLD; 514–582 kg), and middle-aged horses ranged from 12–14 years (MID; 455–500kg). Sample size was determined based on a power-analysis of previous data demonstrating an increase in post-prandial IL-1β concentrations in non-obese horses.
Horses were fed in individual stalls, with access to coastal bermudagrass hay (
Cynodon dactylon), fed at 2% of bodyweight (
Table 1), and ad libitum salt and water. Horses received treatment diets with steam-rolled barley being fed between 07:00 and 08:00 a.m. and a commercial concentrate (SafeChoice Original, Cargill Animal Nutrition, Wayzata, MN, USA,
Table 1). Steam-rolled barley was fed at a rate to provide 1.2 g of NSC per kg BW. The commercial concentrate was fed at a rate of 0.5% bodyweight and split between two feedings; one feeding followed barley consumption, and the other occurred between 15:00 and 16:00 p.m. Horses received the barley diet for 36 days, a longer time frame than Trial 1, to enable a greater chance of capturing gene expression changes.
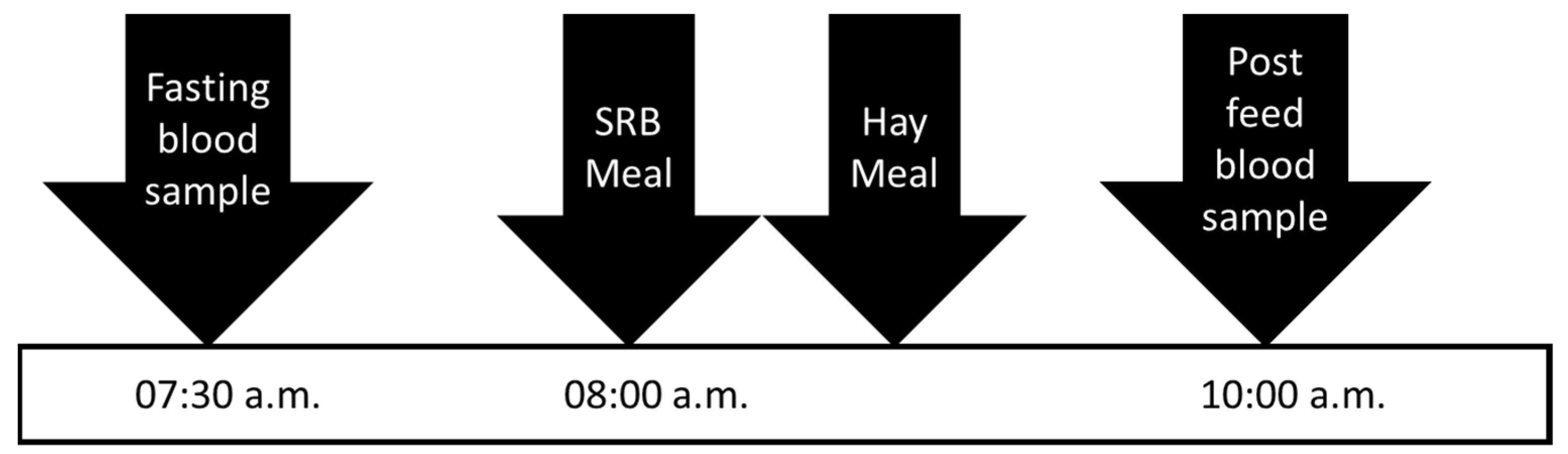
On days 0 and 35, horses were observed to have finished their evening hay by 20:00 p.m. and were not provided additional hay until sample collection the following morning. On days 1 and 36, horses were offered their SRB meal and had blood collected at –30 min (07:30 a.m.), and 2 h post-feeding (
Figure 2). Horses received their hay allotment after they finished consuming their SRB. Blood was centrifuged at 1500×
g for 10 min to separate plasma. Plasma was separated and stored at −20 °C until analysis for concentrations of IL-1β. White blood cells from whole blood were subject to RNA extraction using the PureLink
TM Total RNA Blood Kit (K156001; ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
2.3. Sample Analysis
Plasma was analyzed for concentrations of IL-1β using the Equine IL-1β Do-It-Yourself ELISA (DIY0699E-003; KingFisher Biotech, St. Paul, MN, USA) according to previously published methods [
8]. Briefly, Nunc Maxisorb 96-well plates (VWR, Radnor, PA, USA) were coated with capture antibody at a concentration of 3 µg/mL in phosphate-buffered saline (Product # 28374; BuPH
TM Modified Dulbecco’s PBS Packs, ThermoFisher Scientific, Waltham, MA, USA) and allowed to incubate overnight at 4 °C. Plasma was diluted 1:2 with reagent diluent, which consisted of 4% bovine serum albumin (Product # 126593; Bovine Serum Albumin, Fraction V, EMD Millipore Sigma, Burlington, MA, USA) in PBS, and incubated for one hour at room temperature. The detection antibody was diluted to a concentration of 3 µg/mL in reagent diluent. Optical density values were obtained using a plate reader (SpectraMax, Molecular Devices, San Jose, CA, USA) and converted to concentrations using the plate reader’s software (SoftMax Pro, VWR, Radnor, PA, USA).
To determine host gene expression, horse blood was collected in lithium heparin tubes. Total RNA was extracted via PureLink™ Total RNA Blood Purification Kit (K156001; Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized utilizing Protoscript First Strand cDNA Synthesis Kit (E6300S; New England Biolabs, Ipswich, MA, USA) following the standard protocol guide. qRT-PCR assays were performed using Luna Universal Probe qPCR Master Mix in 20 μL volumes according to the manufacturer’s instructions (New England Biolabs, Ipswich, MA, USA). Cycling conditions were set for an initial denaturation of 95 °C for 60 s for one cycle, and denaturation of 95 °C for 15 s and extension of 60 °C for 30 s for 40 cycles. To check specificity of amplification, melt curve analysis was performed on each amplification. Transcript abundance was calculated utilizing the 2
−ΔΔCT method and normalized to glyceraldehyde-4-phosphate dehydrogenase (GAPDH). Primers used in qRT-PCR analysis are listed in
Table 2.
2.4. Statistical Analysis
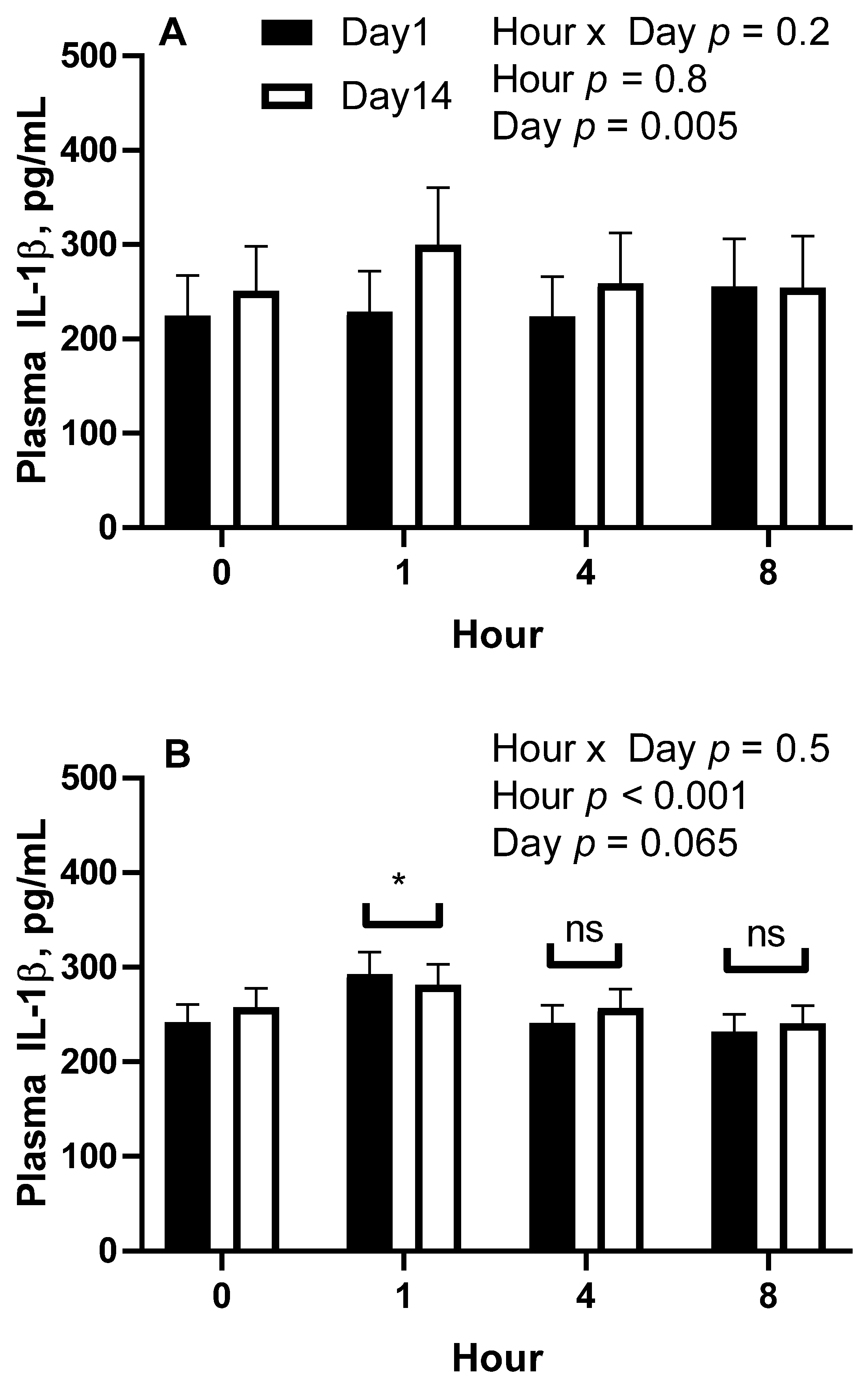
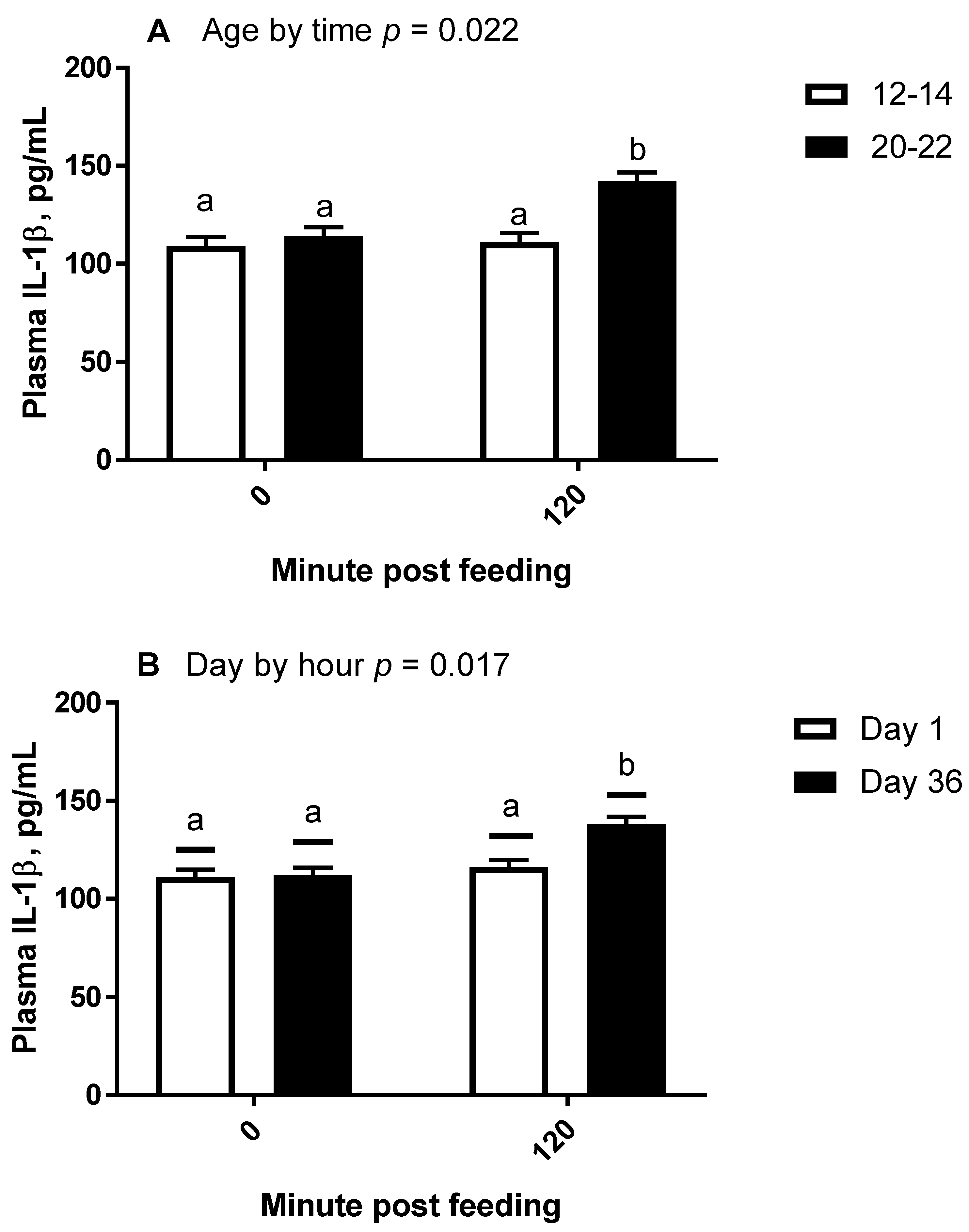
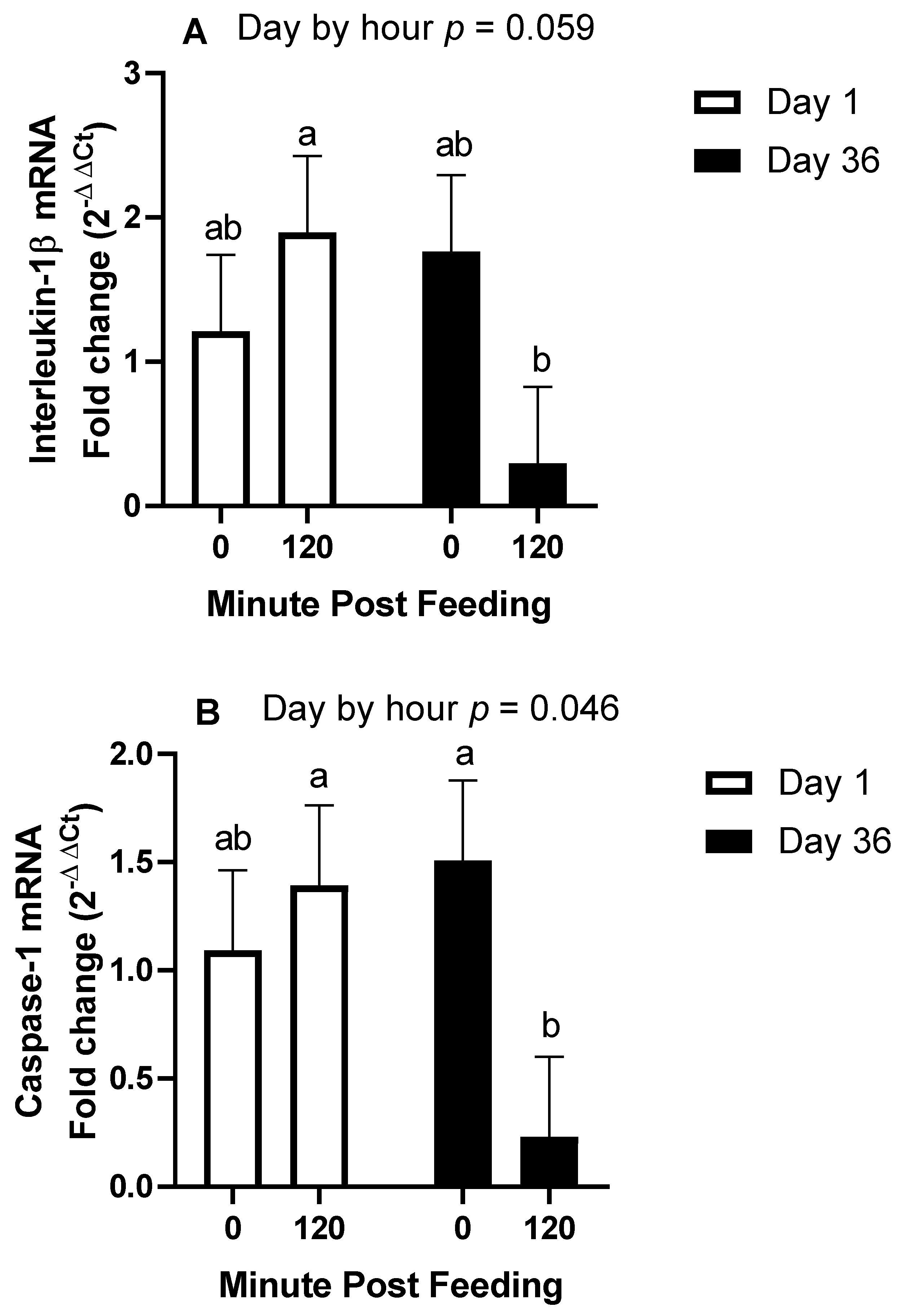
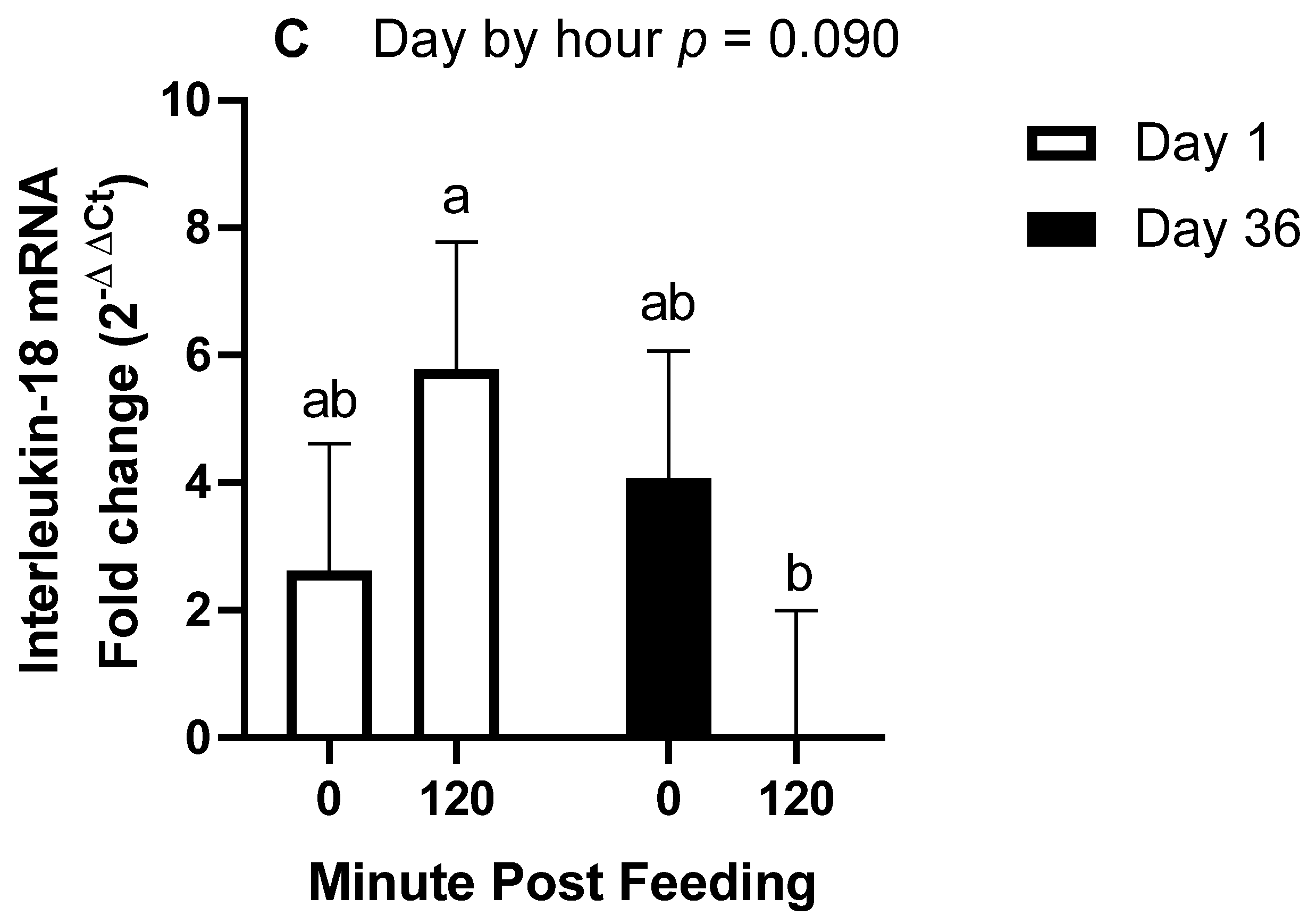
All statistical analyses were conducted using the SAS® OnDemand for Academics webserver (2021 SAS Institute, Cary, NC, USA). For all repeated measures analyses, the covariance matrix yielding the lowest AICC was used. For trial 1, plasma concentrations of IL-1β were analyzed by repeated measures analysis of variance (ANOVA) for the effects and interactions of day (1 vs. 14) and hour (0, 1, 4, and 8) within treatment (overweight vs. lean). Hour within day was the repeated term. Hour 0 concentrations were used as a covariate after confirming significance, and all values were log10 transformed to improve normality and homogeneity of variance. A Dunnett test was used to determine significance of simple effects, which is a test that compares each time point to time 0 only. For trial 2, plasma concentrations of IL-1β and fold change mRNA abundance were analyzed by repeated measures ANOVA for the effects and 2 way-interactions of day (1 vs. 36), hour (0 vs. 2), and age (12–14 years vs. 20–22 years). When 2 × 2 interactions were considered significant, specific contrasts were used to compare simple effects.
4. Discussion
The main objective of this research was to determine the effects of body condition and age on the IL-1β response to ingestion of 1.2 g NSC/kg bodyweight. The main finding of this study was that when controlling for age and only using middle-aged horses, being over-conditioned was associated with a post-prandial increase in IL-1β, whereas lean horses only observed this increase after 14 days of NSC consumption. Secondly, in horses of moderate body condition, older horses had an enhanced IL-1β plasma response after 36 days of NSC consumption compared to middle-aged horses. Our third finding was the downregulation of inflammasome transcript abundance 2 h post-feeding after 36 days, which is intriguing as it contrasts the increase in plasma protein concentrations of IL-1β.
Regarding our first finding, we observed that overweight horses responded to the barley consumption differently from lean horses. We have previously reported an effect of NSC consumption to induce an immediate post-prandial elevation in IL-1β concentrations within 1 h of feeding on the first day of feeding [
7,
9]. In the current study’s first trial, which used horses of middle age, lean horses did not have an IL-1β response on day 1. The differences observed between the current and former studies could be due to our specifically choosing lean horses between a BCS of 4 and 5, whereas previous studies included horses with higher BCS ranges. The overweight horses responded similarly to previous reports and suggests that the day 1 post-prandial IL-1β protein response is specific to horses with higher body condition scores. Of note, the mean IL-1β concentrations increased from day 1 to day 14 in lean horses when they consumed a daily meal providing 1.2 g NSC/kg body weight per day for 14 days. These results could indicate that the capacity to produce and secrete mature IL-1β protein increases in response to regular exposure to a high-starch and -sugar meal and having a higher body condition.
The effects of overweight and obesity on inflammation are well established in human and rodent models [
15]; however, equine literature has not shown consistent data. For instance, [
16] did not find a difference in fasting circulating concentrations of IL-1β between equine metabolic syndrome horses (BCS 7) and healthy horses (BCS 5.75). Similarly, there was no correlation between plasma IL-1β concentration and BCS in mixed breed, sex, and aged horses that had access to hay at the time of sampling [
17]. Alternatively, others have found increased adipose tissue gene expression of IL-1β in obese horses [
11]. The production and release of mature IL-1β is controlled by activating inflammasomes and caspase-1, the enzyme that cleaves pro-IL-1β to its mature form [
18]. Therefore, upregulation of transcript abundance will not correlate to increased plasma concentrations without activation of inflammasomes. In humans and rodent models, IL-1β is tied to the pancreatic β cell dysfunction of type II diabetes through the effect of IL-1β to inhibit glucose-stimulated insulin secretion [
19]. Pancreatic β cells may also produce IL-1β in response to inflammatory stimuli. Hyperglycemia causes oxidative stress in these cells, which in turn promotes inflammasome scaffolding, caspase-1 activation, and production of IL-1β [
20]. Mechanistic studies of the inflammasome in horses have received minimal attention in equine models.
An additional finding of interest came from our second trial, in which we discovered that when selecting horses of moderate body condition (BCS 5–6), older horses had an enhanced IL-1β plasma protein response to barley consumption as compared to younger horses. Higher concentrations of inflammatory proteins, even in an unstimulated state, have been documented in horses older than 20 years [
10]. Similarly, LPS-stimulated IL-1β protein production is greater from elderly human white blood cells as compared to those from young humans when tested in vitro [
21]. The effect of age to enhance inflammation is thought to be due to various influences, including chronic stimulation of the innate immune system throughout the lifespan and a reduced capacity of older individuals to differentiate between the severity of signals that activate inflammation [
22]. Lastly, although we did not design an experiment to test the interaction of age and obesity, this may also be present and should receive further study. In the experiment by Reynolds et al. [
11], which used horses averaging 17.4 to 18.8 years, adipose tissue gene expression of IL-1β was greater in obese animals compared to lean animals. Our study lends evidence to the body of knowledge regarding the effects of age on inflammatory protein production in horses.
To support our findings regarding plasma protein concentrations of IL-1β, we also investigated gene expression of
CASP1, which is translated to caspase-1, the enzyme that cleaves pro-IL-1β to its mature form. We also investigated transcript abundances of IL-1β and IL-18. We expected that the 2 h post-feeding samples would demonstrate a higher abundance of these genes with further elevations on day 36. For instance, the protein levels of caspase-1 and IL-1β were increased in the livers of mice fed a high-fat diet [
23]. In contrast, we found that all three genes exhibited reduced transcript abundance at 2 h post-feeding on day 36, with no influence of age. To our knowledge, this is the first indication of a rapid reduction in gene expression of
CASP1,
IL-1β, or
IL-18 following meal consumption in any species. Potential explanations involve gene degradation and inhibitors of gene transcription. Recently, it was discovered that peroxisome proliferator-activated receptor α (PPARα) induces an antisense codon
Gm15441 which then down-regulates thioredoxin interacting protein (Txnip) [
24]. Expression of TXNIP protein assists with activation of caspase-1 and inflammasome scaffolding [
25]; therefore, increased Txnip would reduce caspase-1. The protein, PPARα, is a nuclear receptor and transcription factor with endogenous ligands that include fatty acids. Although most commonly known for expression in the liver and adipose tissue, PPARα is also expressed in lymphocytes [
26]. Brocker et al. suggested that increased fatty acid mobilization, particularly during fasting, could activate PPARα induced production of
GM15441, which would then downregulate inflammasomes. Although we did not measure lipid metabolism in this study, previous research in horses has noted that 1.2 g NSC/kg body weight daily does induce alterations in lipid metabolism, including the reduced ability of insulin to reduce fasting plasma fatty acid concentrations [
27]. Further research should include more frequent time points to confirm these data, determine the time response of gene suppression, and investigate the potential role of lipids and PPARα in inflammasome activity.
To complete the objectives of the current study, the authors used steam-rolled barley, whereas previous studies relied more heavily on molasses and steam-flaked corn. Although these two diets contributed equivalent total NSC, they did so with varying amounts of simple sugars and starch compositions. Notably, grains contain different amounts of resistant starches [
28], the starch that escapes small intestinal digestion and therefore could impact the degree of starch fermentation in the hindgut. The influence of starch composition on inflammatory responses is unknown and should receive further investigation.
In conclusion, older and heavier conditioned horses appear to have an immediate inflammatory response to NSC consumption. This finding lends further evidence to the importance of limiting NSC intake in these types of horses. Alternatively, younger and leaner horses require repeated exposure for as little as 14 days to induce the same response, suggesting that age and leanness protect them against occasional spikes in NSC consumption. Further investigation should elucidate the downstream effects of IL-1β activation and whether there is a role in acute or chronic diseases. Further, we should investigate the timeline of inflammasome priming and activation in equine models.