Chronic Cholecystitis of Dogs: Clinicopathologic Features and Relationship with Liver
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histopathology
2.3. Histochemistry
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Signalment (Age, Breed, Sex) and Chief Complaint
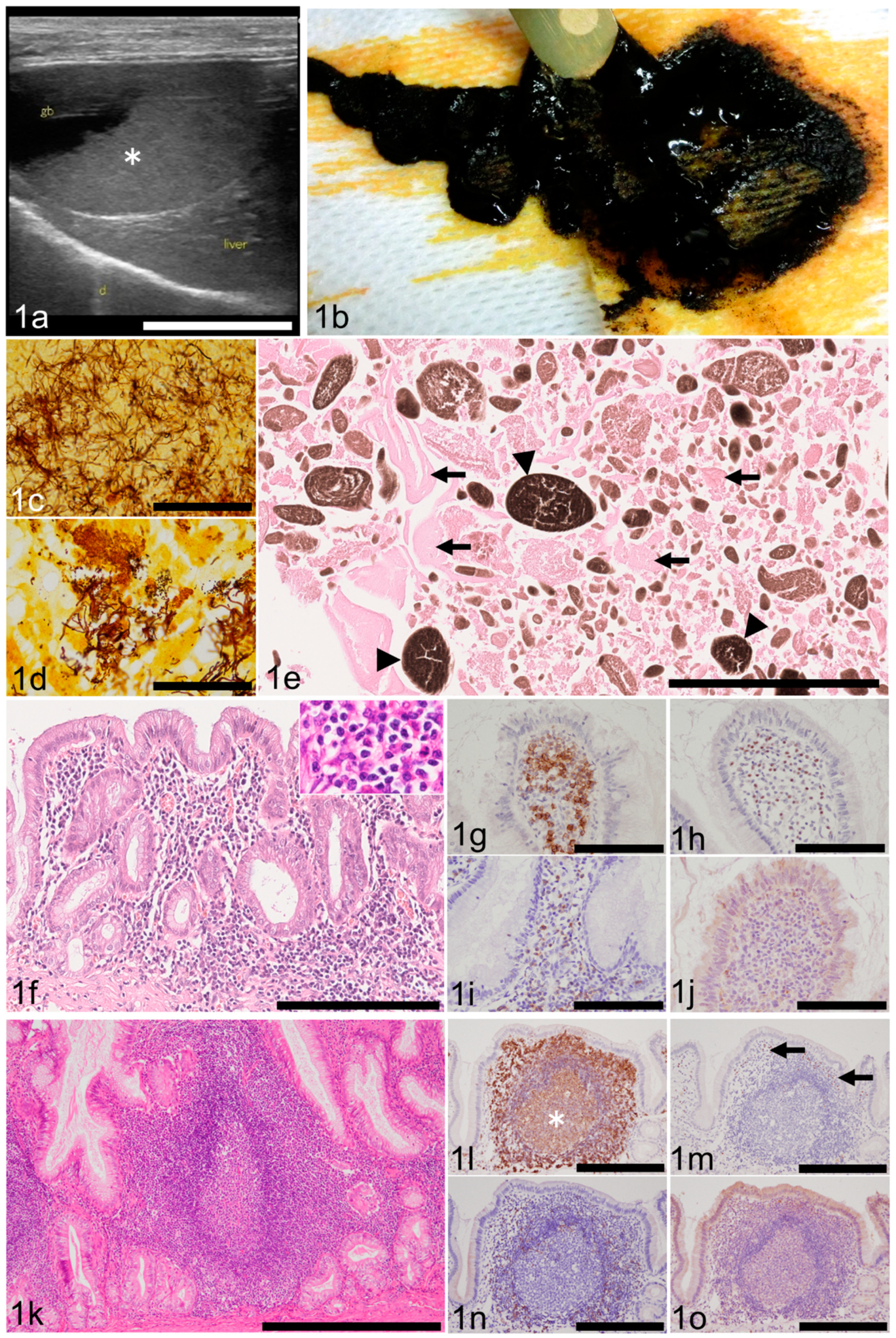
3.2. Ultrasonographic/Gross Abnormalities of the Gallbladder
3.3. Complete Blood Count (CBC) and Blood Chemistry
3.4. Bacterial Culture and Histochemically Detected Bacteria in the Gallbladder
3.5. Contents of the Gallbladder
3.6. Gallbladder Mucosal Inflammation (Cholecystitis)
3.7. Other Histologic Findings of the Gallbladder
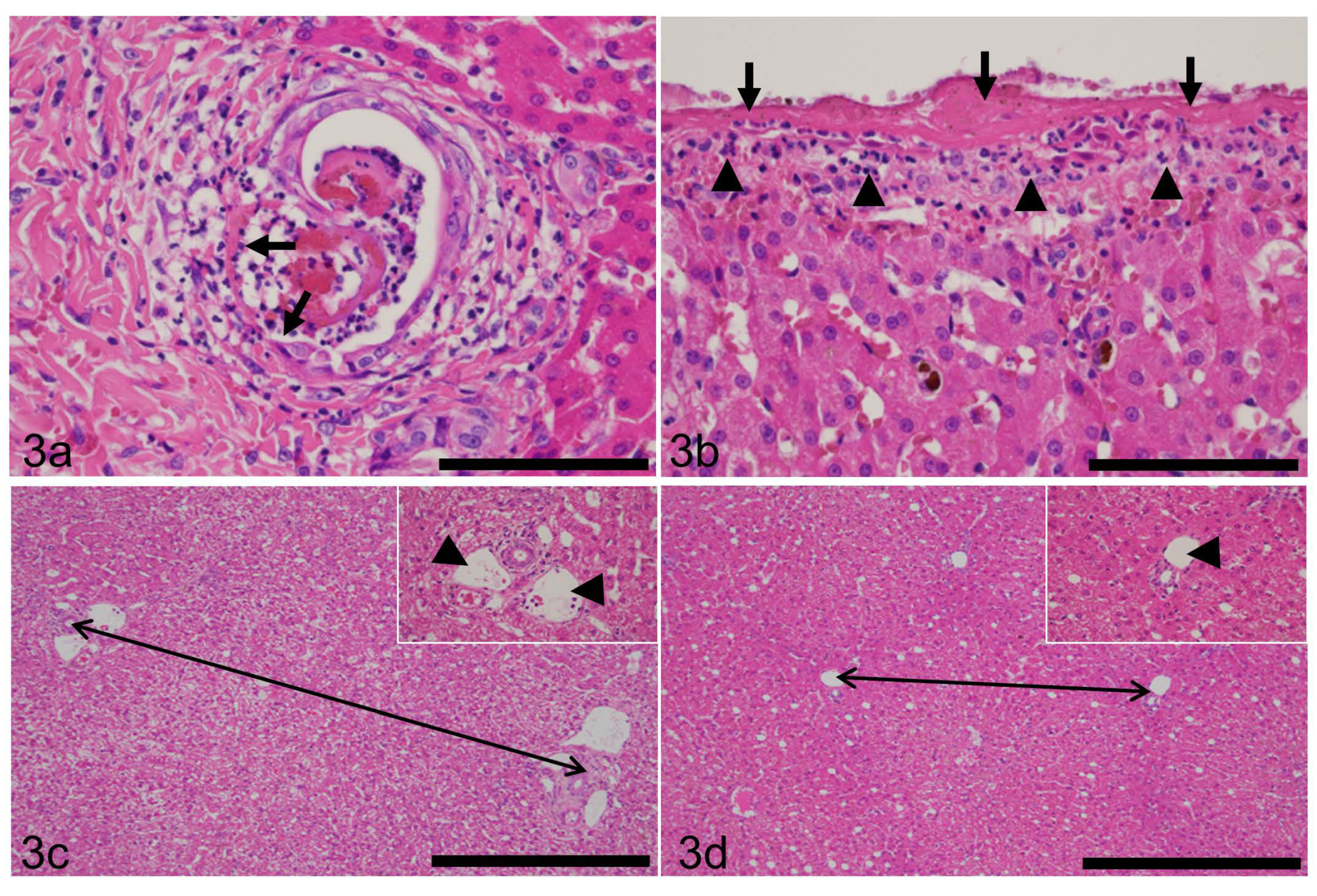
3.8. Liver Inflammation
3.9. Hepatic Lobular Diameter (HLD) and Primary Portal Vein Hypoplasia (PPVH)
3.10. Other Histologic Findings of the Liver
3.11. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre, A.L.; Center, S.A.; Randolph, J.F.; Yeager, A.E.; Keegan, A.M.; Harvey, H.J.; Erb, H.N. Gallbladder disease in Shetland Sheepdogs: 38 cases (1995–2005). J. Am. Vet. Med. Assoc. 2007, 231, 79–88. [Google Scholar] [CrossRef]
- Bargellini, P.; Orlandi, R.; Paloni, C.; Rubini, G.; Fonti, P.; Peterson, M.E.; Rishniw, M.; Boiti, C. Evaluation of Contrast-Enhanced Ultrasonography as a Method for Detecting Gallbladder Necrosis or Rupture in Dogs. Vet. Radiol. Ultrasound 2016, 57, 611–620. [Google Scholar] [CrossRef]
- Birettoni, F.; Porciello, F.; Caivano, D.; Arcelli, R.; Sforna, M.; Antognoni, M.T. Primary neuroendocrine carcinoma of the gallbladder in a dog. Vet. Res. Commun. 2008, 32 (Suppl. 1), 239–242. [Google Scholar] [CrossRef]
- Brömel, C.; Barthez, P.Y.; Léveillé, R.; Scrivani, P.V. Prevalence of gallbladder sludge in dogs as assessed by ultrasonography. Vet. Radiol. Ultrasound 1998, 39, 206–210. [Google Scholar] [CrossRef]
- Corfield, G.; Read, R.; Nicholls, P.; Lester, N. Gall bladder torsion and rupture in a dog. Aust. Vet. J. 2007, 85, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Crews, L.J.; Feeney, D.A.; Jessen, C.R.; Rose, N.D.; Matise, I. Clinical, ultrasonographic, and laboratory findings associated with gallbladder disease and rupture in dogs: 45 cases (1997–2007). J. Am. Vet. Med. Assoc. 2009, 234, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M. Tumors of the Liver and Gallbladder. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Ames, IA, USA, 2017; pp. 602–631. [Google Scholar]
- Gookin, J.L.; Mathews, K.G.; Cullen, J.; Seiler, G. Qualitative metabolomics profiling of serum and bile from dogs with gallbladder mucocele formation. PLoS ONE 2018, 13, e0191076. [Google Scholar] [CrossRef] [Green Version]
- Jaffey, J.; Pavlick, M.; Webster, C.; Moore, G.; McDaniel, K.; Blois, S.; Brand, E.; Reich, C.; Motschenbacher, L.; Hostnik, E.; et al. Effect of clinical signs, endocrinopathies, timing of surgery, hyperlipidemia, and hyperbilirubinemia on outcome in dogs with gallbladder mucocele. Vet. J. 2019, 251, 105350. [Google Scholar] [CrossRef]
- Lovell, S.; Singh, A.; Linden, A.Z.; Hagen, C.; Cuq, B. Gallbladder leiomyoma treated by laparoscopic cholecystectomy in a dog. J. Am. Vet. Med Assoc. 2019, 255, 85–89. [Google Scholar] [CrossRef]
- Mesich, M.L.; Mayhew, P.D.; Paek, M.; Holt, D.E.; Brown, D.C. Gall bladder mucoceles and their association with endocrinopathies in dogs: A retrospective case-control study. J. Small Anim. Pract. 2009, 50, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Torisu, S.; Kaneko, Y.; Yamamoto, S.; Fujimoto, S.; Ong, B.H.E.; Naganobu, K. Retrospective analysis of canine gallbladder contents in biliary sludge and gallbladder mucoceles. J. Vet. Med. Sci. 2017, 79, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, C.N.; Volk, M.V.; Mankowski, J.L. A Carcinoid Tumor in the Gallbladder of a Dog. Vet. Pathol. 2002, 39, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Shibata, S.; Sakai, H.; Konno, H.; Takashima, S.; Kawabe, M.; Mori, T.; Kitagawa, H.; Washizu, M. Gallbladder lymphoma in a miniature dachshund. J. Vet. Med. Sci. 2015, 77, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.M.; Bankoff, B.J.; Rosenstein, P.K.; Clendaniel, D.C.; Sánchez, M.D.; Durham, A.C. Clinical, histopathologic, and immunohistochemical features of 13 cases of canine gallbladder neuroendocrine carcinoma. J. Vet. Diagn. Investig. 2020, 33, 294–299. [Google Scholar] [CrossRef]
- Pike, F.S.; Berg, J.; King, N.W.; Penninck, D.G.; Webster, C.R.L. Gallbladder mucocele in dogs: 30 cases (2000–2002). J. Am. Vet. Med. Assoc. 2004, 224, 1615–1622. [Google Scholar] [CrossRef]
- Rogers, E.; Jaffey, J.A.; Graham, A.; Hostnik, E.T.; Jacobs, C.; Fox-Alvarez, W.; Van Eerde, E.; Arango, J.; Williams, F., 3rd; DeClue, A.E. Prevalence and impact of cholecystitis on outcome in dogs with gallbladder mucocele. J. Vet. Emerg. Crit. Care 2020, 30, 97–101. [Google Scholar] [CrossRef]
- Tamborini, A.; Jahns, H.; McAllister, H.; Kent, A.; Harris, B.; Procoli, F.; Allenspach, K.; Hall, E.; Day, M.J.; Watson, P.J.; et al. Bacterial Cholangitis, Cholecystitis, or both in Dogs. J. Vet. Intern. Med. 2016, 30, 1046–1055. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.; Turek, B.; Brown, D.; Bradley, C.; Clark, J.C. Cholangitis and Cholangiohepatitis in Dogs: A Descriptive Study of 54 Cases Based on Histopathologic Diagnosis (2004–2014). J. Vet. Intern. Med. 2017, 32, 172–180. [Google Scholar] [CrossRef] [Green Version]
- DeMonaco, S.; Grant, D.; Larson, M.; Panciera, D.; Leib, M. Spontaneous Course of Biliary Sludge Over 12 Months in Dogs with Ultrasonographically Identified Biliary Sludge. J. Vet. Intern. Med. 2016, 30, 771–778. [Google Scholar] [CrossRef]
- Gookin, J.; Correa, M.; Peters, A.; Malueg, A.; Mathews, K.; Cullen, J.; Seiler, G. Association of Gallbladder Mucocele Histologic Diagnosis with Selected Drug Use in Dogs: A Matched Case-Control Study. J. Vet. Intern. Med. 2015, 29, 1464–1472. [Google Scholar] [CrossRef] [Green Version]
- Kakimoto, T.; Kanemoto, H.; Fukushima, K.; Tsujimoto, H.; Ohno, K. Bile acid composition of gallbladder contents in dogs with gallbladder mucocele and biliary sludge. Am. J. Vet. Res. 2017, 78, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Secchi, P.; Pöppl, A.; Ilha, A.; Filho, H.K.; Lima, F.; García, A.; González, F. Prevalence, risk factors, and biochemical markers in dogs with ultrasound-diagnosed biliary sludge. Res. Vet. Sci. 2012, 93, 1185–1189. [Google Scholar] [CrossRef]
- Tsukagoshi, T.; Ohno, K.; Tsukamoto, A.; Fukushima, K.; Takahashi, M.; Nakashima, K.; Fujino, Y.; Tsujimoto, H. Decreased gallbladder emptying in dogs with biliary sludge or gallbladder mucocele. Vet. Radiol. Ultrasound 2011, 53, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.D.; Tamborini, A.; Watson, P.J.; Bexfield, N.H. Clinical characteristics and histology of cholecystectomised dogs with nongravity-dependent biliary sludge: 16 cases (2014–2019). J. Small Anim. Pr. 2021, 62, 478–488. [Google Scholar] [CrossRef]
- Saunders, H.; Thornton, L.A.; Burchell, R. Medical and surgical management of gallbladder sludge and mucocoele development in a Miniature Schnauzer. Int. J. Vet. Sci. Med. 2017, 5, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Stalker, M.J. Liver and Biliary System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, pp. 258–352. [Google Scholar]
- Smith, R.P.; Gookin, J.; Smolski, W.; Di Cicco, M.; Correa, M.; Seiler, G. Association between Gallbladder Ultrasound Findings and Bacterial Culture of Bile in 70 Cats and 202 Dogs. J. Vet. Intern. Med. 2017, 31, 1451–1458. [Google Scholar] [CrossRef]
- Wagner, K.A.; Hartmann, F.A.; Trepanier, L.A. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998–2003. J. Vet. Intern. Med. 2007, 21, 417–424. [Google Scholar]
- Besso, J.G.; Wrigley, R.H.; Gliatto, J.M.; Webster, C. Ultrasonographic appearance and clinical findings in 14 dogs with gallbladder mucocele. Vet. Radiol. Ultrasound 2000, 41, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, I.; Ohtsuki, S.; Uchida, K. Lobular diameters of autopsied dog livers give clues for an appropriate liver biopsy methodology. J. Vet. Med. Sci. 2020, 82, 1084–1092. [Google Scholar] [CrossRef]
- Bargellini, P.; Orlandi, R.; Paloni, C.; Rubini, G.; Fonti, P.; Righi, C.; Peterson, M.E.; Rishniw, M.; Boiti, C. Contrast-enhanced ultrasound complements two-dimensional ultrasonography in diagnosing gallbladder diseases in dogs. Vet. Radiol. Ultrasound 2018, 59, 345–356. [Google Scholar] [CrossRef]
- Wennogle, S.A.; Randall, E.K.; Priestnall, S.L.; Twedt, D.C.; Simpson, K.W. Eubacterial fluorescence in situ hybridisation and histologic features in 25 dogs with gallbladder mucocele. J. Small Anim. Pract. 2019, 60, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, D.; Lee, S.P.; Hayashi, A. Long-term culture and partial characterization of dog gallbladder epithelial cells. Lab. Investig. 1991, 64, 682–692. [Google Scholar]
- Kesimer, M.; Cullen, J.; Cao, R.; Radicioni, G.; Mathews, K.G.; Seiler, G.; Gookin, J.L. Excess Secretion of Gel-Forming Mucins and Associated Innate Defense Proteins with Defective Mucin Un-Packaging Underpin Gallbladder Mucocele Formation in Dogs. PLoS ONE 2015, 10, e0138988. [Google Scholar]
- Aicher, K.M.; Cullen, J.M.; Seiler, G.S.; Lunn, K.; Mathews, K.G.; Gookin, J.L. Investigation of adrenal and thyroid gland dysfunction in dogs with ultrasonographic diagnosis of gallbladder mucocele formation. PLoS ONE 2019, 14, e0212638. [Google Scholar] [CrossRef] [PubMed]
- Jessurun, J.; Pambuccian, S. Infectious and Inflammatory Disorders of the Gallbladder and Extrahepatic Biliary Tract. In Sur-Gical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas, 3rd ed.; Odze, R.D., Goldblum, J.R., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 995–1020. [Google Scholar]
- Dooley, J.S.; Gurusamy, K.S.; Davidson, B.R. Gallstones and Benign Biliary Disease. In Sherlock’s Diseases of the Liver and Biliary System, 13th ed.; Dooley, J.S., Lok, A.S.F., Garcia-Tsao, G., Pinzani, M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 256–293. [Google Scholar]
- Lawrence, Y.A.; Ruaux, C.G.; Nemanic, S.; Milovancev, M. Characterization, treatment, and outcome of bacterial chole-cystitis and bactibilia in dogs. J. Am. Vet. Med. Assoc. 2015, 246, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Saka, B.; Memis, B.; Seven, I.E.; Pehlivanoglu, B.; Balci, S.; Bagci, P.; Reid, M.; Dursun, N.; Escalano, O.T.; Roa, J.C.; et al. Follicular Cholecystitis: Reappraisal of Incidence, Definition, and Clinicopathologic Associations in an Analysis of 2550 Cholecystectomies. Int. J. Surg. Pathol. 2020, 28, 826–834. [Google Scholar] [CrossRef]
- Ushio, N.; Chambers, J.K.; Watanabe, K.I.; Kishimoto, T.E.; Shiga, T.; Li, J.Y.; Nakayama, H.; Uchida, K. Chronic Inflammatory and Proliferative Lesions of the Gallbladder in Aged Pigs. Vet. Pathol. 2020, 57, 122–131. [Google Scholar] [CrossRef]
- Vientós-Plotts, A.; Wiggen, K.; Lisciandro, G.; Reinero, C. The utility of point-of-care ultrasound right-sided cardiac markers as a screening test for moderate to severe pulmonary hypertension in dogs. Vet. J. 2019, 250, 6–13. [Google Scholar] [CrossRef]
- Haworth, M.; McEwen, M.; Dixon, B.; Purcell, S.L. Anaphylaxis associated with intravenous administration of alphaxalone in a dog. Aust. Vet. J. 2019, 97, 197–201. [Google Scholar] [CrossRef]
- Gilloteaux, J.; Tomasello, L.M.; Elgison, D.A. Lipid deposits and lipo-mucosomes in human cholecystitis and epithelial metaplasia in chronic cholecystitis. Ultrastruct. Pathol. 2003, 27, 313–321. [Google Scholar] [CrossRef]
- Gilloteaux, J.; Miller, D.; Morrison, R.L. Intracellular Liposomes and Cholesterol Deposits in Chronic Cholecystitis and Biliary Sludge. Ultrastruct. Pathol. 2004, 28, 123–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imano, M.; Satou, T.; Itoh, T.; Takeyama, Y.; Yasuda, A.; Peng, Y.-F.; Shinkai, M.; Haji, S.; Yasuda, C.; Nakai, T.; et al. An immunohistochemical study of osteopontin in pigment gallstone formation. Am. Surg. 2010, 76, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Glanemann, B.; Garden, O.; Szladovits, B. Cytological Findings of 140 Bile Samples from Dogs and Cats and Associated Clinical Pathological Data. J. Vet. Intern. Med. 2015, 30, 123–131. [Google Scholar] [CrossRef]
- Kaminski, D.L.; Feinstein, W.K.; Deshpande, Y.G. The production of experimental cholecystitis by endotoxin. Prostaglandins 1994, 47, 233–245. [Google Scholar] [CrossRef]
- Kutsunai, M.; Kanemoto, H.; Fukushima, K.; Fujino, Y.; Ohno, K.; Tsujimoto, H. The association between gall bladder mucoceles and hyperlipidaemia in dogs: A retrospective case control study. Vet. J. 2014, 199, 76–79. [Google Scholar] [CrossRef]

| Antibody to | Host | Type | Dilution | Source | Catalogue Number |
|---|---|---|---|---|---|
| CD3 | Mouse | Monoclonal | 1:50 | Abcam, Cambridge, UK | ab17143 |
| CD20 | Rabbit | Monoclonal | 1:200 | Abcam, Cambridge, UK | ab64088 |
| MUM1 | Rabbit | Monoclonal | 1:500 | Abcam, Cambridge, UK | ab133590 |
| Granzyme B | Rabbit | Polyclonal | 1:50 | Abcam, Cambridge, UK | ab4059 |
| p-Value | ||||
|---|---|---|---|---|
| Parameter | G0 vs. G1 vs. G2 | G0 vs. G1 | G0 vs. G2 | G1 vs. G2 |
| Age | 0.113 | N/A | N/A | N/A |
| Breed | 0.061 | N/A | N/A | N/A |
| Sex | 0.727 | N/A | N/A | N/A |
| Sludge | 0.025 * | 0.208 | 1.000 | 0.013 ** |
| Mucocele | 0.005 * | 0.044 | 0.002 ** | 0.122 |
| GWTT a | 0.000 * | 1.000 | 0.000 ** | 0.000 ** |
| Bacteria | 0.000 * | 0.724 | 0.000 ** | 0.000 ** |
| Lymphoid follicle | 0.000 * | 0.134 | 0.000 ** | 0.000 ** |
| Smooth muscle thickening | 0.000 * | 0.011 ** | 0.000 ** | 0.000 ** |
| Edema | 0.021 * | 0.010 ** | 0.106 | 0.267 |
| Liver inflammation | 0.000 * | 0.064 | 0.102 | 0.000 ** |
| HLD b | 0.566 | N/A | N/A | N/A |
| PPVH c | 0.304 | N/A | N/A | N/A |
| Parameter | Coefficient of Association | p-Value |
|---|---|---|
| Age | 0.420 (1) | 0.010 * |
| Breed | 0.282 | 0.788 |
| Sex | 0.069 | 0.787 |
| Sludge | 0.183 | 0.010 * |
| GWTT a | 0.160 (2) | 0.018 * |
| Bacteria | 0.116 | 0.113 |
| Lymphoid follicle | 0.066 | 0.389 |
| Smooth muscle thickening | 0.109 | 0.137 |
| Edema | 0.260 | 0.000 * |
| Liver inflammation | 0.120 | 0.093 |
| HLD b | 0.515 (1) | 0.002 * |
| PPVH c | 0.200 | 0.003 * |
| Parameter | Coefficient of Association | p-Value |
|---|---|---|
| Age | 0.087 (1) | 0.518 |
| Breed | 0.326 | 0.443 |
| Sex | 0.094 | 0.583 |
| GWTT a | 0.274 (2) | 0.000 * |
| Bacteria | 0.114 | 0.103 |
| Lymphoid follicle | 0.091 | 0.191 |
| Smooth muscle thickening | 0.070 | 0.330 |
| Edema | 0.062 | 0.417 |
| Liver inflammation | 0.277 | 0.000 * |
| HLD b | 0.122 (1) | 0.373 |
| PPVH c | 0.052 | 0.490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsui, I.; Ohtsuki, S.; Uchida, K. Chronic Cholecystitis of Dogs: Clinicopathologic Features and Relationship with Liver. Animals 2021, 11, 3324. https://doi.org/10.3390/ani11113324
Mitsui I, Ohtsuki S, Uchida K. Chronic Cholecystitis of Dogs: Clinicopathologic Features and Relationship with Liver. Animals. 2021; 11(11):3324. https://doi.org/10.3390/ani11113324
Chicago/Turabian StyleMitsui, Ikki, Shigeaki Ohtsuki, and Kazuyuki Uchida. 2021. "Chronic Cholecystitis of Dogs: Clinicopathologic Features and Relationship with Liver" Animals 11, no. 11: 3324. https://doi.org/10.3390/ani11113324
APA StyleMitsui, I., Ohtsuki, S., & Uchida, K. (2021). Chronic Cholecystitis of Dogs: Clinicopathologic Features and Relationship with Liver. Animals, 11(11), 3324. https://doi.org/10.3390/ani11113324






