Effects of Temperament on the Reproduction of Beef Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
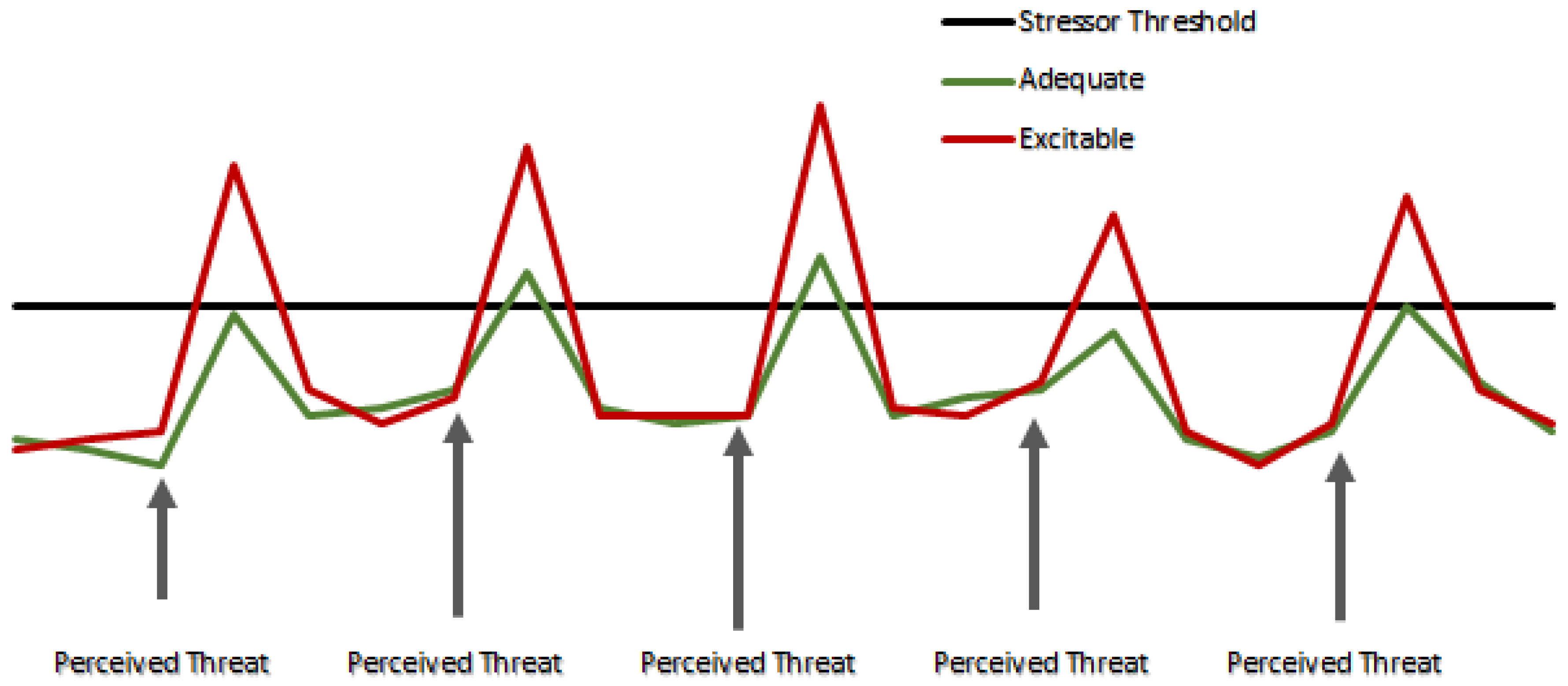
2. Assessment of Temperament in Beef Cattle
3. Temperament, Stress, and Reproductive Efficiency in Beef Cattle
4. Improving Temperament to Optimize Reproductive Outcomes in Beef Operations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merenda, P.F. Toward a four-factor theory of temperament and/or personality. J. Personal. Assess. 1987, 51, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.L.; Buchanan, D.S.; Anderson, V.L.; Ilse, B.R.; Carlin, K.R.; Berg, E.P. Working chute behavior of feedlot cattle can be an indication of cattle temperament and beef carcass composition and quality. Meat Sci. 2011, 89, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.; Yücel, M.; Fornito, A.; Barrett, A.; Wood, S.J.; Lubman, D.I.; Simmons, J.; Pantelis, C.; Allen, N.B. Neuroanatomical correlates of temperament in early adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.; Bill, E. Kunkle Interdisciplinary Beef Symposium: Temperament and acclimation to human handling influence growth, health, and reproductive responses in Bos taurus and Bos indicus cattle. J. Anim. Sci. 2014, 92, 5325–5333. [Google Scholar] [CrossRef] [Green Version]
- Dreschel, N.A. The effects of fear and anxiety on health and lifespan in pet dogs. Appl. Anim. Behav. Sci. 2010, 125, 157–162. [Google Scholar] [CrossRef]
- Grandin, T.; Deesing, M.J. Genetics and Behavior during Handling, Restraint, and Herding, in Genetics and the Behavior of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 2014; pp. 115–158. [Google Scholar]
- Benhajali, H.; Boivin, X.; Sapa, J.; Pellegrini, P.; Boulesteix, P.; Lajudie, P.; Phocas, F. Assessment of different on-farm measures of beef cattle temperament for use in genetic evaluation. J. Anim. Sci. 2010, 88, 3529–3537. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, T.; Watts, J.; Stookey, J.; Buchanan, F.; Waldner, C. Temperament in beef cattle: Methods of measurement and their relationship to production. Can. J. Anim. Sci. 2011, 91, 557–565. [Google Scholar] [CrossRef]
- Parham, J.T.; Tanner, A.E.; Wahlberg, M.L.; Grandin, T.; Lewis, R.M. Subjective methods to quantify temperament in beef cattle are insensitive to the number and biases of observers. Appl. Anim. Behav. Sci. 2019, 212, 30–35. [Google Scholar] [CrossRef]
- Fordyce, G.; Dodt, R.; Wythes, J. Cattle temperaments in extensive beef herds in northern Queensland. 1. Factors affecting temperament. Aust. J. Exp. Agric. 1988, 28, 683–687. [Google Scholar] [CrossRef]
- Whitney, G. Timidity and fearfulness of laboratory mice: An illustration of problems in animal temperament. Behav. Genet. 1970, 1, 77–85. [Google Scholar] [CrossRef]
- Tulloh, N. Behaviour of cattle in yards. II. A study of temperament. Anim. Behav. 1961, 9, 25–30. [Google Scholar] [CrossRef]
- Plasse, D.; Warnick, A.; Koger, M. Reproductive behavior of Bos indicus females in a subtropical environment. IV. Length of estrous cycle, duration of estrus, time of ovulation, fertilization and embryo survival in grade Brahman heifers. J. Anim. Sci. 1970, 30, 63–72. [Google Scholar] [CrossRef]
- Grandin, T. Solving livestock handling problems. Vet. Med. 1994, 89, 989–998. [Google Scholar]
- Cavalcanti, M.S.; Crawshaw, P.G.; Tortato, F.R. Use of electric fencing and associated measures as deterrents to jaguar predation on cattle in the Pantanal of Brazil, in Fencing for Conservation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 295–309. [Google Scholar]
- Matsunaga, M.; Silva, J.; Toledo, L.; da Costa, M.P.; Eler, J.; Ferraz, J. Genetic analysis of temperament in Nelore cattle. Proc. 7th World Congr. Genet. Appl. Livest. Prod. 2002, 14, 16. [Google Scholar]
- Le Neindre, P.; Trillat, G.; Sapa, J.; Ménissier, F.; Bonnet, J.; Chupin, J. Individual differences in docility in Limousin cattle. J. Anim. Sci. 1995, 73, 2249–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, D.; Barnes, A.; Taylor, E.; Pethick, D.; McCarthy, M.; Maloney, S.M. Physiological responses of Bos taurus and Bos indicus cattle to prolonged, continuous heat and humidity. J. Anim. Sci. 2006, 84, 972–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wambura, P.; Gwakisa, P.S.; Silayo, R.S.; Rugaimukamu, E.A. Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Vet. Parasitol. 1998, 77, 63–70. [Google Scholar] [CrossRef]
- Lanier, J.L.; Grandin, T.; Green, R.; Avery, D.; McGee, K. A note on hair whorl position and cattle temperament in the auction ring. Appl. Anim. Behav. Sci. 2001, 73, 93–101. [Google Scholar] [CrossRef]
- Littlejohn, B.; Price, D.; Banta, J.; Lewis, A.; Neuendorff, D.; Carroll, J.; Vann, R.; Welsh Jr, T.; Randel, R. Prenatal transportation stress alters temperament and serum cortisol concentrations in suckling Brahman calves. J. Anim. Sci. 2016, 94, 602–609. [Google Scholar] [CrossRef]
- Herring, A.D. Beef Cattle Production Systems; CABI Publishing: Wallingford, UK, 2014. [Google Scholar]
- Grandin, T. Factors that impeded animal movement at slaughter plants. J. Am. Vet. Mediacl Assoc. 1996, 209, 757–759. [Google Scholar]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: Wallingford, UK, 2000; p. 21. [Google Scholar]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moya, D.; He, M.; Jin, L.; Wang, Y.; Penner, G.; Schwartzkopf-Genswein, K.; McAllister, T. Effect of grain type and processing index on growth performance, carcass quality, feeding behavior, and stress response of feedlot steers. J. Anim. Sci. 2015, 93, 3091–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahringer, R.; Randel, R.; Neuendorff, D. Effects of naloxone and animal temperament on serum luteinizing hormone and cortisol concentrations in seasonally anestrous Brahman heifers. Theriogenology 1990, 34, 393–406. [Google Scholar] [CrossRef]
- Fell, L.; Colditz, I.; Walker, K.; Watson, D. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- King, D.; Pfeiffer, C.S.; Randel, R.; Welsh, T., Jr.; Oliphint, R.; Baird, B.; Curley, K., Jr.; Vann, R.; Hale, D.; Savell, J. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W.; Cappellozza, B.I.; Mueller, C.J.; Delcurto, T. Effects of temperament and acclimation to handling on reproductive performance of Bos taurus beef females. J. Anim. Sci. 2012, 90, 3547–3555. [Google Scholar] [CrossRef]
- Cooke, R.; Schubach, K.; Marques, R.; Peres, R.; Silva, L.; Carvalho, R.; Cipriano, R.; Bohnert, D.; Pires, A.; Vasconcelos, J.L.M. Effects of temperament on physiological, productive, and reproductive responses in Bos indicus beef cows. J. Anim. Sci. 2017, 95, 1–8. [Google Scholar]
- Cooke, R.; Moriel, P.; Cappellozza, B.; Miranda, V.; Batista, L.; Colombo, E.; Ferreira, V.; Miranda, M.; Marques, R.; Vasconcelos, J. Effects of temperament on growth, plasma cortisol concentrations and puberty attainment in Nelore beef heifers. Animals 2019, 13, 1208–1213. [Google Scholar]
- Cooke, R.F.; Arthington, J.D.; Austin, B.R.; Yelich, J.V. Effects of acclimation to handling on performance, reproductive, and physiological responses of Brahman-crossbred heifers. J. Anim. Sci. 2009, 87, 3403–3412. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.; Arthington, J.; Araujo, D.; Lamb, G. Effects of acclimation to human interaction on performance, temperament, physiological responses, and pregnancy rates of Brahman-crossbred cows. J. Anim. Sci. 2009, 87, 4125–4132. [Google Scholar] [CrossRef] [Green Version]
- Burdick, N.; Randel, R.; Carroll, J.; Welsh, T. Interactions between temperament, stress, and immune function in cattle. Int. J. Zool. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chase, C., Jr.; Randel, R.; Riley, D.; Coleman, S.; Phillips, W. Evaluation of tropically adapted straightbred and crossbred beef cattle: Cortisol concentration and measures of temperament at weaning and transport. J. Anim. Sci. 2017, 95, 5253–5262. [Google Scholar] [CrossRef] [Green Version]
- Schubach, K.M.; Cooke, R.F.; Brandao, A.P.; Lippolis, K.D.; Silva, L.G.T.; Marques, R.S.; Bohnert, D.W. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animals 2017, 11, 2260–2267. [Google Scholar] [CrossRef] [Green Version]
- Tilbrook, A.J.; Turner, A.I.; Clarke, I.J. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 2000, 5, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ziomkiewicz, A.; Wichary, S.; Bochenek, D.; Pawlowski, B.; Jasienska, G. Temperament and ovarian reproductive hormones in women: Evidence from a study during the entire menstrual cycle. Horm. Behav. 2012, 61, 535–540. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Sancilio, A.; Galbarczyk, A.; Klimek, M.; Jasienska, G.; Bribiescas, R.G. Evidence for the cost of reproduction in humans: High lifetime reproductive effort is associated with greater oxidative stress in post-menopausal women. PLoS ONE 2016, 11, e0145753. [Google Scholar] [CrossRef] [Green Version]
- Da Rosa, G.; Wagner, W. Adrenal-gonad interactions in cattle: Corpus luteum function in intact and adrenalectomized heifers. J. Anim. Sci. 1981, 52, 1098–1105. [Google Scholar] [CrossRef]
- Li, P.; Wagner, W. In vivo and in vitro studies on the effect of adrenocorticotropic hormone or cortisol on the pituitary response to gonadotropin releasing hormone. Biol. Reprod. 1983, 29, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat-stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60, 535–547. [Google Scholar] [CrossRef]
- Dobson, H.; Tebble, J.; Smith, R.; Ward, W. Is stress really all that important? Theriogenology 2001, 55, 65–73. [Google Scholar] [CrossRef]
- Goldstein, D.S. Catecholamines and stress. Endocr. Regul. 2003, 37, 69–80. [Google Scholar] [PubMed]
- Nwe, T.; Hori, E.; Manda, M.; Watanabe, S. Significance of catecholamines and cortisol levels in blood during transportation stress in goats. Small Rumin. Res. 1996, 20, 129–135. [Google Scholar] [CrossRef]
- Burdick, N.; Carroll, J.; Hulbert, L.; Dailey, J.; Willard, S.; Vann, R.; Welsh, T., Jr.; Randel, R. Relationships between temperament and transportation with rectal temperature and serum concentrations of cortisol and epinephrine in bulls. Livest. Sci. 2010, 129, 166–172. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Ballou, M.A.; Randel, R.D.; Willard, S.T.; Vann, R.C.; Welsh, T.H., Jr. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 2011, 17, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Alsemgeest, S.; Lambooy, I.; Wierenga, H.; Dieleman, S.; Meerkerk, B.; Van Ederen, A.; Niewold, T.A. Influence of physical stress on the plasma concentration of serum amyloid-a (SAA) and haptoglobin (HP) in calves. Vet. Q. 1995, 17, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Ceciliani, F.; Ceron, J.; Eckersall, P.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Cooke, R.; Bohnert, D. Bovine acute-phase response after corticotrophin-release hormone challenge. J. Anim. Sci. 2011, 89, 252–257. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W.; Meneghetti, M.; Losi, T.C.; Vasconcelos, J.L.M. Effects of temperament on pregnancy rates to fixed-timed AI in Bos indicus beef cows. Livest. Sci. 2011, 142, 108–113. [Google Scholar] [CrossRef]
- Bagley, C. Nutritional management of replacement beef heifers: A review. J. Anim. Sci. 1993, 71, 3155–3163. [Google Scholar] [CrossRef]
- Lesmeister, J.; Burfening, P.J.; Blackwell, R. Date of first calving in beef cows and subsequent calf production. J. Anim. Sci. 1973, 36, 1–6. [Google Scholar] [CrossRef]
- Amstalden, M.; Cardoso, R.; Alves, B.; Williams, G. Reproduction Symposium: Hypothalamic neuropeptides and the nutritional programming of puberty in heifers. J. Anim. Sci. 2014, 92, 3211–3222. [Google Scholar] [CrossRef]
- Day, M.; Imakawa, K.; Garcia-Winder, M.; Zalesky, D.; Schanbacher, B.; Kittok, R.J.; Kinder, J. Endocrine mechanisms of puberty in heifers: Estradiol negative feedback regulation of luteinizing hormone secretion. Biol. Reprod. 1984, 31, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Patterson, D.; Perry, R.; Kiracofe, G.; Bellows, R.; Staigmiller, R.; Corah, L. Management considerations in heifer development and puberty. J. Anim. Sci. 1992, 70, 4018–4035. [Google Scholar] [CrossRef] [PubMed]
- Voisinet, B.; Grandin, T.; Tatum, J.; O’connor, S.; Struthers, J. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafe, L.; Robinson, D.L.; Ferguson, D.; McIntyre, B.L.; Geesink, G.; Greenwood, P. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- Turner, S.; Navajas, E.; Hyslop, J.; Ross, D.; Richardson, R.; Prieto, N.; Bell, M.; Jack, M.; Roehe, R. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef]
- Ajmone-Marsan, P.; Garcia, J.F.; Lenstra, J.A. On the origin of cattle: How aurochs became cattle and colonized the world. Evol. Anthropol. Issues News Rev. 2010, 19, 148–157. [Google Scholar] [CrossRef]
- Morris, C.; Cullen, N.G.; Kilgour, R.; Bremner, K.J. Some genetic factors affecting temperament in Bos taurus cattle. N. Zealand J. Agric. Res. 1994, 37, 167–175. [Google Scholar] [CrossRef]
- Haskell, M.J.; Simm, G.; Turner, S.P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 368. [Google Scholar] [CrossRef]
- Roberts, A.; Petersen, M.; Funston, R. Beef Species Symposium: Can we build the cowherd by increasing longevity of females? J. Anim. Sci. 2015, 93, 4235–4243. [Google Scholar] [CrossRef]
- Emmerson, D. Commercial approaches to genetic selection for growth and feed conversion in domestic poultry. Poult. Sci. 1997, 76, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.; Richardson, E.; Herd, R.; Arthur, P. Potential for selection to improve efficiency of feed use in beef cattle: A review. Aust. J. Agric. Res. 1999, 50, 147–162. [Google Scholar] [CrossRef]
- Notter, D.R. The importance of genetic diversity in livestock populations of the future. J. Anim. Sci. 1999, 77, 61–69. [Google Scholar] [CrossRef]
- Blumstein, D.T. Habituation and sensitization: New thoughts about old ideas. Anim. Behav. 2016, 120, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Echternkamp, S. Relationship between LH and cortisol in acutely stressed beef cows. Theriogenology 1984, 22, 305–311. [Google Scholar] [CrossRef]
- Jago, J.; Krohn, C.; Matthews, L. The influence of feeding and handling on the development of the human–animal interactions in young cattle. Appl. Anim. Behav. Sci. 1999, 62, 137–151. [Google Scholar] [CrossRef]
- Krohn, C.; Jago, J.; Boivin, X. The effect of early handling on the socialisation of young calves to humans. Appl. Anim. Behav. Sci. 2001, 74, 121–133. [Google Scholar] [CrossRef]
- Curley, K., Jr.; Paschal, J.P.; Welsh, T.H.; Randel, R.D. Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Asay, M.; Schroeder, S.; Kasimanickam, V.; Gay, J.M.; Kastelic, J.P.; Hall, J.B.; Whittier, W.D. Calm temperament improves reproductive performance of beef cows. Reprod. Domest. Animals. 2014, 49, 1063–1067. [Google Scholar] [CrossRef]
- Mello, B.P.; Maturana Filho, M.; Lemes, K.M.; Gonçalves, R.L.; Lollato, J.P.M.; Zanella, A.J.; Ferreira, T.F.V.; Pugliesi, G.; Madureira, E.H.; Gonella-Diaza, A.; et al. Importance of temperament in the pregnancy by timed insemination in bovine females Bos taurus indicus. Livest. Sci. 2020, 240, 104104. [Google Scholar] [CrossRef]
| Item | Adequate | Excitable | p-Value | Type of Cattle |
|---|---|---|---|---|
| Serum cortisol, ng/mL [27] | 26.1 (tame; n = 10) 32.3 (normal; n = 9) | 53.7 (wild; n = 5) | <0.02 | B.indicus heifers (Brahman) |
| Plasma cortisol, ng/mL [28] | 30.5 (n = 12) | 46.3 (n = 12) | <0.01 | B. taurus steer calves (Angus × Hereford) |
| Serum cortisol, ng/mL [29] | 10.18 (calm; n = 22) 11.91 (intermediate; n = 79) | 14.99 (n = 28) | <0.01 | B. taurus steers (Bonsmara × Romosinuano and Angus) |
| Plasma cortisol, ng/mL [30] | 17.8 (n = 324) | 22.7 (n = 109) | <0.01 | B. taurus cows (Angus × Hereford) |
| Serum cortisol, ng/mL [31] | 39.1 (n = 726) | 49.1 (n = 227) | <0.01 | B.indicus cows (Nelore) |
| Hair cortisol, pg/mg hair [31] | 4.31 (n = 726) | 4.23 (n = 227) | 0.91 | B.indicus cows (Nelore) |
| Plasma cortisol, ng/mL [32] | 35.8 (n = 96) | 50.8 (n = 74) | <0.01 | B.indicus heifers (Nelore) |
| Item | Adequate | Excitable | p-Value |
|---|---|---|---|
| B. indicus [31] | n = 726 | n = 227 | |
| Pregnancy rates, % 3 | 47.3 | 41.0 | 0.09 |
| Pregnancy loss, % 4 | 5.9 | 9.9 | 0.05 |
| Calving rate, % | 74.8 | 68.3 | 0.04 |
| Weaning rate, % | 69.4 | 63.9 | 0.09 |
| Calf weaning BW, kg | 210 | 204 | 0.04 |
| Kg of calf weaned per cow exposed, kg | 146 | 130 | 0.04 |
| B. taurus [30] | n = 324 | n = 109 | |
| Pregnancy rates, % | 94.6 | 88.7 | 0.03 |
| Pregnancy loss, % 5 | 2.83 | 3.74 | 0.63 |
| Calving rate, % | 91.8 | 85 | 0.04 |
| Weaning rate, % | 89.9 | 83.9 | 0.09 |
| Calf weaning BW, kg | 248 | 247 | 0.71 |
| Kg of calf weaned per cow exposed, kg | 223 | 207 | 0.08 |
| Item | Acclimated | Non-Acclimated | p-Value |
|---|---|---|---|
| B. indicus × B. taurus cows [34] | (n = 197) 2 | (n = 197) 2 | |
| Plasma cortisol, after acclimation ng/mL | 33.3 | 33.6 | 0.88 |
| Chute score after acclimation | 1.98 | 1.96 | 0.59 |
| Body weight change, kg | −43 | −50 | 0.38 |
| B. indicus × B. taurus heifers [33] | (n = 40) | (n = 40) | |
| Plasma cortisol after acclimation, ng/mL | 37.8 | 50.5 | <0.01 |
| Chute score after acclimation | 1.37 | 1.84 | <0.01 |
| Growth rate until breeding season, kg/d | 0.50 | 0.58 | <0.01 |
| B. taurus heifers [30] | (n = 44) | (n = 44) | |
| Plasma cortisol after acclimation, ng/mL | 26.1 | 32.8 | 0.01 |
| Growth rate until breeding season, kg/d | 0.47 | 0.46 | 0.37 |
| Exit velocity at breeding season, m/s | 2.10 | 2.56 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, A.P.; Cooke, R.F. Effects of Temperament on the Reproduction of Beef Cattle. Animals 2021, 11, 3325. https://doi.org/10.3390/ani11113325
Brandão AP, Cooke RF. Effects of Temperament on the Reproduction of Beef Cattle. Animals. 2021; 11(11):3325. https://doi.org/10.3390/ani11113325
Chicago/Turabian StyleBrandão, Alice Poggi, and Reinaldo Fernandes Cooke. 2021. "Effects of Temperament on the Reproduction of Beef Cattle" Animals 11, no. 11: 3325. https://doi.org/10.3390/ani11113325
APA StyleBrandão, A. P., & Cooke, R. F. (2021). Effects of Temperament on the Reproduction of Beef Cattle. Animals, 11(11), 3325. https://doi.org/10.3390/ani11113325






