Simple Summary
Tannin has been extensively assessed for its potential and utilisation as a ruminant feed additive in recent years and is becoming important due to its beneficial effects on modulating ruminant performance and health and mitigating methane emissions. However, evidence concerning the effect of tannin in extracted forms on ruminants appears to be inconclusive on whether it can genuinely provide either beneficial or detrimental effects for ruminants. Moreover, the effects of various sources, types of tannin extract, or appropriate levels of supplementation on ruminants remain unclear. Therefore, there is a need for a systematic evaluation concerning the effects of tannin extract on rumen fermentation, digestibility, performance, methane emissions, and metabolism of ruminants.
Abstract
The objective of this meta-analysis was to elucidate whether there are general underlying effects of dietary tannin extract supplementation on rumen fermentation, digestibility, methane production, performance, as well as N utilisation in ruminants. A total of 70 papers comprised of 348 dietary treatments (from both in vivo and in situ studies) were included in the study. The database was then statistically analysed by the mixed model methodology, in which different experiments were considered as random effects and tannin-related factors were treated as fixed effects. The results revealed that an increased level of tannin extract inclusion in the diet lowered ruminant intake, digestibility, and production performance. Furthermore, the evidence also showed that an increased level of tannin extract decreased animal N utilisation where most of rumen by-pass protein was not absorbed well in the small intestine and directly excreted in the faeces. Due to the type of tannin extract, HT is more favourable to maintain nutrient intake, digestibility, and production performance and to mitigate methane production instead of CT, particularly when supplemented at low (<1%) to moderate (~3%) levels.
1. Introduction
Tannin is known for its anti-nutritional properties due to its detrimental effects on feed intake, rumen microorganisms, nutrient utilisation, and production performance of ruminant livestock, particularly when present at a high concentration in the diet [1]. However, when present at a low to moderate level, tannin may provide beneficial effects to modulate ruminant performance, health, and environmental sustainability [2]. Its molecular structure enables it to modulate ruminal fermentation by binding to protein through hydrogen bonds and forming a tannin–protein complex, thus influencing protein degradation in the rumen [3]. The tannin–protein bound in the rumen is stable at a normal pH environment and resistant to rumen microbial degradation, but it dissociates at a low pH environment in the abomasum [4]. Thus, tannin supplementation commits to lowering the amount of protein that is degraded in the rumen and increases the flow of by-pass protein to the small intestine. Tannin may also alleviate the toxic effect of high rumen ammonia concentration and improve nitrogen efficiency [5,6].
Another beneficial effect of tannin is its ability to decrease enteric methane emissions [7]. Enteric methane emissions are an important issue to consider since ruminants contribute to approximately 17% of global methane emissions or about 47% of the global livestock sector for global greenhouse gases [8,9]. A number of experiments have demonstrated the methane-mitigating property of tannin. For instance, Zhang et al. [10] found that the supplementation of 60 g/kg extracted hydrolysable tannin (HT) from Chinese nutgall decreased methane production up to 30–36% in sheep, while Pineiro-Vazquez et al. [11] found that the supplementation of 30 g/kg extracted condensed tannin (CT) from Mimosa decreased sheep methane production up to 38%. However, there were contrasting results regarding the methane mitigating effect of tannin; some other experiments did not observe any reduction in the methane emissions of ruminants after being supplemented with tannin. These variations depend on the level, type of tannin applied, plant sources, and form of tannin [7].
Tannin may be supplemented into the diet either as tannin-containing plants or as its extracted form. The use of tannin extract instead of tannin-containing plants is typically preferable for a large-scale and commercialised ruminant production system such as in a feedlot. The commonly used tannin extract originates from acacia, quebracho, chestnut, and mimosa. Such various sources of tannin extract and different doses of dietary supplementation may lead to their inconsistent and highly variable effects on ruminant production such as nutrient intake, digestibility, production performance, methane emissions, product quality, and other parameters. Therefore, there is a need for a systematic evaluation concerning the dietary supplementation of tannin extract in ruminants. The objective of this study was to examine the effects of tannin extract supplementation at various levels and sources (types) on nutrient intake, rumen fermentation, digestibility, methane production, blood metabolites, production performance, and nitrogen utilisation of ruminants by employing a meta-analysis method.
2. Materials and Methods
2.1. Database Development
A database was constructed from various experiments reported in the literature where tannin extract was supplemented into ruminant diets. All constructed data were based on in vivo and in situ experiments (did not include in vitro experiments), obtained from various electronic journal platforms such as Web of Science, Scopus, Google Scholar, and Science Direct. The selection of studies included in the database is graphically presented in Figure 1.
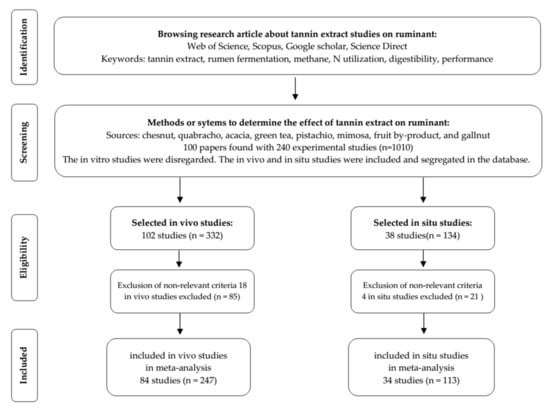
Figure 1.
Diagram flow for selection of the studies on the influence of tannin extract on ruminants.
A total of 118 experimental studies, both in vivo and in situ, from 70 papers and comprised of 360 dietary treatments were finally integrated into the database (summarised in Table 1). Experimental studies were treated individually even when published within an article. The database was segregated into two categories based on the study methods, i.e., in vivo studies (84 experiments, 247 treatments) and in situ studies (34 experiments, 113 treatments). Animals that were involved in the in vivo and in situ experiments were large ruminants (lactating dairy cows, heifers, and beef cattle, both steers and bulls) and small ruminants (goats and sheep). Parameters included in the meta-analysis were nutrient intakes such as the digestibility of dry matter (DMD), organic matter (OMD), crude protein (CPD), and neutral detergent fibre (NDFD); production performance such as weight gain and feed efficiency; methane production; milk production and composition; rumen fermentation and microbial profiles; ruminal feed disappearance; blood plasma metabolites; N utilisation; and urinary purine profile.

Table 1.
Studies included in the meta-analysis of the influence of dietary tannin extract concentration on ruminants.
The tannin form was specified as HT, CT, or unspecified or represented a mixture of HT and CT. The unspecified tannin then was categorised as CT or HT based on the primary tannin content. Overall, the sources of extracted tannin were obtained from chestnut, quebracho, acacia, green tea, pistachio, mimosa, fruit by-product such as grape pomace and pomegranate peel, gallnut, as well as commercial or unspecified tannin. Extracted tannin sources from acacia, Cistus ladanifer L., grape pomace, mimosa, pomegranate peel, quebracho, and Vaccinium vitis idaea were classified as a source of CT. Meanwhile, extracted tannin sources from chestnut, gallnut, green tea, pistachio, valonia, and tara were classified as a source HT. The supplementation level of tannin extract was presented as g/kg DM of feed, and measurements expressed in other units (mg/mL, % v/v, or % w/v) were converted to g/kg DM from available information in the papers. Supplemented tannin extract in the diet ranged from 0 (typically in the control diet) to 140 g/kg DM. The data points of animals treated with polyethylene glycol were not included in the database since this compound is known to be a tannin-deactivating agent [12].
The measurement of CH4 emissions in the in vivo experiments was performed by using a respiration calorimetry system equipped with an infrared CH4 detector. The units for milk composition and milk N utilisation were converted and presented as g/100 g, while the units for rumen fermentation profiles, rumen ammonia, milk urea N, or blood plasma were converted and presented as mmol or mg/dL. The unit for production performance, digestibility, and milk production parameters was presented as g/d, kg/d, or converted to g/kg metabolic body weight (g/kg BW0.75). The unit for the in situ degradation kinetics was uniformed in percentage (%) unit. The statistical summary of the database is presented in Table 2.

Table 2.
Descriptive statistics of the variables in the database were used to evaluate the influence of tannin extract supplementation on ruminant parameters.
2.2. Statistical Analysis
The database was analysed by employing the mixed model methodology [79,80], using the MIXED procedure of SAS software (version 9.2, SAS Institute Inc., 2008). Different experiments were considered as random effects and tannin-related factors (either concentration or type of tannin) were treated as fixed effects, followed Jayanegara et al. [12] and Yanza et al. [9] with some modifications. The assessment of the tannin extract supplementation level and tannin type (CT or HT) was accomplished with the following statistical model:
where Yij = dependent variable, µ = overall mean, si = random effect of the -ith experiment, τj = fixed effect of the -jth level of factor τ, sτij = random interaction between the -ith experiment and the -jth level of factor τ, B0 = overall intercept across all experiments (fixed effect), B1 = linear regression coefficient of Y on X (fixed effect), Xij = value of the continuous predictor variable (tannin extract level), bi = random effect of study on the regression coefficient of Y on X in study -i, and eij = the unexplained residual error. The CLASS statement was declared based on the tannin type and the study variable since they did not contain any quantitative information. The RANDOM statement was declared based on different studies included. The number of replicates in the studies was declared in the WEIGHT statement available in SAS as performed by Jayanegara et al. [12] and Yanza et al. [9]. The model was considered significant at p ≤ 0.05 or tends when the p-value was >0.05 and ≤0.10.
Yij = µ + si + τj + sτij + B0 + B1Xij + B2X2ij + biXij + eij
3. Results
The addition of tannin extract did not affect ruminant performance, such as average daily gain expressed as gram/d (ADG), gross energy intake (GEI/BW0.75), digestible energy intake (DEI/BW0.75), and metabolizable energy intake (MEI/BW0.75) (Table 3). However, when expressed as ADG/DMI (g/kg DM intake; feed efficiency), animal weight gain tended to increase with the increased tannin extract concentration following a quadratic response (p = 0.092). Concerning nutrient intake, although the OMI and CPI were not affected by tannin extract supplementation, daily DMI (kg/d) and DMI per kg metabolic body weight (DMI/BW0.75) were decreased by quadratic response (p = 0.002) and linear response (p < 0.001), respectively. The concentration of tannin extract also decreased the daily NDF intake (p = 0.025) as well as CPI/BW0.75 (p = 0.005) and NDFI/BW0.75 (p = 0.003) in a linear response. The OMI/BW0.75 (p = 0.058) tended to decrease linearly by the increased level of tannin extract supplementation. The DMD, OMD, CPD, and NDFD digestibility were also decreased with increased levels of tannin extract by quadratic responses (p < 0.010). In regard to the type of tannin supplementation (CT vs. HT), there were significant interaction on the NDFD (p = 0.044) and a tendency (p = 0.096) of interaction on NDFI/BW0.75.

Table 3.
Regression equations on the influence of tannin extract supplementation (T, in g/kg DM; independent factor) on ruminant intake, digestibility, ruminant performance, methane production, as well as milk production and milk composition.
Methane emissions expressed as CH4/DMI and CH4/BW0.75 were lowered by the increased level of supplementary tannin extract with a linear response (p < 0.010). Significant responses were also shown on the methane production expressed as CH4 (L/d; p = 0.047) and CH4/BW0.75 (L/kg; p = 0.046), as well as tended to different for CH4/DMI (L/kg; p = 0.051) in the case of tannin type. Milk yields expressed in kg/d tended to decrease with increased concentrations of tannin extract (p = 0.083) with a quadratic response, but were not affected when expressed as Milk yield/BW0.75 and Milk/DM intake. However, FPCM, solid non-fat, total solid, and urea-N in milk were decreased by the level of tannin extract supplementation (p ≤ 0.01), where FPCM showed a quadratic response while others showed linear responses. Although there is no effect by tannin extract concentration, protein (p = 0.094; tended to be significant) and lactose (p = 0.022, significant) content in milk were influenced by the different types of tannin extract.
The rumen fermentation parameters such as pH and Iso-C5 proportion were not affected by tannin extract supplementation (Table 4). However, the TVFA, C2, C5, and the ratio of C2:C3 were decreased by increasing the concentration of tannin extract (p < 0.01), where the NH3, TVFA, C2, and the ratio of C2:C3 showed a linear response and C5 had a quadratic response. In contrast, C3, Iso-C4, and C4 proportions were increased by the concentration of tannin extract supplementation (p < 0.050), where Iso-C4 showed a quadratic response while C3 and C4 showed a linear response for their models. Concerning the rumen microbial population, the levels of tannin extract supplementation had no significant effect on the bacterial population but tended to linearly decrease the protozoa population (p = 0.058). Nonetheless, only C2 and C4 had significant differences by the type of tannin extract (p < 0.050). Meanwhile, digestibility aspects such as ruminal total N, ruminal OM-N, ruminal total protein, and intestinal protein were decreased following a linear response due to increasing the concentration of tannin extract (p < 0.05), but no effect was observed on duodenal total protein digestibility.

Table 4.
Regression equations on the influence of tannin extract supplementation (T, in g/kg DM; independent factor) on rumen fermentation profile and feed disappearance in the rumen.
The plasma urea-N (PUN) was decreased by a quadratic response (p = 0.002) (Table 5) when the supplementation of tannin extract increased and tended to be significantly influenced by the type of tannin extract (p = 0.089). Although the albumin was not influenced by the tannin extract concentration, the type of tannin tended to affect the albumin concentration in the blood plasma (p = 0.060). Concerning N utilisation, the concentration of tannin extract did not affect milk-N and urine-N output. However, the faeces-N output was significantly increased linearly by the level of tannin extract supplementation (p < 0.001). N retention was also increased by the concentration of tannin extract with a quadratic model (p < 0.001) and was significantly influenced by different types of tannin extract supplementation (p = 0.012). However, the ENU tended to decrease by the concentration of tannin extract with a quadratic response (p = 0.070). Based on urinary purine, the concentration of allantoin and microbial N supply were not influenced by the level of tannin extract supplementation in ruminants. However, uric acids and purine derivative concentration tended to be lowered by the level of tannin extract supplementation (p < 0.010) and significantly depended on the type of tannin extract (p < 0.001). Meanwhile, the effectiveness of microbial protein supply (EMPS) was significantly lowered by the increase in concentration of tannin extract (p = 0.043), and the type of tannin significantly affected the EMPS reduction (p < 0.001).

Table 5.
Regression equations on the influence of tannin extract supplementation (T, in g/kg DM; independent factor) on ruminant blood plasma, percentage of N utilisation, and ruminant urinary purine.
In the in situ studies, the concentration of tannin extract supplementation significantly decreased a, a + b, and c coefficients followed by a decrease in the ERD percentage at 2%, 5%, and 8% (p < 0.001) of DM and CP (Table 6). The coefficient of the non-soluble fraction (b coefficient) of DM and CP was increased quadratically by the level of supplemented tannin extract (p ≤ 0.001), which was also influenced by the type of tannin (p = 0.072 and p < 0.001, respectively). On the other hand, a-dm, a-cp, ERM 2%, and ERM 8% of DM were significantly affected by the type of tannin (p < 0.050). The a and a + b of CP were also assigned for the type of tannin (p < 0.005). Meanwhile, there was no significant dependence on ERM percentages of CP degradability. Moreover, the concentration of tannin extract decreased the ID but increased the RUP percentage, and both variables were changed in a linear response (p < 0.001).

Table 6.
Regression equations on the influence of tannin extract supplementation (T, in g/kg DM; independent factor) on in situ dry matter kinetic degradability and protein kinetic degradability of ruminants.
4. Discussion
4.1. Influence of Tannin Extract on Performance, Digestibility, Rumen Parameters, Milk Production, and Methane Production
Investigations on the influence of dietary tannin extract supplementation in animals have been growing massively in the last two decades, especially on ruminants [12]. The intervention with tannin obtained large variability in the outputs, whether beneficial and/or detrimental on ruminants’ health and production. Tannin is generally known for its capability to bind with protein in feed, forming a tannin–protein complex that is stable at ruminal pH conditions but dissociates at abomasal acidic pH or duodenal alkaline pH. Accordingly, most of the tannin–protein complex is skipped from ruminal protein degradation and is non-denatured protein for further metabolic processes in the intestine, which is beneficial for metabolism efficiency, optimising dietary energy utilisation when supplemented at appropriate doses [4,81,82]. Another beneficial effect is the toxic effect of tannin that could diminish undesirable ruminal microorganisms involved in methane formation, resulting in lower methane production [7]. Nonetheless, due to the presence of other bioactive molecules in the whole plant that might interfere with the tannin effect such as phenolic acid, flavonoids, diterpenes [83,84], saponins [85], lipids [9], and essential oils [86], studies regarding the effect of tannin on ruminants have been moving forward to specifically determine the influence of tannin in extracted or purified form on ruminant methane production, digestibility, and performance [87,88]. It is expected that the effects of extracted tannin on those parameters would be more obvious corresponding to the type of tannin used, i.e., CT and HT.
In this meta-analysis, supplementation with tannin extract (HT and CT) had an adverse effect on the nutrient intake of ruminants. It is generally known that tannin in the diet influences ruminant palatability. Thus, under this aversion, a decrease in feed intake and rate of digestion in the rumen might occur [89]. On the contrary, some studies reported a non-detrimental effect of tannin extract on ruminant intake [16,26,51]. Meanwhile, decreases in nutrient intake were more obvious in the present study, probably because ruminants had a limited adaptation period to the supplementary tannin extract in the diets. Similar results were reported in our previous meta-analysis study where tannin supplementation impaired ruminant dry matter intake and performance [7]. We suspected that the unaffected nutrient intake may be attributed to the presence of tannin extract in a low concentration, about 0.5–3% of the total diet [11]. Another reason that should be noticed is that some treated animals were fed a diet composed of molasses, which can improve animal palatability [25]. Thus, the effect of tannin on animal palatability was resolved. However, the decrease in nutrient intake was concomitant with a depression in nutrient digestibility (Table 4), especially on NDF. Tannin extract tended to impair the NDF intake and digestibility rate, in which the type of tannin (CT and HT) might also influence the ruminant digestibility rate differently. This is plausible because tannins are acknowledged for their detrimental effects on ruminant digestibility by coating the physical attributes of feed particles due to the tannin–fibre or tannin–protein complex binding. In addition, tannin also caused intoxication in ruminal microorganisms, especially fibre-degrading bacteria, thus preventing them from rumen degradation [51,90,91]. In accordance with the type of tannin, we assume that the condensed tannin exerted a greater repercussion on a nutritional and digestibility perspective than HT. This is because CT had a greater affinity for more solid feed particles and is more difficult to hydrolyse than HT, which is easier to degrade by rumen microbes.
Such conditions also influenced methane production, which was confirmed by the reduced methane production in the present study, and this was associated with the decrease in ruminal fibre degradation. Limited fibre degradation as a result of fibre–tannin bonding is unfavourable to synthesising optimum VFA by rumen microorganisms; hence, the H2 supply is also limited for methanogens to perform methanogenesis. Therefore, the increasing level of tannin extract in the diet tremendously suppressed rumen methane formation due to the decrease in acetate formation from pyruvate [7,92], although there was no significant effect on total VFA by increasing the supplementary level of tannin extract. The enhancement of propionate concentration occurred by the lack of activity of acetogenic bacteria due to tannin biological activity, while H2 utilisation was shifted to propionate formation where free-H2 is more approbatory for propionic bacteria agents [83,93]. Hence, a lowered C2:C3 ratio was also confirmed in the present study.
If we compare the effectiveness between tannin types on reducing methane production, HT seems to have a greater ability to reduce methane production than CT. According to Jayanegara et al. [81], a decrease in methane production is strongly related to the protein precipitation degree caused by tannin–protein complexes. In such a way, HT is more susceptible to microbial degradation involved in the methanogenesis process (fibrolytic bacteria and methanogens) due to the fact that the HT hydrogen bond is easily attached to microbial cells or enzymes that are toxic to rumen microbes; thus, this condition may impair the microbial metabolism. Although the bacterial population in the present study was not clearly affected by tannin biological activity, tannin is generally known to decrease bacterial attachment to plant particles and cause subsequent decreases in N and NDF digestibility [45,81,94]. Perhaps this condition could explain the unaffected VFA concentration in the rumen by the increased level of tannin in the diet. Moreover, such tannin mechanisms could be associated with the decrease in the protozoa population where this microbe is involved in methanogenesis [31,95,96].
The decrease in ammonia (NH3) concentration also showed an obvious relationship with the increased level of tannin, whereas the feed particles that formed fibre–tannin and protein–tannin complex bonds are difficult to degrade by proteolytic bacteria. Thus, protein and amino acids protected by tannin to pass rumen fermentation are favourable because this would increase protein absorption in the small intestine, which in turn increases N use efficiency. On the contrary, most reports showed that most of the rumen by-pass protein and amino acids were undigested in the small intestine due to the strong protein–tannin molecule bonds that are difficult to break down by the intestinal enzyme. This explains why somehow N and amino acid supplies for animal metabolism were lower than the expectation. Likewise, although tannin is propitious in decreasing methane production, both tannin types may be supplemented in a low dose; hence, their adverse effect on performance and nutrient digestibility can be averted [51].
Moreover, our meta-analysis has shown that ruminant performance was also decreased. The decrease in animal weight gain (ADG) was robustly correlated to the decreased nutrient intake and digestibility, but feed efficiency (ADG/DMI) tended to be increased. The lower ADG might reflect the negative association between tannin intervention and nutrient intake and digestibility that might not meet the animal growth requirements [34]. On the other hand, the decrease in milk yield in the present study was not observed as of kg/DM intake or milk yield/metabolic BW (g/kg0.75). Although there was a potential decrease in milk production (kg/d), which might not be related to tannin intervention directly, this aspect needs further assessment. The fat protein corrected milk (FPCM), which represents the general model of milk fat and protein composition as well as general milk yield (kg/d), was consistently lowered due to increasing levels of tannin extract supplementation. Toral et al. [73] reported that the inconsistent effect of tannin on milk production is probably related to ruminant species, dietary treatment period, type of tannin, and dose. However, no significant difference was noticed in the present study regarding milk yield. It was in the range of FCPM value according to the Dutch feeding system for dairy cows as reported by Herremans et al. [42], which is between 23.9 and 26.1 FPCM. Although the total solid and solid non-fat were also influenced by the level of tannin supplementation, they were decreased only if the dairy animal was fed with a high dosage of tannin extract. This finding emphasised that tannin inclusion in the diet would only slightly affect the milk yield and total solid in milk with or without fat composition, where tannin did not increase the quantity of digestible proteins, thus explaining milk N stability [42,56].
Milk yield and milk composition results were inconsistent, but the changes in milk components such as protein and lactose were largely dependent on the different types of tannins. HT and CT showed different effects on protein and lactose contents in milk, whereas HT seems to have a better beneficial value compared with CT. This is likely because the hydrogen bond of HT derivative in the rumen is weaker, thus it is easier to degrade, with the consequence that the by-pass protein is preserved for further metabolism processes, e.g., glucose and protein deposition in milk is higher when compared to the case of CT. Such HT inclusion in ruminant diet may provide better protein and lactose composition in milk rather than CT [73]. Above all, it is critical to consider the type and levels of tannins supplemented by dairy cows.
4.2. Influence of Tannin Extract on Ruminal N Digestibility, Blood Plasma, N Utilisation, and Urinary Purine Derivative of Ruminants
Since the beneficial effects of tannin are primarily known to protect the feed by-pass protein (degradable) and distribute their amino derivative to further metabolism processes, the protected protein was expected to be absorbed in the small intestine and accumulated in the liver. Plasma urea nitrogen (PUN) and albumin concentration in the blood are considered as parameters to clarify animal protein status [18]. The albumin concentration from CT and HT interventions might appear differently due to the difference in digestibility index associated with them. Meanwhile, the decrease in PUN concentration occurred due to the undissociated by-pass protein in the small intestine. Moreover, PUN is not absorbed but produced in the liver or from ammonia coming from the rumen or gut epithelium or amino acids used in the liver for gluconeogenesis. For example, Orlandi et al. [59], who observed steers and offered Acacia mearnsii tannin extract at the rates of 20, 40, or 60 g/kg DM, found a linear decrease in ruminal ammonia while the faecal nitrogen (N) excretion, N retention, and the efficiency of N utilisation increased. In their report, they found an increase in N duodenal flux, α-amino N, and non-ammonia non-microbial N. However, Wischer et al. [76] also found an increase in faecal-N but without any difference in N retention and urinary N in sheep treated with chestnut and valonea tannin at 20 g/kg DM.
Although the increased level of tannin showed a positive relationship with N retention and the efficiency of N utilisation in ruminants, nevertheless, instead of being absorbed, most of the protein–tannin complexes were not dissociated in the small intestine, which is also confirmed in the present study. Consequently, N excretion might also increase, thus expected higher growth did not occur. When animals are fed with high dietary protein in parallel with elevated tannin supplementation, unfortunately, the intestinal enzymes are disabled to degrade most of those tannin–protein complexes, making it less available for further metabolism. Both tannin types had similar effects on the decrease in PUN. The present findings agreed with Henke et al. [1], who observed the effect of quebracho tannin extract at 15 and 30 g/kg DM on dairy cows. They suggested that tannins are less effective at improving feed intake and protein use efficiency. However, if the tannin–protein complexes disassociated post-ruminally and amino acids could be absorbed in excess, absorbed PUN would be expected to be similar in cattle fed an excessive protein diet without tannin [47].
Sequential effects by increasing the level of tannin extract presence in the diet cannot be evaded. It can be seen by the indirect effect on the reduction in milk urea N (MUN). The MUN concentration is a necessary parameter to estimate and monitor the nutritional status of lactating dairy cows as well as to improve dairy herd nutrition [36]. This condition is believed to be correlated with the effect of tannin inclusion that influences lower N intake, provides insufficient absorbable N in the small intestine, and is distributed below the required concentration in the blood; hence, the MUN deposition in milk was also reduced. Although N retention was potentially increased, most of the protein was poorly absorbed due to tannin extract supplementation, indicated by the increase in faecal N concentration. Although N-urine was not affected, the uric acids, purine derivatives (PD), and effectiveness of microbial protein supply (EMPS) were decreased. Urinary PD is commonly used as an indicator for the effectiveness of rumen MCP synthesis [36]. A lower urinary PD excretion pinpoints that the tannin extract reduces the microbial protein reaching the duodenum. In such a case, it showed that by-pass protein was not thoroughly absorbed and distributed for metabolic purposes as it was shown to increase N in faeces and urine as well as the concentration of uric acids, PD, and EMPS rates. Koenig et al. [36] suggested that amino acids from feed absorbed in excess or with an imbalanced profile with maintenance production requirements are extracted and deaminated in the liver and the N is also excreted in the form of urea N in urine. Due to the different biological characters of tannin, it seems that the CT tannin–protein bond is difficult to hydrolyse post-ruminally; therefore, feed protein bonded with the HT tannin was more available to be absorbed in the ruminant hindgut.
4.3. Influence of Tannin Extract on Kinetics Degradability In Situ
The distinct effects of tannin extract on ruminant digestibility can be observed thoroughly from the kinetics degradability of in situ experiments. The decrease in non-soluble fractions of DM and CP indicates an inhibitory effect on endoglucanases and cellulose degradation of feed particles due to the protein–tannin or fibre–tannin complex bonds. Moreover, some proteolytic bacteria are noticed to be able to modify their metabolism, i.e., adapt with a selective advantage environment to grow in the presence of phenolic compounds such as tannin [97,98]. Thus, rumen degradation was potentially reduced by the increased levels of tannin because ruminal microbes are also sensitive to the presence of tannin extract. Our evidence showed that a low dosage of tannin extract inclusion might not adversely affect the rumen bacterial population. However, they persistently impair ruminant digestibility and productivity. The presence of tannin extract is toxic to several species of rumen bacteria. Therefore, inhibitory effects on protein proteolysis often occur, and in some conditions, the polymer–tannin bond fails to be absorbed as rumen undegradable protein (RUP) in the intestine. Nasehi et al. [57] reported that tannin reduced the ruminal degradability of plant proteins and enhanced the intestinal bioavailability of amino acids in ruminants. By contrast, our evidence showed that the presence of tannin extract negatively influenced rumen protein degradability and total tract apparent digestibility. Concerning the difference in effectivity between tannin types, the reduced ruminal degradability was also influenced by the difference in the biological activity of tannin as we described above (Section 4.1).
4.4. Noticeable Effect by the Divergence between Tannin Extracts
In the present study, types of tannin were distinguished into CT and HT as those types have different chemical properties [12]. HT is a hydroxyl group of which they are partially, or fully, esterified with either gallic or hexahydroxydiphenic acid and may have long chains of gallic acid coming from the central glucose core [99]. HT is hydrolysed into their constituent phenolic acids with acid or enzymes. Meanwhile, CT includes polymers formed by the condensation of flavans molecules such as procyanidin, or higher oligomers of substituted flavan-3-ols, but they do not contain any sugar residues [100]. CT monomers are favourable to link with carbon bonds and difficult to break down where the molecule bond stability is vigorous. The molecules can be broken down by heating or strong acids.
However, their mechanism can be explained chemically based on the data analysed in the present study. HT had a stronger protein precipitation ability than CT; thus, methane emissions were decreased effectively, and by-pass protein might escape from the rumen. However, a higher level of HT presence in the diet may not effectively alter ruminant metabolism in a further condition since the HT–protein or HT–fibre bonds are hydrolysed by ruminal microbes or intestinal enzymes. HT might camouflage the bonds of protein or fibre; hence, in such a way the absorbable nutrient might escape for further metabolism processes. Meanwhile, when CT bonded with carbonic groups of feeds, the ruminal microbes found it difficult to break down the CT–protein or CT–fibre in the rumen due to their solid bonds. Escaped rumen CT–feed bonds were also difficult to degrade. Therefore, the adverse effect of CT on ruminant digestibility is potentially greater than HT. Moreover, although the faecal-N and urinary-N were increased, HT seems to support N retention more than CT due to their sequential effects before escaping the rumen. N supply and available amino acids might be greater when ruminants are fed a diet with HT supplementation compared to CT. This condition might also reflect on animal production such as milk yield and milk composition. However, it should be underlined that the presence of HT can be absorbed in the digestive tract to some extent, whereas HT consumption with excessive amounts can be toxic to ruminants [12]. On the other hand, CT is notably vigorous for ruminal microbial or digestive tract enzymes to absorb. Accordingly, the readily absorbable nutrients are limited in the lower gut [41]. Despite their detrimental effects, both types of tannins may provide some beneficial effects if consumed at a low or moderate dosage.
5. Conclusions
The present meta-analysis study evaluated experimental evidence concerning the effects of tannin extract in a beneficial perspective on methane emission reduction and providing higher rumen by-pass protein with the appropriate level of tannin extract. However, some detrimental effects such as decreased animal intake, digestibility, and performance also occurred with excessive levels of tannin extract supplementation. Such a condition occurred due to tannin’s ability to limit proteolysis in the rumen and digestive tract; however, the by-pass protein was less available for absorption in the intestine due to strong CT–protein or CT–fibre bonds that were difficult to dissociate. Thus, ruminant weight gain and milk yield were distinctly impaired by tannin. Otherwise, tannin mechanisms on those parameters were also specified by different types of tannin and their chemical properties. HT seems to be more favourable for ruminants instead of CT. However, HT and CT tannin supplementation were distinctly effective at a low dosage of supplementation to enhance more beneficial outcomes.
Author Contributions
Conceptualisation, methodology, analytical software, data curation, original draft preparation, data analysis, Y.R.Y.; resources, data curation, data analysis, original draft preparation, A.F.; conceptualisation, resources, data curation, supervision, review and editing, visualisation, project administration, A.J.; project administration, review, and visualisation, N.R.K., N.H., E. and B.S.; data curation, data analysis and figure illustration, review, A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Education, Culture, Research and Technology, Republic of Indonesia, through the World Class Research grant, year 2021, grant no. 077/SP2H/LT/DRPM/2021.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not available.
Acknowledgments
All authors are grateful to the Animal Feed and Nutrition Modelling (AFENUE) Research Group, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Indonesia, for providing a collaborative platform across various institutions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henke, A.; Westreicher-Kristen, E.; Molkentin, J.; Dickhoefer, U.; Knappstein, K.; Hasler, M.; Susenbeth, A. Effect of dietary quebracho tannin extract on milk fatty acid composition in cows. J. Dairy Sci. 2017, 100, 6229–6238. [Google Scholar] [CrossRef] [PubMed]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M.; Morgavi, D.P. Replacing urea with nitrate as a non-protein nitrogen source increases lambs’ growth and reduces methane production, whereas acacia tannin has no effect. Anim. Feed Sci. Technol. 2020, 259, 114360. [Google Scholar] [CrossRef]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Fischer, M.L.; Anschau, F.A.; Venturini, T.; Tinini, R.C.R.; Dessbesell, J.G.; Faciola, A.P. Effects of black wattle (Acacia mearnsii) condensed tannins on intake, protozoa population, ruminal fermentation, and nutrient digestibility in jersey steers. Animals 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Pal, K.; Patra, A.; Sahoo, A.; Soren, N.M. Effects of nitrate and fumarate in tree leaves-based diets on nutrient utilizaton, rumen fermentation, microbial protein supply and and blood profiles in sheep. Livest. Sci. 2015, 172, 5–15. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of lipid-encapsulated acacia tannin extract on feed intake, nutrient digestibility and methane emission in sheep. Animals 2019, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; p. 1. [Google Scholar]
- Yanza, Y.R.; Szumacher-Strabel, M.; Jayanegara, A.; Kasenta, A.M.; Gao, M.; Huang, H.; Patra, A.K.; Warzych, E.; Cieślak, A. The effects of dietary medium-chain fatty acids on ruminal methanogenesis and fermentation in vitro and in vivo: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 874–889. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of origanum oil, hydrolysable tannins and tea saponin in mitigating ruminant methane: In vitro and in vivo methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef]
- Pineiro-Vazquez, A.T.; Jimenez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Perez, C.F.; Ku-Vera, J.C. Effects of quebracho tannin extract on intake, digestibility, rumen fermentation, and methane production in crossbred heifers fed low-quality tropical grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Jayanegara, A.; Sujarnoko, T.U.P.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage quality as influenced by concentration and type of tannins present in the material ensiled: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of ruminal Quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Alipour, D.; Rouzbehan, Y. Effects of several levels of extracted tannin from grape pomace on intestinal digestibility of soybean meal. Livest. Sci. 2010, 128, 87–91. [Google Scholar] [CrossRef]
- Al-Kindi, A.; Dickhoefer, U.; Schlecht, E.; Sundrum, A.; Schiborra, A. Effects of quebracho tannin extract (Schinopsis balansae Engl.) and activated charcoal on nitrogen balance, rumen microbial protein synthesis and faecal composition of growing Boer goats. Arch. Anim. Nutr. 2016, 70, 307–321. [Google Scholar] [CrossRef]
- Aprianita, A.; Donkor, O.N.; Moate, P.J.; Williams, S.R.O.; Auldist, M.J.; Greenwood, J.S.; Hannah, M.C.; Wales, W.J.; Vasiljevic, T. Effects of dietary cottonseed oil and tannin supplements on protein and fatty acid composition of bovine milk. J. Dairy Res. 2014, 81, 183–192. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Abo-Donia, F.M.; Yang, L.Y.; Hristov, A.N.; Wang, M.; Tang, S.X.; Zhou, C.S.; Han, X.F.; Kang, J.H.; Tan, Z.L.; He, Z.X. Effects of tannins on the fatty acid profiles of rumen fluids and milk from lactating goats fed a total mixed ration containing rapeseed oil. Livest. Sci. 2017, 204, 16–24. [Google Scholar] [CrossRef]
- Ávila, S.C.; Kozloski, G.V.; Orlandi, T.; Mezzomo, M.P.; Stefanello, S. Impact of a tannin extract on digestibility, ruminal fermentation and duodenal flow of amino acids in steers fed maize silage and concentrate containing soybean meal or canola meal as protein source. J. Agric. Sci. 2015, 153, 943–953. [Google Scholar] [CrossRef]
- Baah, J.; Ivan, M.; Hristov, A.N.; Koenig, K.M.; Rode, L.M.; McAllister, T.A. Effects of potential dietary antiprotozoal supplements on rumen fermentation and digestibility in heifers. Anim. Feed Sci. Technol. 2007, 137, 126–137. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Viti, C.; Minieri, S.; Pallara, G.; Roscini, V.; Rapaccini, S.; Marinucci, M.T.; Lupi, P.; Conte, G.; et al. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. J. Dairy Sci. 2015, 98, 1145–1156. [Google Scholar] [CrossRef]
- Buccioni, A.; Serra, A.; Minieri, S.; Mannelli, F.; Cappucci, A.; Benvenuti, D.; Rapaccini, S.; Conte, G.; Mele, M. Milk production, composition, and milk fatty acid profile from grazing sheep fed diets supplemented with chestnut tannin extract and extruded linseed. Small Rumin. Res. 2015, 130, 200–207. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Henke, A.; Molkentin, J.; Knappstein, K.; Susenbeth, A.; Dickhoefer, U. Relationship between milk odd and branched-chain fatty acids and urinary purine derivatives in dairy cows supplemented with quebracho tannins-A study to test milk fatty acids as predictors of rumen microbial protein synthesis. Anim. Feed Sci. Technol. 2016, 214, 22–33. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Colombini, S.; Colombari, G.; Crovetto, G.M.; Galassi, G.; Rapetti, L. Tannin treated lucerne silage in dairy cow feeding. Ital. J. Anim. Sci. 2009, 8, 289–291. [Google Scholar] [CrossRef]
- Dallastra, L.J.H.; Alves, T.P.; Dal-Pizzol, J.; Fonseca, B.L.; Camera, M.; Raupp, G.T.; Nunes Ribeiro-Filho, H.M. Tannin extract of Acacia mearnsii for lactating ewes. Semin. Agrar. 2018, 39, 2741–2748. [Google Scholar] [CrossRef]
- Deaville, E.R.; Givens, D.I.; Mueller-Harvey, I. Chestnut and mimosa tannin silages: Effects in sheep differ for apparent digestibility, nitrogen utilisation and losses. Anim. Feed Sci. Technol. 2010, 157, 129–138. [Google Scholar] [CrossRef]
- Denninger, T.M.; Schwarm, A.; Birkinshaw, A.; Terranova, M.; Dohme-Meier, F.; Münger, A.; Eggerschwiler, L.; Bapst, B.; Wegmann, S.; Clauss, M.; et al. Immediate effect of Acacia mearnsii tannins on methane emissions and milk fatty acid profiles of dairy cows. Anim. Feed Sci. Technol. 2020, 261, 114388. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Moreira, O.C.; Pereira, M.S.; Bessa, R.J.B. The use of a tannin crude extract from Cistus ladanifer L. to protect soya-bean protein from degradation in the rumen. Animal 2007, 1, 645–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, ruminal fermentation and microbial nitrogen supply in sheep fed soybean meal treated with Cistus ladanifer L. tannins. Small Rumin. Res. 2014, 119, 57–64. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Paulos, K.; Francisco, A.; Belo, A.T.; Jerónimo, E.; Almeida, J.; Bessa, R.J.B.; Santos-Silva, J. Effect of soybean meal treatment with Cistus ladanifer condensed tannins in growth performance, carcass and meat quality of lambs. Livest. Sci. 2020, 236, 104021. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Ahnert, S.; Susenbeth, A. Effects of quebracho tannin extract on rumen fermentation and yield and composition of microbial mass in heifers. J. Anim. Sci. 2016, 94, 1561–1575. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef]
- Duval, B.D.; Aguerre, M.; Wattiaux, M.; Vadas, P.A.; Powell, J.M. Potential for Reducing On-Farm Greenhouse Gas and Ammonia Emissions from Dairy Cows with Prolonged Dietary Tannin Additions. Water. Air. Soil Pollut. 2016, 227, 329. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Fernández, M.; Mantecón, A.R. Digestive utilisation of quebracho-treated soya bean meals in sheep. J. Agric. Sci. 2000, 134, 101–108. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Griffiths, W.M.; Clark, C.E.F.; Clark, D.A.; Waghorn, G.C. Supplementing lactating dairy cows fed high-quality pasture with black wattle (Acacia mearnsii) tannin. Animal 2013, 7, 1789–1795. [Google Scholar] [CrossRef]
- Henke, A.; Dickhoefer, U.; Westreicher-Kristen, E.; Knappstein, K.; Molkentin, J.; Hasler, M.; Susenbeth, A. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch. Anim. Nutr. 2016, 71, 37–53. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Cantalapiedra-Hijar, G.; Beckers, Y.; Froidmont, E. Effects of hydrolysable tannin-treated grass silage on milk yield and composition, nitrogen partitioning and nitrogen isotopic discrimination in lactating dairy cows. Animal 2020, 14, 771–779. [Google Scholar] [CrossRef]
- Hervas, G.; Frutos, P.; Serrano, E.; Mantecon, A.R.; Giraldez, F.J. Effect of tannic acid on rumen degradation and intestinal digestion of treated soya bean meals in sheep. J. Agric. Sci. 2000, 135, 305–310. [Google Scholar] [CrossRef][Green Version]
- Hervás, G.; Frutos, P.; Ramos, G.; Giráldez, F.J.; Mantecón, A.R. Intraruminal administration of two doses of quebracho tannins to sheep: Effect on rumen degradation and total tract digestibility, faecal recovery and toxicity. J. Anim. Feed Sci. 2004, 13, 111–120. [Google Scholar] [CrossRef][Green Version]
- Jolazadeh, A.R.; Dehghan-banadaky, M.; Rezayazdi, K. Effects of soybean meal treated with tannins extracted from pistachio hulls on performance, ruminal fermentation, blood metabolites and nutrient digestion of Holstein bulls. Anim. Feed Sci. Technol. 2015, 203, 33–40. [Google Scholar] [CrossRef]
- Perna Junior, F.; Vásquez, D.C.Z.; Gardinal, R.; Meyer, P.M.; Berndt, A.; Friguetto, R.T.S.; de Abreu Demarchi, J.J.A.; Rodrigues, P.H.M. Short-term use of monensin and tannins as feed additives on digestibility and methanogenesis in cattle. Rev. Bras. Zootec. 2020, 49. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distillers grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef]
- Komolong, M.K.; Barber, D.G.; McNeill, D.M. Post-ruminal protein supply and N retention of weaner sheep fed on a basal diet of lucerne hay (Medicago sativa) with increasing levels of quebracho tannins. Anim. Feed Sci. Technol. 2001, 92, 59–72. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Härter, C.J.; Hentz, F.; de ávila, S.C.; Orlandi, T.; Stefanello, C.M. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin. Res. 2012, 106, 125–130. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura, L.M.L.; Nascimento, J.C.S.; Rodrigues, R.T.S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.Á.; et al. Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Anim. Feed Sci. Technol. 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhou, D.W.; Li, K. Effects of chestnut tannins on performance and antioxidative status of transition dairy cows. J. Dairy Sci. 2013, 96, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.F.; Moyano, F.J.; Díaz, M.; Barroso, F.G.; Alarcón, F.J. Ruminal degradation of tannin-treated legume meals. J. Sci. Food Agric. 2004, 84, 1979–1987. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Monnerat, J.P.I.S.; Duarte, M.S.; Silva, L.H.P.; Moura, L.S. Influence of condensed tannin on intake, digestibility, and efficiency of protein utilisation in beef steers fed high concentrate diet. Livest. Sci. 2011, 141, 1–11. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Barbosa, M.M.; da Silva Martins, T.; Paulino, M.F.; Alves, K.S.; Gomes, D.I.; dos Santos Monnerat, J.P.I. Performance and carcass characteristics of young cattle fed with soybean meal treated with tannins. Anim. Sci. J. 2016, 87, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarpour, A.; Naserian, A.A.; Pourmollae, F.; Ghaffari, M.H. Effect of treating alfalfa silage with pistachio by-products extract on Saanen dairy goats performance and microbial nitrogen synthesis. J. Anim. Physiol. Anim. Nutr. 2016, 100, 758–767. [Google Scholar] [CrossRef]
- Nasehi, M.; Torbatinejad, N.M.; Rezaie, M.; Ghoorchi, T. The effect of green tea waste extract on ruminal degradability and intestinal digestibility of barley grain. Turk. J. Vet. Anim. Sci. 2018, 42, 624–632. [Google Scholar] [CrossRef]
- Norris, A.B.; Crossland, W.L.; Tedeschi, L.O.; Foster, J.L.; Muir, J.P.; Pinchak, W.E.; Fonseca, M.A. Inclusion of quebracho tannin extract in a high-roughage cattle diet alters digestibility, nitrogen balance, and energy partitioning. J. Anim. Sci. 2020, 98, skaa047. [Google Scholar] [CrossRef]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
- Orlandi, T.; Stefanello, S.; Mezzomo, M.P.; Pozo, C.A.; Kozloski, G.V. Impact of a tannin extract on digestibility and net flux of metabolites across splanchnic tissues of sheep. Anim. Feed Sci. Technol. 2020, 261, 114384. [Google Scholar] [CrossRef]
- Orlandi, T.; Pozo, C.A.; Mezzomo, M.P.; Kozloski, G.V. Acacia mearnsii tannin extract as a feed additive: Impact on feed intake, digestibility and nitrogen excretion by sheep fed a tropical grass-based diet. Cienc. Rural 2020, 50, 1–6. [Google Scholar] [CrossRef]
- Perna Junior, F.; Cassiano, E.C.O.; Martins, M.F.; Romero, L.A.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of tannins-rich extract from Acacia mearnsii or monensin as feed additives on ruminal fermentation efficiency in cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- Poncet, C.; Rémond, D. Rumen digestion and intestinal nutrient flows in sheep consuming pea seeds: The effect of extrusion or chestnut tannin addition. Anim. Res. 2002, 51, 201–216. [Google Scholar] [CrossRef]
- Salami, S.A.; Valenti, B.; Bella, M.; O’Grady, M.N.; Luciano, G.; Kerry, J.P.; Jones, E.; Priolo, A.; Newbold, C.J. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol. Ecol. 2018, 94, 1–13. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hovell, F.D.D.B. Quebracho tannins with or without Browse Plus (a commercial preparation of polyethylene glycol) in sheep diets: Effect on digestibility of nutrients in vivo and degradation of grass hay in sacco and in vitro. Anim. Feed Sci. Technol. 1997, 69, 67–78. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hovell, F.D.D.B.; McKay, I. Assessment of the nutritive value of Calliandra calothyrsus: In sacco degradation and in vitro gas production in the presence of Quebracho tannins with or without Browse Plus. Anim. Feed Sci. Technol. 1997, 69, 219–232. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hvelplund, T.; Weisbjerg, M.R. The use of tannins as silage additives: Effects on silage composition and mobile bag disappearance of dry matter and protein. Anim. Feed Sci. Technol. 1999, 82, 243–259. [Google Scholar] [CrossRef]
- Shakeri, P.; Reiasi, A.; Tahmasbi, R. The effect of pistachio by-product extracts treatment in protecting soybean meal and canola meal protein from rumen microbial degradation. J. Sci. Food Agric. 2020, 100, 5222–5229. [Google Scholar] [CrossRef]
- Sharifi, A.; Chaji, M. Effects of processed recycled poultry bedding with tannins extracted from pomegranate peel on the nutrient digestibility and growth performance of lambs. S. Afr. J. Anim. Sci. 2019, 49, 291–300. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Wettstein, H.R.; Machmüller, A. Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Arch. Anim. Nutr. Tierernahr. 2002, 56, 379–392. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Sutter, F.; Machmüller, A.; Wettstein, H.R. Performance, body nitrogen conversion and nitrogen emission from manure of dairy cows fed diets supplemented with different plant extracts. J. Anim. Feed Sci. 2004, 13, 73–91. [Google Scholar] [CrossRef]
- Szczechowiak, J.; Szumacher-Strabel, M.; El-Sherbiny, M.; Pers-Kamczyc, E.; Pawlak, P.; Cieslak, A. Rumen fermentation, methane concentration and fatty acid proportion in the rumen and milk of dairy cows fed condensed tannin and/or fish-soybean oils blend. Anim. Feed Sci. Technol. 2016, 216, 93–107. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Bichi, E.; Belenguer, Á.; Frutos, P. Tannins as feed additives to modulate ruminal biohydrogenation: Effects on animal performance, milk fatty acid composition and ruminal fermentation in dairy ewes fed a diet containing sunflower oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Belenguer, A.; Bichi, E.; Frutos, P. Effect of the inclusion of quebracho tannins in a diet rich in linoleic acid on milk fatty acid composition in dairy ewes. J. Dairy Sci. 2013, 96, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, S.A.; Cibils, A.F.; Estell, R.E.; Soto-Navarro, S.A.; Chen, L.; Hallford, D.M. Effects of adding protein, condensed tannins, and polyethylene glycol to diets of sheep and goats fed one-seed juniper and low quality roughage. Small Rumin. Res. 2013, 112, 56–68. [Google Scholar] [CrossRef]
- Wischer, G.; Greiling, A.M.; Boguhn, J.; Steingass, H.; Schollenberger, M.; Hartung, K.; Rodehutscord, M. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Animal 2014, 8, 938–948. [Google Scholar] [CrossRef]
- Zimmer, N.; Cordesse, R. Digestibility and ruminal digestion of non-nitrogenous compounds in adult sheep and goats: Effects of chestnut tannins. Anim. Feed Sci. Technol. 1996, 61, 259–273. [Google Scholar] [CrossRef]
- Orskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- St-Pierre, N.R. Invited review. Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef]
- Sauvant, D.; Schmidely, P.; Daudin, J.J.; St-Pierre, N.R. Metaanalyses of experimental data in animal nutrition. Animal 2008, 2, 1203–1214. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Estrada-Angulo, A.; Castro-Pérez, B.I.; Urías-Estrada, J.D.; Ríos-Rincón, F.G.; Arteaga-Wences, Y.J.; Barreras, A.; López-Soto, M.A.; Plascencia, A.; Zinn, R.A. Influence of protein level on growth performance, dietary energetics and carcass characteristics of Pelibuey × Katahdin lambs finished with isocaloric diets. Small. Rum. Res. 2018, 160, 59–64. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Bryszak, M.; Gao, M.; Kolodziejski, P.; Stochmal, A.; Slusarczyk, S.; Patra, A.K.; Cieslak, A. Coleus amboinicus (Lour.) leaves as a modulator of ruminal methanogenesis and biohydrogenation in vitro. J. Anim. Sci. 2018, 96, 4868–4881. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Cieślak, A.; Yanza, Y.R.; Szumacher-Strabel, M.; Varadyova, Z.; Stafiniak, M.; Wojnicz, D.; Matkowski, A. Phytochemical Profile and Antioxidant Activities of Coleus amboinicus Lour. Cultivated in Indonesia and Poland. Molecules 2021, 26, 2915. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Stochmal, A.; Pecio, L.; Oleszek, W.; Pers-Kamczyc, E.; Szczechowiak, J.; Nowak, A.; Szumacher-Strabel, M. Rumen antimethanogenic effect of Saponaria officinalis L. phytochemicals in vitro. J. Agric. Sci. 2014, 152, 981–983. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545–546, 556–558. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, 2–16. [Google Scholar] [CrossRef]
- Niderkorn, V.; Jayanegara, A. Opportunities Offered by Plant Bioactive Compounds to Improve Silage Quality, Animal Health and Product Quality for Sustainable Ruminant Production: A Review. Agronomy 2021, 11, 86. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Woodward, S.L.; Waghorn, G.C.; Laboyrie, P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 160–164. [Google Scholar]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. De Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Ren, H.; Su, X.; Bai, H.; Yang, Y.; Wang, H.; Dan, Z.; Lu, J.; Wu, S.; Cai, C.; Cao, Y.; et al. Specific enrichment of microbes and increased ruminal propionate production: The potential mechanism underlying the high energy efficiency of Holstein heifers fed steam-flaked corn. AMB Express 2019, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Bae, H.D.; Jones, G.A.; Cheng, K.J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Irawan, A.; Noviandi, C.T.; Widyobroto, B.P.; Astuti, A.; Ates, S. Effect of Leucaena leucocephala and corn oil on ruminal fermentation, methane production and fatty acid profile: An in vitro study. Anim. Prod. Sci. 2021, 61, 459–469. [Google Scholar] [CrossRef]
- O’donovan, L.; Brooker, J.D. Effect of hydrolysable and condensed tannins on growth, morphology and metabolism of Streptococcus gallolyticus (S. caprinus) and Streptococcus bovis. Microbiology 2001, 147, 1025–1033. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, Á.R.; Álvarez Del Pino, M.C. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2004, 109, 65–78. [Google Scholar] [CrossRef]
- Hill, G.D. Plant antinutritional factors. In Encyclopedia of Food Science & Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4578–4587. [Google Scholar] [CrossRef]
- Schofield, P.; Mbagua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).