An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section II
Abstract
:Simple Summary
Abstract
1. Introduction
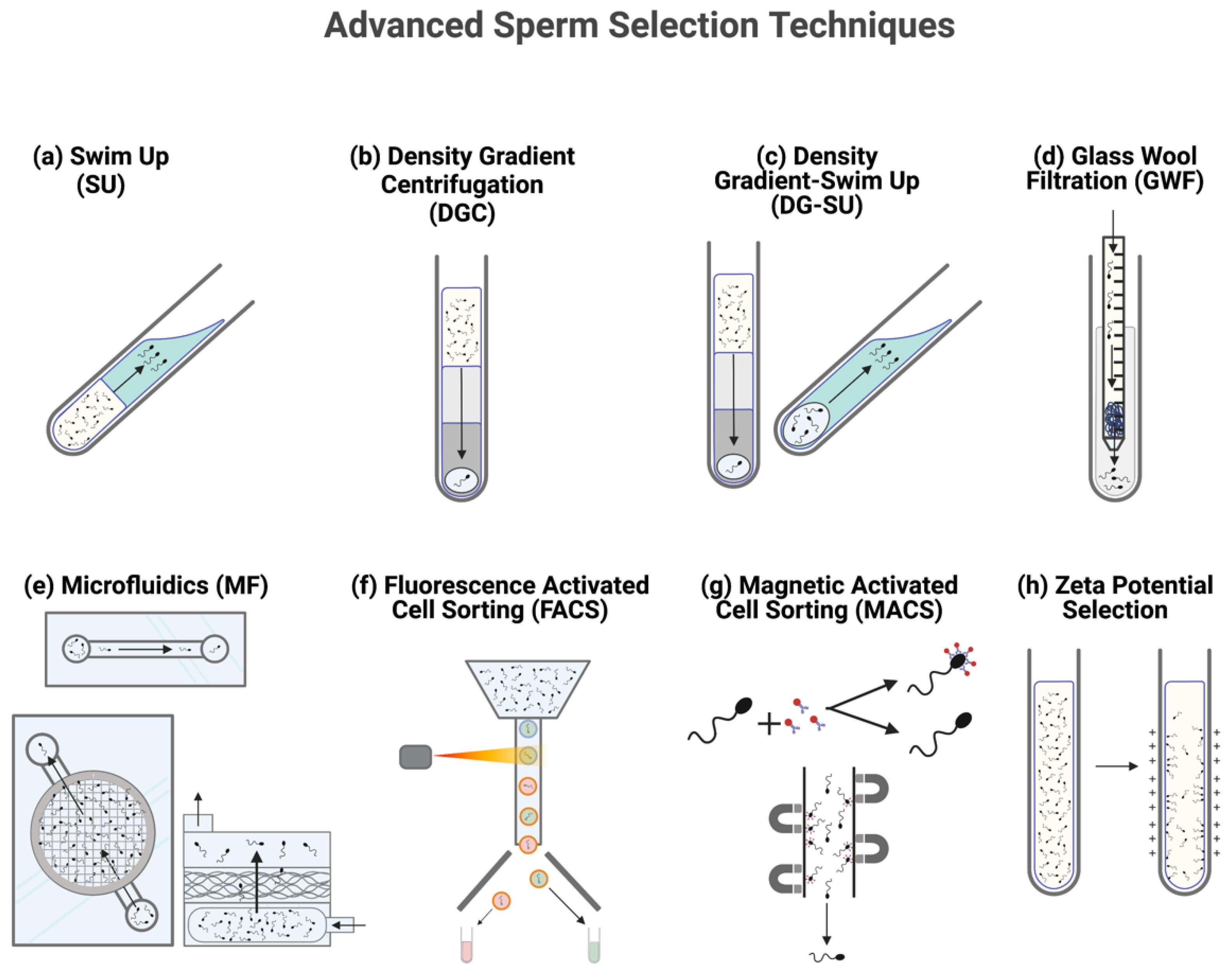
2. Sorting Semen: Significance and Method
2.1. Density Gradient Centrifugation
2.2. Swim Up
2.3. Combination Density Gradient-Swim Up
2.4. Glass Wool Filtration
2.5. Fluorescent Activated Cell Sorting
2.6. Microfluidic Sorting
2.7. Magnetic Activated Cell Sorting
2.8. Zeta Potential Selection
3. Sexing Semen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hinrichs, K. Assisted reproductive techniques in mares. Reprod. Domest. Anim. 2018, 53, 4–13. [Google Scholar] [CrossRef]
- Choi, Y.H.; Hinrichs, K. Vitrification of in vitro-produced and in vivo-recovered equine blastocysts in a clinical program. Theriogenology 2017, 87, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.L.; McCue, P.M.; Vanderwall, D. The current status of equine embryo transfer. Theriogenology 1999, 51, 91–104. [Google Scholar] [CrossRef]
- Galli, C.; Crotti, G.; Turini, P.; Duchi, R.; Mari, G.; Zavaglia, G. Frozen-thawed embryos produced by ovum pick up and ICSI are capable to establish pregnancies in the horse. Theriogenology 2002, 58, 713–715. [Google Scholar]
- Galli, C.; Duchi, R.; Colleoni, S.; Lagutina, I.; Lazzari, G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: From the research laboratory to clinical practice. Theriogenology 2014, 81, 138–151. [Google Scholar] [CrossRef]
- Choi, Y.H.; Love, C.C.; Love, L.B.; Varner, D.D.; Brinsko, S.; Hinrichs, K. Developmental competence in vivo and in vitro of in vitro-matured equine oocytes fertilized by intracytoplasmic sperm injection with fresh or frozen-thawed spermatozoa. Reproduction 2002, 123, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Colleoni, S.; Duchi, R.; Lagutina, I.; Lazzari, G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim. Reprod. Sci. 2007, 98, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Morikawa, M.I.; Bello, M.B.; von Meyeren, M.; Eusebio Centeno, J.; Dufourq, P.; Martinez, M.M.; Llorente, J. Setting up equine embryo gender determination by preimplantation genetic diagnosis in a commercial embryo transfer program. Theriogenology 2014, 81, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Gustafson-Seabury, A.; Velez, I.C.; Hartman, D.L.; Bliss, S.; Riera, F.L.; Roldán, J.E.; Chowdhary, B.; Hinrichs, K. Viability of equine embryos after puncture of the capsule and biopsy for preimplantation genetic diagnosis. Reproduction 2010, 140, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Squires, E.L. Breakthroughs in equine embryo cryopreservation. Vet. Clin. N. Am. Equine. Pr. 2016, 32, 415–424. [Google Scholar] [CrossRef]
- Hinrichs, K. The equine oocyte: Factors affecting meiotic and developmental competence. Mol. Reprod. Dev. 2010, 77, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Dini, P.; Bogado Pascottini, O.; Ducheyne, K.; Hostens, M.; Daels, P. Holding equine oocytes in a commercial embryo-holding medium: New perspective on holding temperature and maturation time. Theriogenology 2016, 86, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.E.; Goodwin, P.; Klein, N.A.; Soules, M.R. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod. 1996, 11, 2217–2222. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.; Stout, T.A.E.; Cristarella, S.; Quartuccio, M.; Kops, G.J.P.L.; De Ruijter-Villani, M. Compromised MPS1 activity induces multipolar spindle formation in oocytes from aged mares: Establishing the horse as a natural animal model to study age-induced oocyte meiotic spindle instability. Front. Cell. Dev. Biol. 2021, 9, 657366. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Hall, H.; Hunt, P. The origin of human aneuploidy: Where we have been, where we are going. Hum. Mol. Genet. 2007, 16, R203–R208. [Google Scholar] [CrossRef]
- Ge, Z.J.; Schatten, H.; Zhang, C.L.; Sun, Q.Y. Oocyte ageing and epigenetics. Reproduction 2015, 149, R103–R114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, W.A.; Desjardins, M.; Xu, K.P.; Bousquet, D. Chromosome analysis of horse oocytes cultured in vitro. Genet. Sel. Evol. 1990, 22, 151. [Google Scholar] [CrossRef]
- Hinrichs, K.; Schmidt, A.L. Meiotic competence in horse oocytes: Interactions among chromatin configuration, follicle size, cumulus morphology, and season. Biol. Reprod. 2000, 62, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Ortiz, I.; Dorado, J.; Diaz-Jimenez, M.; Consuegra, C.; Demyda-Peyras, S.; Hidalgo, M. The effect of different vitrification and staining protocols on the visibility of the nuclear maturation stage of equine oocytes. J. Equine. Vet. Sci. 2020, 90, 103021. [Google Scholar] [CrossRef]
- Alm, H.; Hinrichs, K. Effect of cycloheximide on nuclear maturation of horse oocytes and its relation to initial cumulus morphology. J. Reprod. Fertil. 1996, 107, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.; Hinrichs, K.; Leese, H.J.; McG Argo, C.; Brison, D.R.; Sturmey, R. Energy metabolism of the equine cumulus oocyte complex during in vitro maturation. Sci. Rep. 2020, 10, 3493. [Google Scholar] [CrossRef] [Green Version]
- Botigelli, R.C.; Razza, E.M.; Pioltine, E.M.; Nogueira, M.F.G. New approaches regarding the in vitro maturation of oocytes: Manipulating cyclic nucleotides and their partners in crime. JBRA Assist. Reprod. 2017, 21, 35–44. [Google Scholar] [CrossRef]
- Holt, W.V.; Van Look, K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; De Schauwer, C.; Nelis, H.; Hoogewijs, M.; Van Soom, A. Why doesn’t conventional IVF work in horses. Reproduction 2016, 152, R233–R245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tournaye, H. Male factor infertility and ART. Asian J. Androl. 2012, 14, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinrichs, K. Update on equine ICSI and cloning. Theriogenology 2005, 64, 535–541. [Google Scholar] [CrossRef]
- Loomis, P.R.; Graham, J.K. Commercial semen freezing: Individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim. Reprod. Sci. 2008, 105, 119–128. [Google Scholar] [CrossRef]
- Loomis, P.R. The equine frozen semen industry. Anim. Reprod. Sci. 2001, 68, 191–200. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakagata, N. Improvement in the long-term stability of freeze-dried mouse spermatozoa by adding of a chelating agent. Cryobiology 2006, 53, 279–282. [Google Scholar] [CrossRef]
- Watanabe, H.; Asano, T.; Abe, Y.; Fukui, Y.; Suzuki, H. Pronuclear formation of freeze-dried canine spermatozoa microinjected into mouse oocytes. J. Assist. Reprod. Genet. 2009, 26, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Olaciregui, M.; Gil, L. Freeze-dried spermatozoa: A future tool? Reprod. Domest. Anim. 2017, 52, 248–254. [Google Scholar] [CrossRef]
- Meyers, S.A.; Li, M.W.; Enders, A.C.; Overstreet, J.W. Rhesus macaque blastocysts resulting from intracytoplasmic sperm injection of vacuum-dried spermatozoa. J. Med. Primatol. 2009, 38, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, H.; Yanagimachi, R.; Kamiguchi, Y. Mouse and human spermatozoa can be freeze-dried without damaging their chromosomes. Hum. Reprod. 2008, 23, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Varner, D.D.; Love, C.C.; Hartman, D.L.; Hinrichs, K. Production of live foals via intracytoplasmic injection of lyophilized sperm and sperm extract in the horse. Reproduction 2011, 142, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L. Sperm selection in natural conception: What can we learn from Mother Nature to improve assisted reproduction outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cerezales, S.; Ramos-Ibeas, P.; Acuna, O.S.; Aviles, M.; Coy, P.; Rizos, D.; Gutierrez-Adan, A. The oviduct: From sperm selection to the epigenetic landscape of the embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, A.; Ng, S.C. Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool. 1999, 284, 696–704. [Google Scholar] [CrossRef]
- Henkel, R.R.; Schill, W.-B. Sperm Preparation for ART. Reprod. Biol. Endocrinol. 2003, 1, 108. [Google Scholar] [CrossRef] [Green Version]
- Oseguera-Lopez, I.; Ruiz-Diaz, S.; Ramos-Ibeas, P.; Perez-Cerezales, S. novel techniques of sperm selection for improving IVF and ICSI outcomes. Front. Cell. Dev. Biol. 2019, 7, 298. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Waberski, D.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007, 134, 3–17. [Google Scholar] [CrossRef]
- Foote, R.H. Fertility estimation: A review of past experience and future prospects. Anim. Reprod. Sci. 2003, 75, 119–139. [Google Scholar] [CrossRef]
- Love, C.C. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology 2011, 76, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Katigbak, R.D.; Turchini, G.M.; de Graaf, S.P.; Kong, L.; Dumee, L.F. Review on sperm sorting technologies and sperm properties toward new separation methods via the interface of biochemistry and material science. Adv. Biosyst. 2019, 3, e1900079. [Google Scholar] [CrossRef]
- Brahmkshtri, B.P.; Edwin, M.J.; John, M.C.; Nainar, A.M.; Krishnan, A.R. Relative efficacy of conventional sperm parameters and sperm penetration bioassay to assess bull fertility in vitro. Anim. Reprod. Sci. 1999, 54, 159–168. [Google Scholar] [CrossRef]
- Said, T.M.; Land, J.A. Effects of advanced selection methods on sperm quality and ART outcome: A systematic review. Hum. Reprod. Update 2011, 17, 719–733. [Google Scholar] [CrossRef] [Green Version]
- Lannou, D.L.; Blanchard, Y. Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J. Reprod. Fert. 1988, 84, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paasch, U.; Grunewald, S.; Glander, H.J. Sperm selection in assisted reproductive techniques. Soc. Reprod. Fertil. Suppl. 2007, 65, 515–525. [Google Scholar] [PubMed]
- Oumaima, A.; Tesnim, A.; Zohra, H.; Amira, S.; Ines, Z.; Sana, C.; Intissar, G.; Lobna, E.; Ali, J.; Meriem, M. Investigation on the origin of sperm morphological defects: Oxidative attacks, chromatin immaturity, and DNA fragmentation. Env. Sci. Pollut. Res. Int. 2018, 25, 13775–13786. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.G.; Jurisicova, A.; Casper, R.F. Detection of deoxyribonucleic acid fragmentation in human sperm: Correlation with fertilization in vitro. Biol. Reprod. 1997, 56, 602–607. [Google Scholar] [CrossRef]
- Jones, H.W., Jr.; Oehninger, S.; Bocca, S.; Stadtmauer, L.; Mayer, J. Reproductive efficiency of human oocytes fertilized in vitro. Facts Views Vis. Obgyn. 2010, 2, 169–171. [Google Scholar]
- Wang, Q.; Sun, Q.Y. Evaluation of oocyte quality: Morphological, cellular and molecular predictors. Reprod. Fertil. Dev. 2007, 19, 1–12. [Google Scholar] [CrossRef]
- Ruvolo, G.; Fattouh, R.R.; Bosco, L.; Brucculeri, A.M.; Cittadini, E. New molecular markers for the evaluation of gamete quality. J. Assist. Reprod. Genet. 2013, 30, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trounson, A.; Anderiesz, C.; Jones, G. Maturation of human oocytes in vitro and their developmental competence. Reproduction 2001, 121, 51–75. [Google Scholar] [CrossRef]
- Yildiz, C.; Ottaviani, P.; Law, N.; Ayearst, R.; Liu, L.; McKerlie, C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction 2007, 133, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.T.; Keefer, C.L.; Downey, B.R. Activation of bovine oocytes following intracytoplasmic sperm injection (ICSI). Theriogenology 2000, 53, 1273–1284. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.E.; Sánchez, R.; Felmer, R. Effect of anisomycin, a protein synthesis inhibitor, on the in vitro developmental potential, ploidy and embryo quality of bovine ICSI embryos. Zygote 2016, 24, 724–732. [Google Scholar] [CrossRef]
- Hara, H.; Abdalla, H.; Morita, H.; Kuwayama, M.; Hirabayashi, M.; Hochi, S. Procedure for bovine ICSI, not sperm freeze-drying, impairs the function of the microtubule-organizing center. J. Reprod. Dev. 2011, 57, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Malcuit, C.; Maserati, M.; Takahashi, Y.; Page, R.; Fissore, R.A. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca2+]i responses and oocyte activation. Reprod. Fertil. Dev. 2005, 18, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Grupen, C.G. The evolution of porcine embryo in vitro production. Theriogenology 2014, 81, 24–37. [Google Scholar] [CrossRef]
- Wang, W.-H.; Abeydeera, L.R.; Prather, R.S.; Day, B.N. Morphologic comparison of ovulated and in vitro–matured porcine oocytes, with particular reference to polyspermy after in vitro fertilization. Mol. Reprod. Dev. 1998, 49, 308–316. [Google Scholar] [CrossRef]
- Palmer, E.; Bézard, J.; Magistrini, M.; Duchamp, G. In vitro fertilization in the horse. A retrospective study. J. Reprod. Fertil. Suppl. 1991, 44, 375–384. [Google Scholar] [PubMed]
- Foss, R.; Ortis, H.; Hinrichs, K. Effect of potential oocyte transport protocols on blastocyst rates after intracytoplasmic sperm injection in the horse. Equine. Vet. J. Suppl. 2013, 45, 39–43. [Google Scholar] [CrossRef]
- Boulet, S.L.; Mehta, A.; Kissin, D.M.; Warner, L.; Kawwass, J.F.; Jamieson, D.J. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 2015, 313, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, E.T.; Lewis, S.E.; McNally, J.A.; Thompson, W. In vitro fertilization and pregnancy rates: The influence of sperm motility and morphology on IVF outcome. Fertil. Steril. 1998, 70, 305–314. [Google Scholar] [CrossRef]
- Kruger, T.F.; Acosta, A.A.; Simmons, K.F.; Swanson, R.J.; Matta, J.F.; Oehninger, S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil. Steril. 1988, 49, 112–117. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Y.; Qu, X.; Wang, P.; Zhao, L.; Gao, M.; Shi, H.; Jin, X. Decreased sperm motility retarded ICSI fertilization rate in severe oligozoospermia but good-quality embryo transfer had achieved the prospective clinical outcomes. PLoS ONE 2016, 11, e0163524. [Google Scholar] [CrossRef]
- Verheyen, G.; Tournaye, H.; Staessen, C.; De Vos, A.; Vandervorst, M.; Van Steirteghem, A. Controlled comparison of conventional in-vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Hum. Reprod. 1999, 14, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.Z.; Hossepian de Lima, V.F.; Levenhagen, M.A.; Santos, R.M.; Assumpção, T.I.; Jacomini, J.O.; Andrade, A.F.; Arruda, R.P.; Beletti, M.E. Transmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoa. J. Vet. Sci. 2011, 12, 267–272. [Google Scholar] [CrossRef]
- Arias, M.E.; Andara, K.; Briones, E.; Felmer, R. Bovine sperm separation by Swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reprod. Biol. 2017, 17, 126–132. [Google Scholar] [CrossRef]
- Marzano, G.; Chiriacò, M.S.; Primiceri, E.; Dell’Aquila, M.E.; Ramalho-Santos, J.; Zara, V.; Ferramosca, A.; Maruccio, G. Sperm selection in assisted reproduction: A review of established methods and cutting-edge possibilities. Biotechnol. Adv. 2020, 40, 107498. [Google Scholar] [CrossRef]
- Bolton, V.N.; Braude, P.R. Preparation of human spermatozoa for in vitro fertilization by isopycnic centrifugation on self-generating density gradients. Arch. Androl. 1984, 13, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pousette, A.; Akerlöf, E.; Rosenborg, L.; Fredricsson, B. Increase in progressive motility and improved morphology of human spermatozoa following their migration through Percoll gradients. Int. J. 1986, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Martin, H.; Miranda, E.P.; Cocuzza, M.S.; Monteleone, P.A.A. Density gradient centrifugation and swim-up for ICSI: Useful, unsafe, or just unsuitable? J. Assist. Reprod. Genet. 2019, 36, 2421–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.; Nagy, Z.P.; Van de Velde, H.; Joris, H.; Bocken, G.; Van Steirteghem, A. Percoll gradient centrifugation can be omitted in sperm preparation for intracytoplasmic sperm injection. Hum. Reprod. 1997, 12, 1980–1984. [Google Scholar] [CrossRef] [Green Version]
- Arcidiacono, A.; Walt, H.; Campana, A.; Balerna, M. The use of Percoll gradients for the preparation of subpopulations of human spermatozoa. Int. J. 1983, 6, 433–445. [Google Scholar] [CrossRef]
- Strehler, E.; Baccetti, B.; Sterzik, K.; Capitani, S.; Collodel, G.; De Santo, M.; Gambera, L.; Piomboni, P. Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa (Notulae seminologicae 13). Hum. Reprod. 1998, 13, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Fishel, S.; Jackson, P.; Webster, J.; Faratian, B. Endotoxins in culture medium for human in vitro fertilization. Fertil. Steril. 1988, 49, 108–111. [Google Scholar] [CrossRef]
- Söderlund, B.; Lundin, K. The use of silane-coated silica particles for density gradient centrifugation in in-vitro fertilization. Hum. Reprod. 2000, 15, 857–860. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, M.; Blanchard, T.; Love, C.; Brinsko, S.; Varner, D. Use of a silane-coated silica particle solution to enhance the quality of ejaculated semen in stallions. Theriogenology 2002, 58, 317–320. [Google Scholar]
- Varner, D.; Love, C.; Brinsko, S.; Blanchard, T.; Hartman, D.; Bliss, S.; Carroll, B.; Eslick, M. Semen Processing for the Subfertile Stallion. J. Equine Vet. Sci. 2008, 28, 677–685. [Google Scholar] [CrossRef]
- Edmond, A.J.; Teague, S.; Brinsko, S.; Comerford, K.; Waite, J.A.; Mancill, S.; Love, C.; Varner, D. Effect of density gradient centrifugation on quality and recovery of equine spermatozoa. Anim. Reprod. Sci. 2008, 107, 318. [Google Scholar] [CrossRef]
- Stoll, A.; Stewart, B.; Brum, A.; Liu, I.; Ball, B. Evaluation of cryopreserved-thawed stallion sperm before and after density gradient centrifugation with silane-coated silica particles (EquiPure®). Theriogenology 2008, 70, 590–591. [Google Scholar] [CrossRef]
- Jayaraman, V.; Upadhya, D.; Narayan, P.K.; Adiga, S.K. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J. Assist. Reprod. Genet. 2012, 29, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, I.; Ghorbani, M.; Heshmati, S. Comparison of the DNA Fragmentation and the Sperm Parameters after Processing by the Density Gradient and the Swim up Methods. J. Clin. Diagn. Res. 2012, 6, 1451–1453. [Google Scholar] [CrossRef]
- Zheng, W.-W.; Song, G.; Wang, Q.-L.; Liu, S.-W.; Zhu, X.-L.; Deng, S.-M.; Zhong, A.; Tan, Y.-M.; Tan, Y. Sperm DNA damage has a negative effect on early embryonic development following in vitro fertilization. Asian J. Androl. 2018, 20, 75–79. [Google Scholar] [CrossRef]
- Simon, L.; Lutton, D.; McManus, J.; Lewis, S.E. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil. Steril. 2011, 95, 652–657. [Google Scholar] [CrossRef]
- Oguz, Y.; Guler, I.; Erdem, A.; Mutlu, M.F.; Gumuslu, S.; Oktem, M.; Bozkurt, N.; Erdem, M. The effect of swim-up and gradient sperm preparation techniques on deoxyribonucleic acid (DNA) fragmentation in subfertile patients. J. Assist. Reprod. Genet. 2018, 35, 1083–1089. [Google Scholar] [CrossRef]
- Domínguez, L.A.; Burgos, M.H.; Fornés, M.W. Morphometrical comparison of human spermatozoa obtained from semen and swim-up methodology. Andrologia 1999, 31, 23–26. [Google Scholar] [CrossRef]
- Sieme, H.; Martinsson, G.; Rauterberg, H.; Walter, K.; Aurich, C.; Petzoldt, R.; Klug, E. Application of techniques for sperm selection in fresh and frozen-thawed stallion semen. Reprod. Domest. Anim. 2003, 38, 134–140. [Google Scholar] [CrossRef]
- Yamanaka, M.; Tomita, K.; Hashimoto, S.; Matsumoto, H.; Satoh, M.; Kato, H.; Hosoi, Y.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. J. Reprod. Dev. 2016, 62, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Ng, F.L.; Liu, D.Y.; Baker, H.W. Comparison of Percoll, mini-Percoll and swim-up methods for sperm preparation from abnormal semen samples. Hum. Reprod. 1992, 7, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, A.G.; Junior, N.B.; Arruda, N.S.; Corbellini, A.O.; Chiappetta, C.M.; Pavão, D.L.; D’Angelo, M.; Canal, C.W.; Rodrigues, J.L. Evaluation of the effectiveness of semen processing techniques to remove bovine viral diarrhea virus from experimentally contaminated semen samples. J. Virol. Meth. 2013, 187, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, J.M.; Geraghty, R.M. Effective removal of equine arteritis virus from stallion semen. Equine. Vet. J. 2006, 38, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Nani, J.M.; Jeyendran, R.S. Sperm processing: Glass wool column filtration. Arch. Androl. 2001, 47, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bergh, M.; Revelard, P.; Bertrand, E.; Biramane, J.; Vanin, A.S.; Englert, Y. Glass wool column filtration, an advantageous way of preparing semen samples for intracytoplasmic sperm injection: An auto-controlled randomized study. Hum. Reprod. 1997, 12, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Henkel, R.R.; Franken, D.R.; Lombard, C.J.; Schill, W.-B. Selective capacity of glass-wool filtration for the separation of human spermatozoa with condensed chromatin: A possible therapeutic modality for male-factor cases? J. Assist. Reprod. Genet. 1994, 11, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, K.; Erasmus, E.L.; Kruger, T.F.; Menkveld, R.; Lombard, C.J. Glass wool filter preparation of cryopreserved spermatozoa. Andrologia 1994, 26, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-L.; Kim, S.-H.; Ji, D.-B.; Kim, Y.-J. A comparative study of Sephadex, glass wool and Percoll separation techniques on sperm quality and IVF results for cryopreserved bovine semen. J. Vet. Sci. 2009, 10, 249–255. [Google Scholar] [CrossRef]
- Nie, G.J.; Johnson, K.E.; Wenzel, J.G. Pregnancy outcome in mares following insemination deep in the uterine horn with low numbers of sperm selected by glass wool/Sephadex filtration, Percoll separation or absolute number. Anim. Reprod. Sci. 2003, 79, 103–109. [Google Scholar] [CrossRef]
- Sherman, J.K.; Paulson, J.D.; Liu, K.C. Effect of glass wool filtration on ultrastructure of human spermatozoa. Fertil. Steril. 1981, 36, 643–647. [Google Scholar] [CrossRef]
- Rhemrev, J.; Jeyendran, R.S.; Vermeiden, J.P.W.; Zaneveld, L.J.D. Human sperm selection by glass wool filtration and two-layer, discontinuous Percoll gradient centrifugation*†. Fertil. Steril. 1989, 51, 685–690. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodríguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 67–78. [Google Scholar] [CrossRef]
- Hoogendijk, C.F.; Kruger, T.F.; Bouic, P.J.; Henkel, R.R. A novel approach for the selection of human sperm using annexin V-binding and flow cytometry. Fertil. Steril. 2009, 91, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Chaveiro, A.; Santos, P.; da Silva, F.M. Assessment of sperm apoptosis in cryopreserved bull semen after swim-up treatment: A flow cytometric study. Reprod. Domest. Anim. 2007, 42, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Funaro, M.G.; Kim, H.H.; Mazel, S.; Bolyakov, A.; Goldstein, M.; Schlegel, P.N.; Paduch, D.A. A novel sorting technology allows for highly efficient selection of sperm without chromatin damage. Syst. Biol. Reprod. Med. 2013, 59, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, A.P.; Amaral, A.; Baptista, M.; Tavares, R.; Caballero Campo, P.; Caballero Peregrín, P.; Freitas, A.; Paiva, A.; Almeida-Santos, T.; Ramalho-Santos, J. Not all sperm are equal: Functional mitochondria characterize a subpopulation of human sperm with better fertilization potential. PLoS ONE 2011, 6, e18112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.A. Sexing mammalian sperm for production of offspring: The state-of-the-art. Anim. Reprod. Sci. 2000, 60-61, 93–107. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Baumber, J.; Meyers, S.A. Changes in membrane lipid order with capacitation in rhesus macaque (Macaca mulatta) spermatozoa. J. Androl. 2006, 27, 578–587. [Google Scholar] [CrossRef] [Green Version]
- De Geyter, C.; Gobrecht-Keller, U.; Ahler, A.; Fischer, M. Removal of DNA-fragmented spermatozoa using flow cytometry and sorting does not improve the outcome of intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2019, 36, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Evans, K.M.; Seidel, G.E. Sex-sorting sperm using flow cytometry/cell sorting. Methods Mol. Biol. 2013, 927, 279–295. [Google Scholar] [CrossRef]
- Buchanan, B.R.; Seidel, G.E.; McCue, P.M.; Schenk, J.L.; Herickhoff, L.A.; Squires, E.L. Insemination of mares with low numbers of either unsexed or sexed spermatozoa. Theriogenology 2000, 53, 1333–1344. [Google Scholar] [CrossRef]
- Rath, D.; Barcikowski, S.; de Graaf, S.; Garrels, W.; Grossfeld, R.; Klein, S.; Knabe, W.; Knorr, C.; Kues, W.; Meyer, H.; et al. Sex selection of sperm in farm animals: Status report and developmental prospects. Reproduction 2013, 145, R15–R30. [Google Scholar] [CrossRef] [Green Version]
- Aurich, C.; Schneider, J. Sex determination in horses—Current status and future perspectives. Anim. Reprod. Sci. 2014, 146, 34–41. [Google Scholar] [CrossRef]
- Samper, J.C.; Morris, L.; Plough, T.A. The use of sex-sorted stallion semen in embryo transfer programs. J. Equine. Vet. Sci. 2012, 32, 387–389. [Google Scholar] [CrossRef]
- Balao da Silva, C.M.; Ortega-Ferrusola, C.; Morrell, J.M.; Rodriguez Martínez, H.; Peña, F.J. Flow cytometric chromosomal sex sorting of stallion spermatozoa induces oxidative stress on mitochondria and genomic DNA. Reprod. Domest. Anim. 2016, 51, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L. Flow cytometric sexing of mammalian sperm. Theriogenology 2006, 65, 943–957. [Google Scholar] [CrossRef]
- Carvalho, J.O.; Sartori, R.; Machado, G.M.; Mourão, G.B.; Dode, M.A.N. Quality assessment of bovine cryopreserved sperm after sexing by flow cytometry and their use in in vitro embryo production. Theriogenology 2010, 74, 1521–1530. [Google Scholar] [CrossRef]
- Rath, D.; Johnson, L. Application and commercialization of flow cytometrically sex-sorted semen. Reprod. Domest. Anim. 2008, 43, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Zaferani, M.; Cheong, S.H.; Abbaspourrad, A. Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. Proc. Natl. Acad. Sci. USA 2018, 115, 8272–8277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.J.; Maeng, J.H.; Hwang, S.Y.; Ahn, Y. Design, fabrication, and testing of a microfluidic device for thermotaxis and chemotaxis assays of sperm. SLAS Technol. 2018, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.P.B.; Endo, K.; Ogata, K.; Yamanaka, K.; Egashira, J.; Katafuchi, N.; Yamanouchi, T.; Matsuda, H.; Goto, Y.; Sakatani, M.; et al. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc. Natl. Acad. Sci. USA 2018, 115, e3087–e3096. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.S.; Schuster, T.G.; Zhu, X.; Chang, D.; Smith, G.D.; Takayama, S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef] [Green Version]
- Asghar, W.; Velasco, V.; Kingsley, J.L.; Shoukat, M.S.; Shafiee, H.; Anchan, R.M.; Mutter, G.L.; Tüzel, E.; Demirci, U. Selection of functional human sperm with higher DNA integrity and fewer reactive oxygen species. Adv. Health Mater. 2014, 3, 1671–1679. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Carnevale, E.M. Use of microfluidics to sort stallion sperm for intracytoplasmic sperm injection. Anim. Reprod. Sci. 2019, 202, 1–9. [Google Scholar] [CrossRef]
- Matsuura, K.; Uozumi, T.; Furuichi, T.; Sugimoto, I.; Kodama, M.; Funahashi, H. A microfluidic device to reduce treatment time of intracytoplasmic sperm injection. Fertil. Steril. 2013, 99, 400–407. [Google Scholar] [CrossRef]
- Sano, H.; Matsuura, K.; Naruse, K.; Funahashi, H. Application of a microfluidic sperm sorter to the in-vitro fertilization of porcine oocytes reduced the incidence of polyspermic penetration. Theriogenology 2010, 74, 863–870. [Google Scholar] [CrossRef]
- Li, J.; Zhu, S.; He, X.; Sun, R.; He, Q.; Gan, Y.; Liu, S.; Funahashi, H.; Li, Y. Application of a microfluidic sperm sorter to in vitro production of dairy cattle sex-sorted embryos. Theriogenology 2016, 85, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Vollmer, M.; Eamer, L.; San Gabriel, M.C.; Zeidan, K.; Zini, A.; Sinton, D. Rapid selection of sperm with high DNA integrity. Lab. Chip. 2014, 14, 1142–1150. [Google Scholar] [CrossRef]
- El-sherry, T.M.; Abdel-Ghani, M.A.; Abou-Khalil, N.S.; Elsayed, M.; Abdelgawad, M. Effect of pH on rheotaxis of bull sperm using microfluidics. Reprod. Domest. Anim. 2017, 52, 781–790. [Google Scholar] [CrossRef]
- Eamer, L.; Nosrati, R.; Vollmer, M.; Zini, A.; Sinton, D. Microfluidic assessment of swimming media for motility-based sperm selection. Biomicrofluidics 2015, 9, 044113. [Google Scholar] [CrossRef] [Green Version]
- Hamacher, T.; Berendsen, J.T.W.; Kruit, S.A.; Broekhuijse, M.L.W.J.; Segerink, L.I. Effect of microfluidic processing on the viability of boar and bull spermatozoa. Biomicrofluidics 2020, 14, 044111. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Agarwal, A.; Zborowski, M.; Grunewald, S.; Glander, H.J.; Paasch, U. Utility of magnetic cell separation as a molecular sperm preparation technique. J. Androl. 2008, 29, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, S.; Paasch, U.; Glander, H.-J. Enrichment of non-apoptotic human spermatozoa after cryopreservation by immunogenetic cell sorting. Cell Tissue Bank. 2001, 2, 127–133. [Google Scholar] [CrossRef]
- Falchi, L.; Khalil, W.A.; Hassan, M.; Marei, W.F.A. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2018, 6, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Huang, S.-H.; Juang, R.-S. Biochemical and biomedical applications of multifunctional magnetic nanoparticles: A review. J. Nanoparticle Res. 2011, 13, 4411. [Google Scholar] [CrossRef]
- Yousef, M.S.; Lopez-Lorente, A.I.; Diaz-Jimenez, M.; Consuegra, C.; Dorado, J.; Pereira, B.; Ortiz, I.; Cardenas, S.; Hidalgo, M. Nano-depletion of acrosome-damaged donkey sperm by using lectin peanut agglutinin (PNA)-magnetic nanoparticles. Theriogenology 2020, 151, 103–111. [Google Scholar] [CrossRef]
- Faezah, S.S.; Zuraina, F.M.; Farah, J.H.; Khairul, O.; Hilwani, N.I.; Iswadi, M.I.; Fang, C.N.; Zawawi, I.; Abas, O.M.; Fatimah, S.I. The effects of magnetic separation on cryopreserved bovine spermatozoa motility, viability and cryo-capacitation status. Zygote 2014, 22, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, S.; Paasch, U.; Said, T.M.; Rasch, M.; Agarwal, A.; Glander, H.-J. Magnetic-activated cell sorting before cryopreservation preserves mitochondrial integrity in human spermatozoa. Cell Tissue Bank. 2006, 7, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Grunewald, S.; Fitzl, G.; Glander, H.J. Deterioration of plasma membrane is associated with activated caspases in human spermatozoa. J. Androl. 2003, 24, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Grunewald, S.; Paasch, U.; Glander, H.J.; Baumann, T.; Kriegel, C.; Li, L.; Agarwal, A. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod. Biomed. Online 2005, 10, 740–746. [Google Scholar] [CrossRef]
- Feugang, J.M.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.; Willard, S.T.; Ryan, P.L. Lectin-functionalized magnetic iron oxide nanoparticles for reproductive improvement. JFIV Reprod. Med. Genet. 2015, 3, 17–19. [Google Scholar]
- Domínguez, E.; Moreno-Irusta, A.; Castex, H.R.; Bragulat, A.F.; Ugaz, C.; Clemente, H.; Giojalas, L.; Losinno, L. Sperm sexing mediated by magnetic nanoparticles in donkeys, a preliminary in vitro study. J. Equine. Vet. Sci. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Giuliani, V.; Pandolfi, C.; Santucci, R.; Pelliccione, F.; Macerola, B.; Focarelli, R.; Rosati, F.; Della Giovampaola, C.; Francavilla, F.; Francavilla, S. Expression of gp20, a human sperm antigen of epididymal origin, is reduced in spermatozoa from subfertile men. Mol. Reprod. Dev. 2004, 69, 235–240. [Google Scholar] [CrossRef]
- Ionov, M.; Gontarek, W.; Bryszewska, M. Zeta potential technique for analyzing semen quality. MethodsX 2020, 7, 100895. [Google Scholar] [CrossRef]
- Ainsworth, C.; Nixon, B.; Aitken, R.J. Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum. Reprod. 2005, 20, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Calzada, L.; Salazar, E.L.; Pedron, N. Presence and chemical composition of glycoproteic layer on human spermatozoa. Arch. Androl. 1994, 33, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.A.; Okuno, M.; Mohri, H. Zeta potential of human X- and Y-bearing sperm. Int. J. Androl. 1991, 14, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.J.; Jacobson, J.D.; Corselli, J.U.; Patton, W.C. A simple zeta method for sperm selection based on membrane charge. Fertil. Steril. 2006, 85, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahi-Kouhestani, M.; Razavi, S.; Tavalaee, M.; Deemeh, M.R.; Mardani, M.; Moshtaghian, J.; Nasr-Esfahani, M.H. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum. Reprod. 2009, 24, 2409–2416. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.H.; Nasr-Esfahani, M.H.; Deemeh, M.R.; Shayesteh, M.; Tavalaee, M. Evaluation of zeta and HA-binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia 2010, 42, 13–19. [Google Scholar] [CrossRef]
- Zarei-Kheirabadi, M.; Shayegan Nia, E.; Tavalaee, M.; Deemeh, M.R.; Arabi, M.; Forouzanfar, M.; Javadi, G.R.; Nasr-Esfahani, M.H. Evaluation of ubiquitin and annexin V in sperm population selected based on density gradient centrifugation and zeta potential (DGC-Zeta). J. Assist. Reprod. Genet. 2012, 29, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Nasr Esfahani, M.H.; Deemeh, M.R.; Tavalaee, M.; Sekhavati, M.H.; Gourabi, H. Zeta Sperm selection improves pregnancy rate and alters sex ratio in male factor infertility patients: A double-blind, randomized clinical trial. Int. J. Fertil. Steril. 2016, 10, 253–260. [Google Scholar] [CrossRef]
- Lansford, N.; Freeman, D.W.; Topliff, D.R.; Walker, O.L. Hedonic pricing of race-bred yearling quarter horses produced by quarter horse sires and dams. J. Agribus. 1998, 16, 90443. [Google Scholar]
- Panarace, M.; Pellegrini, R.O.; Basualdo, M.O.; Belé, M.; Ursino, D.A.; Cisterna, R.; Desimone, G.; Rodríguez, E.; Medina, M.J. First field results on the use of stallion sex-sorted semen in a large-scale embryo transfer program. Theriogenology 2014, 81, 520–525. [Google Scholar] [CrossRef]
- Chezum, B.; Wimmer, B. Roses or lemons: Adverse selection in the market for thoroughbred yearlings. Rev. Econ. Stat. 1997, 79, 521–526. [Google Scholar] [CrossRef]
- Hendriksen, P.J.; Welch, G.R.; Grootegoed, J.A.; Van der Lende, T.; Johnson, L.A. Comparison of detergent-solubilized membrane and soluble proteins from flow cytometrically sorted X- and Y-chromosome bearing porcine spermatozoa by high resolution 2-D electrophoresis. Mol. Reprod. Dev. 1996, 45, 342–350. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C.G. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.C.; Schenk, J.L.; Graham, J.K.; Bruemmer, J.E.; Squires, E.L. Hysteroscopic insemination of low numbers of flow sorted fresh and frozen/thawed stallion spermatozoa. Equine. Vet. J. 2002, 34, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Clulow, J.R.; Buss, H.; Sieme, H.; Rodger, J.A.; Cawdell-Smith, A.J.; Evans, G.; Rath, D.; Morris, L.H.; Maxwell, W.M. Field fertility of sex-sorted and non-sorted frozen-thawed stallion spermatozoa. Anim. Reprod. Sci. 2008, 108, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Spinaci, M.; Volpe, S.; Bernardini, C.; de Ambrogi, M.; Tamanini, C.; Seren, E.; Galeati, G. Sperm sorting procedure induces a redistribution of Hsp70 but not Hsp60 and Hsp90 in boar spermatozoa. J. Androl. 2006, 27, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Suh, T.K.; Schenk, J.L.; Seidel, G.E., Jr. High pressure flow cytometric sorting damages sperm. Theriogenology 2005, 64, 1035–1048. [Google Scholar] [CrossRef]
- Gibb, Z.; Morris, L.H.; Maxwell, W.M.; Grupen, C.G. Use of a defined diluent increases the sex-sorting efficiency of stallion sperm. Theriogenology 2011, 75, 610–619. [Google Scholar] [CrossRef]
- Ainsworth, C.J.; Nixon, B.; Aitken, R.J. The electrophoretic separation of spermatozoa: An analysis of genotype, surface carbohydrate composition and potential for capacitation. Int. J. Androl. 2011, 34, e422–e434. [Google Scholar] [CrossRef]
- Engelmann, U.; Krassnigg, F.; Schatz, H.; Schill, W.-B. Separation of human X and Y spermatozoa by free-flow electrophoresis. Gamete. Res. 1988, 19, 151–160. [Google Scholar] [CrossRef]
- Kaneko, S.; Oshio, S.; Kobayashi, T.; Iizuka, R.; Mohri, H. Human X- and Y-bearing sperm differ in cell surface sialic acid content. Biochim. Biophys. Acta. Biomembr. 1984, 124, 950–955. [Google Scholar] [CrossRef]
- Blottner, S.; Bostedt, H.; Mewes, K.; Pitra, C. Enrichment of bovine X and Y spermatozoa by free-flow electrophoresis. J. Vet. Med. A 1994, 41, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.A.; Okuno, M.; Odagiri, H.; Mohri, T.; Mohri, H. Separation of X- and Y-chromosome-bearing murine sperm by free-flow electrophoresis: Evaluation of separation using PCR. Zool. Sci. 1992, 9, 601–606. [Google Scholar]
- Della Giovampaola, C.; Flori, F.; Sabatini, L.; Incerti, L.; La Sala, G.B.; Rosati, F.; Focarelli, R. Surface of human sperm bears three differently charged CD52 forms, two of which remain stably bound to sperm after capacitation. Mol. Reprod. Dev. 2001, 60, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Fukushima, M.; Harayama, H. Premature capacitation of frozen-thawed spermatozoa from subfertile Japanese black cattle. J. Reprod. Dev. 2007, 53, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, L.; Murphy, K.; Aston, K.I.; Emery, B.R.; Hotaling, J.M.; Carrell, D.T. Optimization of microelectrophoresis to select highly negatively charged sperm. J. Assist. Reprod. Genet. 2016, 33, 679–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magdanz, V.; Gebauer, J.; Sharan, P.; Eltoukhy, S.; Voigt, D.; Simmchen, J. Sperm–particle interactions and their prospects for charge mapping. Adv. Biosyst. 2019, 3, 1900061. [Google Scholar] [CrossRef]
- Pommer, A.C.; Linfor, J.J.; Meyers, S.A. Capacitation and acrosomal exocytosis are enhanced by incubation of stallion spermatozoa in a commercial semen extender. Theriogenology 2002, 57, 1493–1501. [Google Scholar] [CrossRef]
| Method | Selects Based On: | Benefits: | Detriments |
|---|---|---|---|
| Density Gradient Centrifugation | Morphology Cell density Motility | Enriches for: Morphology; Motility; Viability; Mitochondrial membrane integrity; Pregnancy rates; DNA integrity Removes cell and protein debris | Toxicity of Percoll® Centrifugation causes DNA damage |
| Swim Up | Progressive motility | Enriches for: Motility; Morphology; DNA integrity Removes cell and protein debris | Low recovery rate |
| Density Gradient-Swim Up | Morphology Cell density Motility | Enriches for: Motility; Morphology; DNA integrity Removes pathogens | Toxicity of Percoll® Centrifugation causes DNA damage |
| Glass Wool Filtration | In vivo fertility Motility | Enriches for: Motility; Morphology; Chromatin Condensation; Membrane, Integrity; Cleavage rates; Blastocyst rates; Pregnancy rates High Recovery Rate | Possible damage to sperm head and acrosome ultrastructure Glass wool contamination of final product |
| Fluorescent Activated Cell Sorting | Variable physiological markers (membrane integrity, apoptotic markers, mitochondrial membrane potential, sex chromosome) | Enriches for: Pregnancy rates; Live birth rates Removes unwanted cells | May cause oxidative and DNA damage Mechanical Stress Time consuming High operating expenses Inability to select for numerous factors |
| Microfluidic Sorting | Motility Rheotactic, Chemotactic, and Thermotactic behavior | Enriches for: Motility; Viability; DNA integrity Reduced ROS generaton Removes extracellular debris Can be combined with IVF | May impose stress May reduce viability in some species |
| Magnetic Activated Cell Sorting | Variable physiological markers (removes sperm with apoptotic markers, acrosome reacted sperm) | Enriches for: Motility; Viability; Morphology; Survival, motility, and mitochondrial integrity after cryopreservation; Sperm binding rates; Fertilization rates; Embryo development rates | May possess cytotoxic effects |
| Zeta Potential Selection | Greater net negative membrane charges | Enriches for: Maturity; Morphology; DNA integrity; Protamine content; Fertilization rates; Pregnancy rates | Not shown to increase motility and viability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsolini, M.F.; Meyers, S.A.; Dini, P. An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section II. Animals 2021, 11, 3319. https://doi.org/10.3390/ani11113319
Orsolini MF, Meyers SA, Dini P. An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section II. Animals. 2021; 11(11):3319. https://doi.org/10.3390/ani11113319
Chicago/Turabian StyleOrsolini, Morgan F., Stuart A. Meyers, and Pouya Dini. 2021. "An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section II" Animals 11, no. 11: 3319. https://doi.org/10.3390/ani11113319
APA StyleOrsolini, M. F., Meyers, S. A., & Dini, P. (2021). An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section II. Animals, 11(11), 3319. https://doi.org/10.3390/ani11113319







