Efficacy and Safety of Dexmedetomidine Premedication in Balanced Anesthesia: A Systematic Review and Meta-Analysis in Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
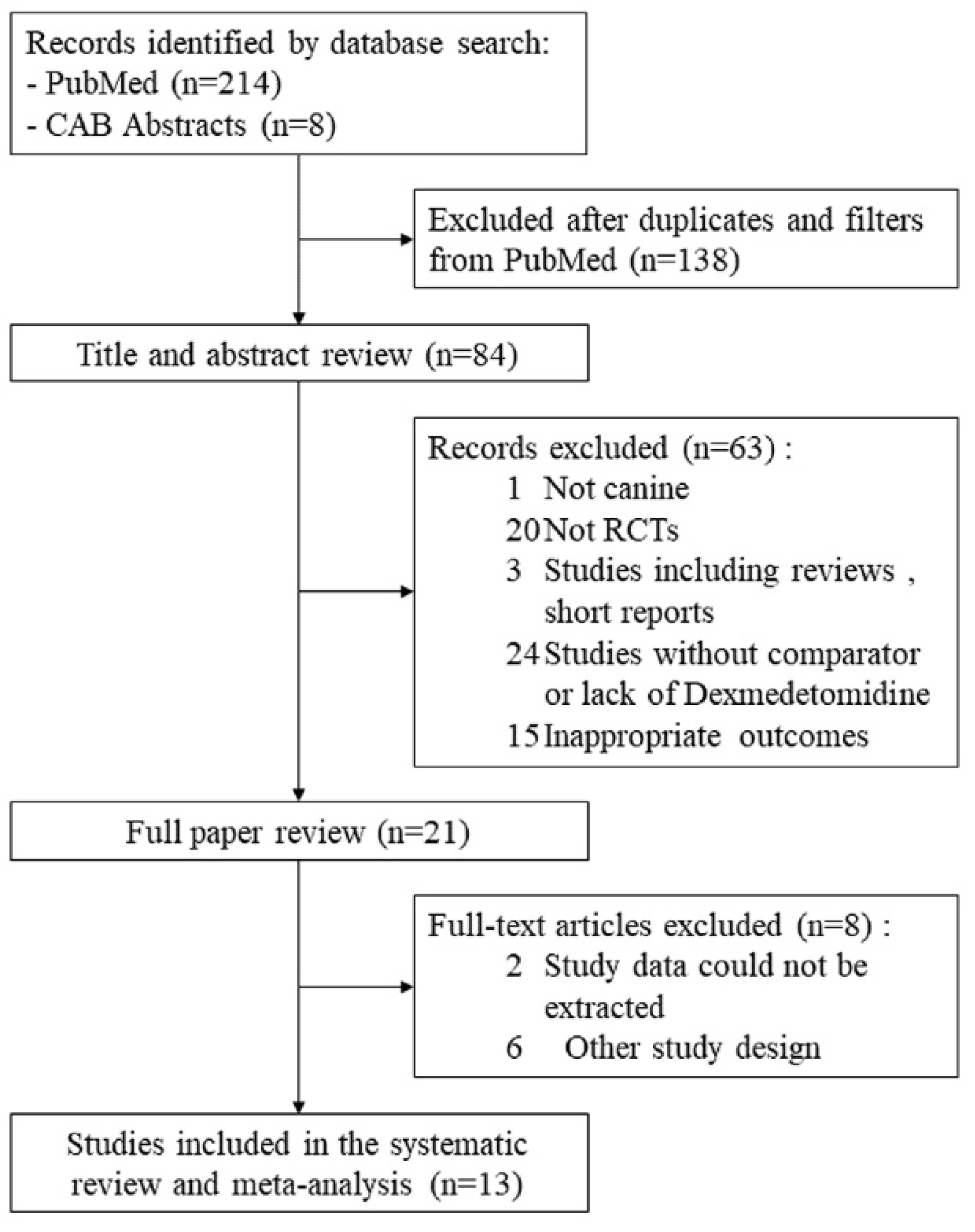
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Outcomes
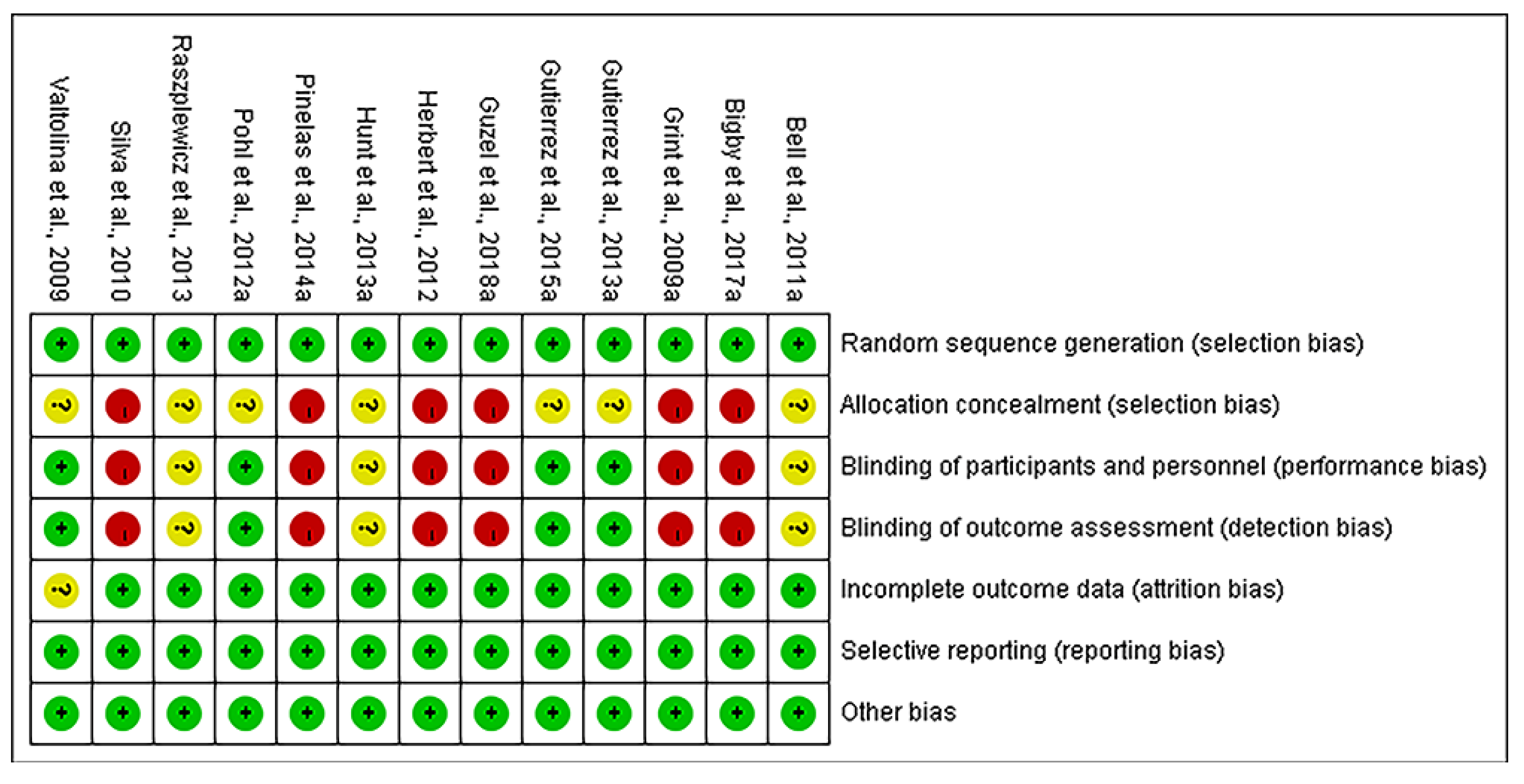
2.3. Quality Assessment
2.3.1. Assessment of Risk of Bias
2.3.2. Certainty of Evidence
2.4. Data Extraction and Analysis
3. Results
3.1. Characteristics of Studies
3.2. Primary Outcomes
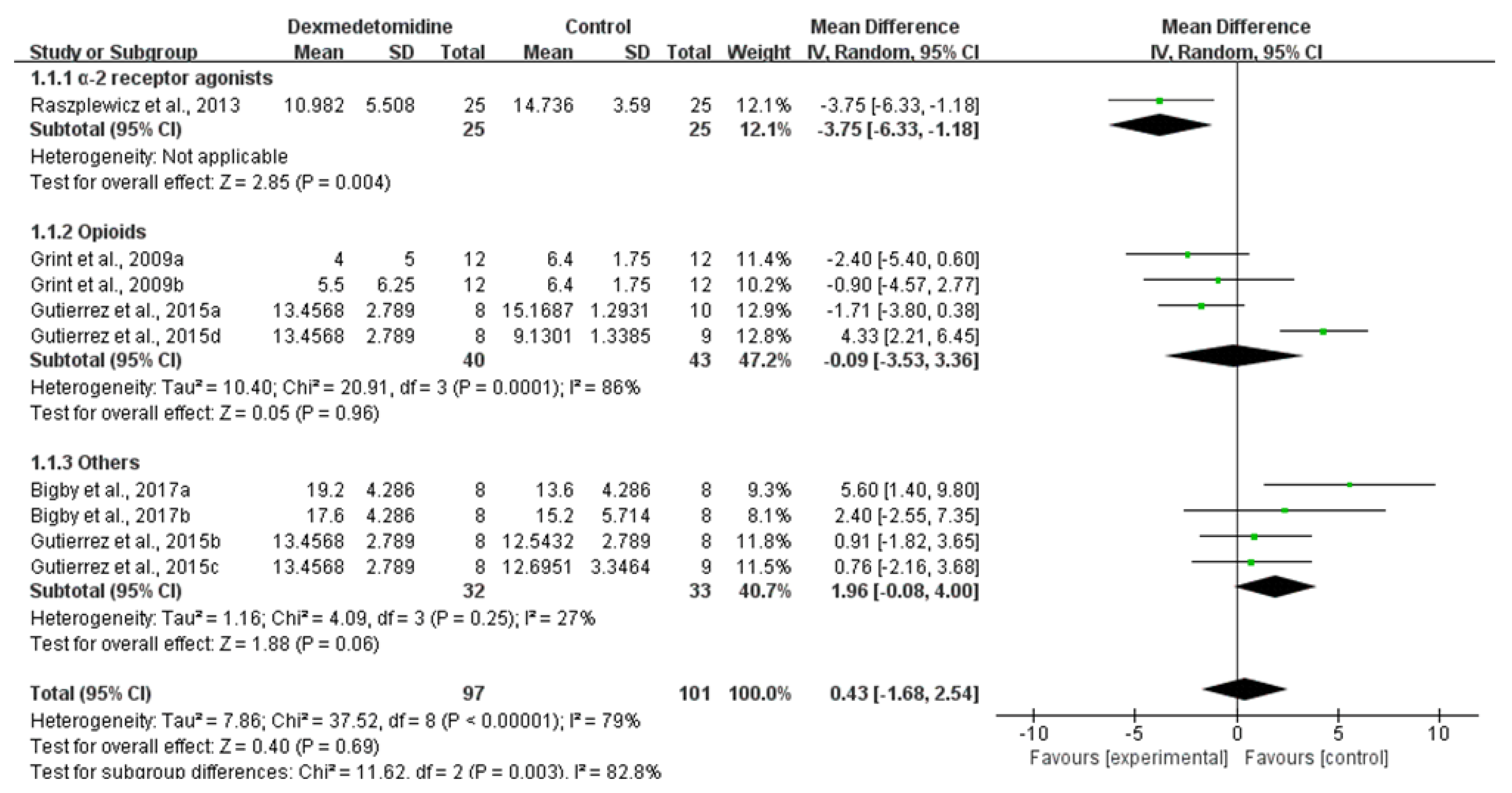
3.2.1. Sedation Score
3.2.2. Pain Score
3.3. Secondary Outcomes
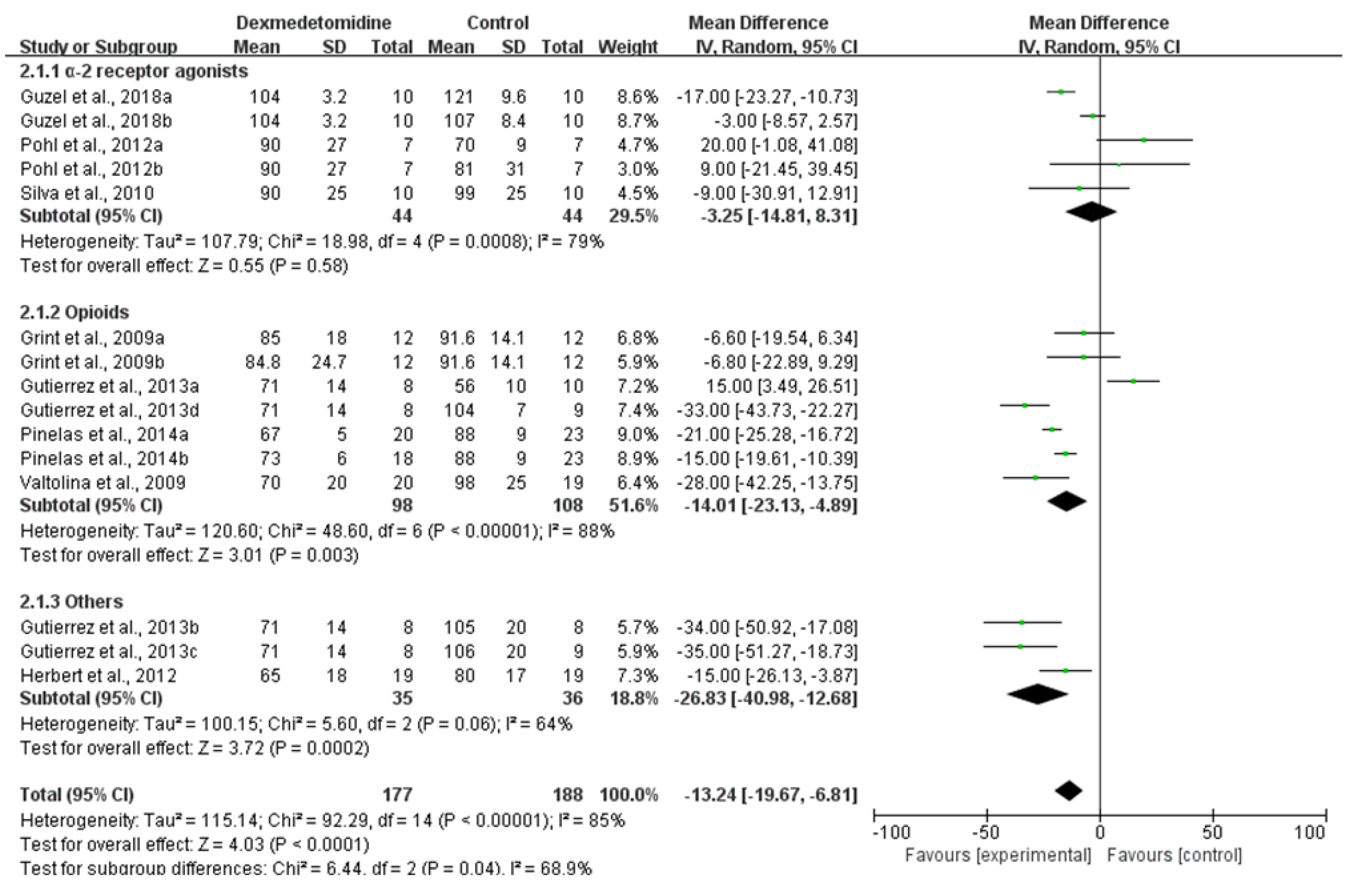
3.3.1. HR
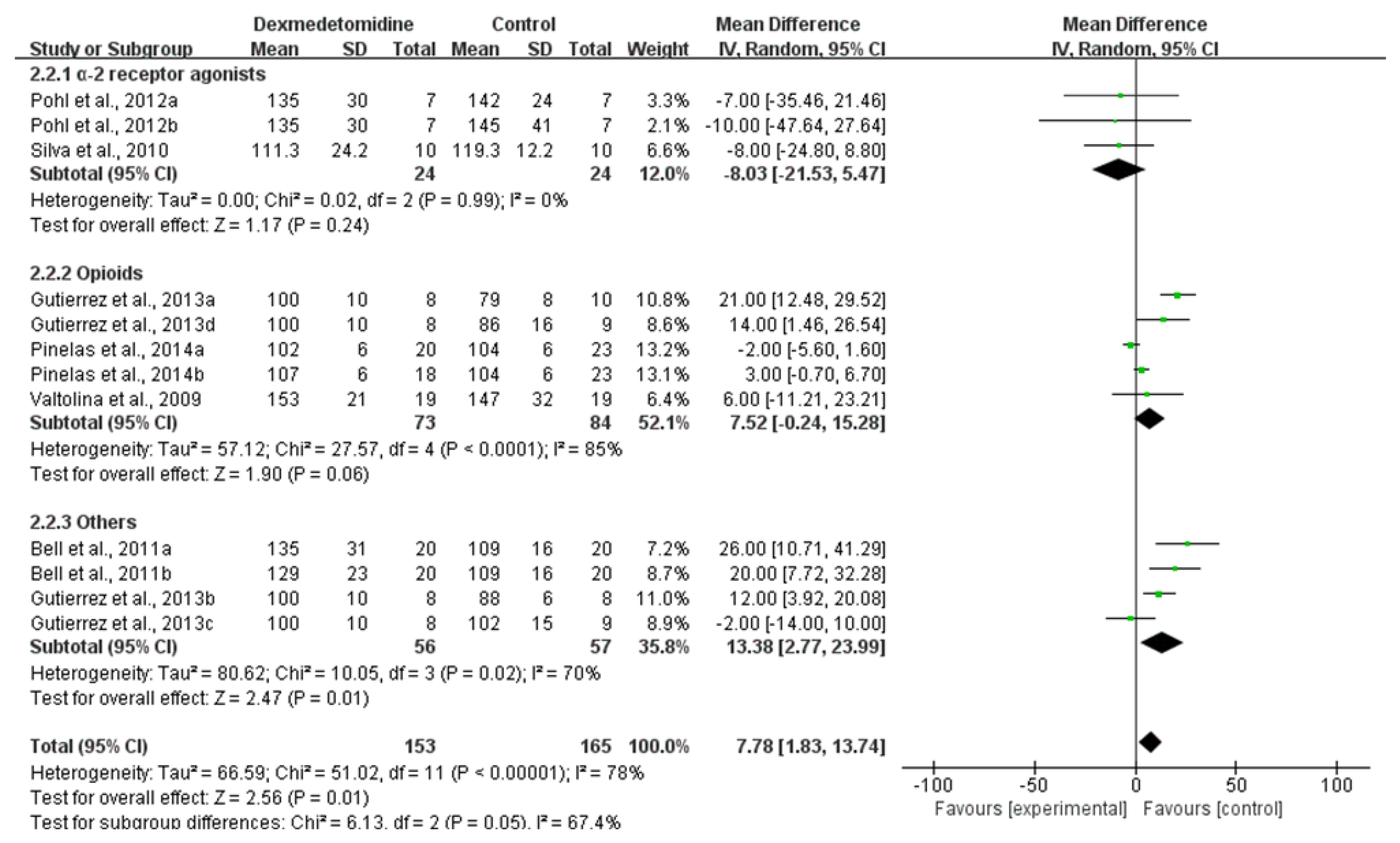
3.3.2. SAP
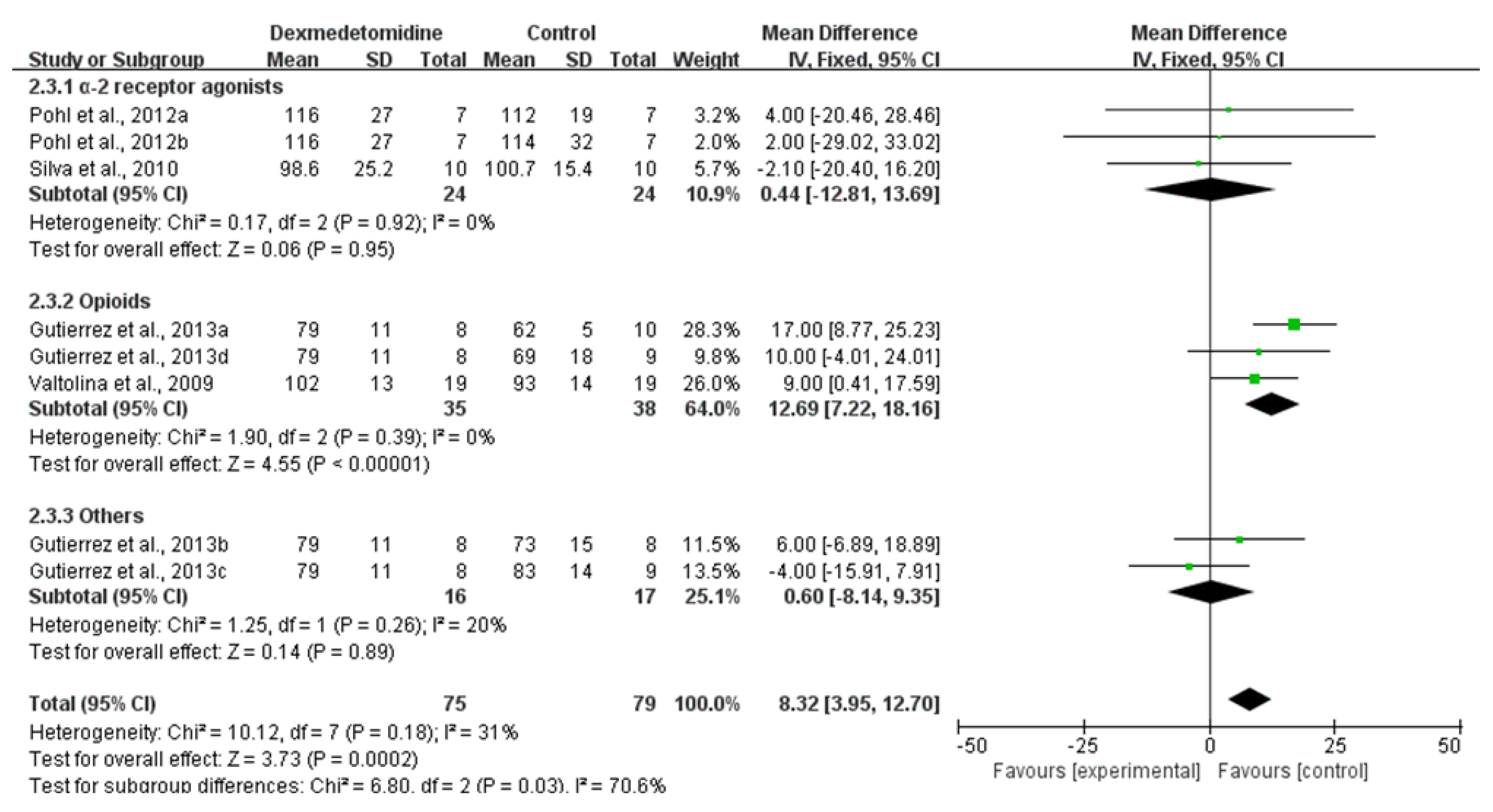
3.3.3. MAP
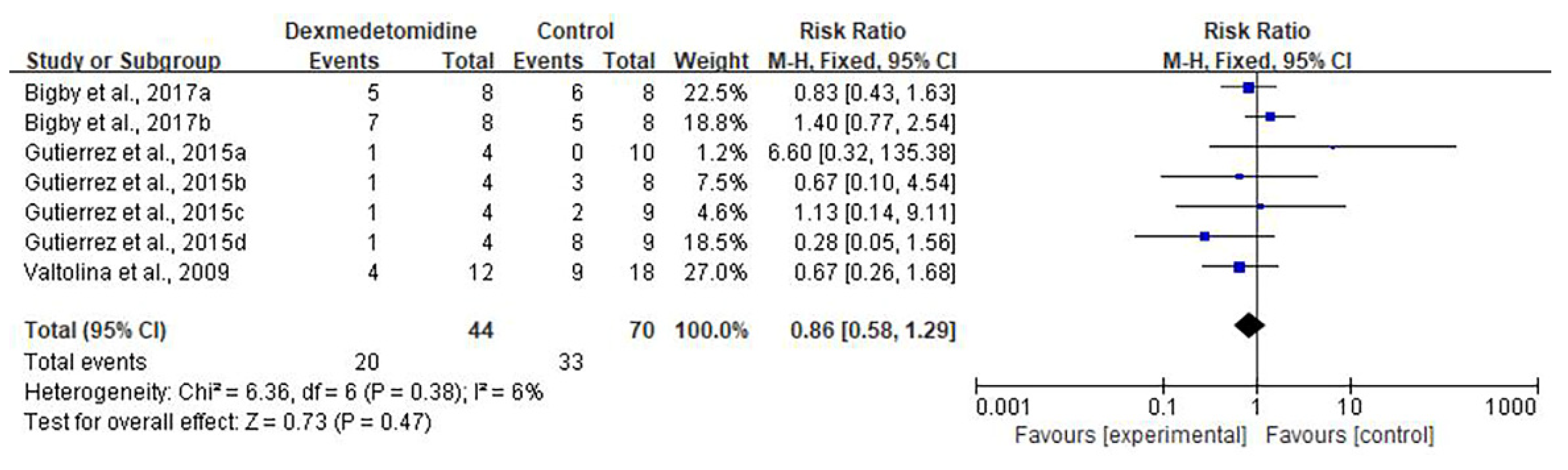
3.4. Safety Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeGroot, W.D.; Tobias, K.M.; Browning, D.C.; Zhu, X. Examination of laryngeal function of healthy dogs by using sedation protocols with dexmedetomidine. Vet. Surg. 2020, 49, 124–130. [Google Scholar] [CrossRef]
- Simon, B.T.; Scallan, E.M.; Coursey, C.D.; Kiehl, W.M.; Moore, E.J. The clinical effects of a low dose dexmedetomidine constant rate infusion in isoflurane anesthetized cats. Vet. J. 2018, 234, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Lu, J.; Guo, T.; Saper, C.B.; Franks, N.P.; Maze, M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003, 98, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, K.A.; Lamont, L.A.; Tranquilli, W.J.; Greene, S.A.; Robertson, S.A. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 188–203. [Google Scholar]
- Kim, J.H.; Ham, S.Y.; Kim, D.H.; Chang, C.H.; Lee, J.S. Efficacy of Single-Dose Dexmedetomidine Combined with Low-Dose Remifentanil Infusion for Cough Suppression Compared to High-Dose Remifentanil Infusion: A Randomized, Controlled, Non-Inferiority Trial. Int J. Med. Sci. 2019, 16, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.; Mason, K.P. Dexmedetomidine: Review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br. J. Anaesth. 2015, 115, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.; Barbi, E.; Mason, K.P. Dexmedetomidine: What’s New for Pediatrics? A Narrative Review. J. Clin. Med. 2020, 9, 2724. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.C.; Hellebrekers, L.J. Medetomidine and dexmedetomidine: A review of cardiovascular effects and antinociceptive properties in the dog. Vet. Anaesth. Analg. Vet. 2005, 32, 117–127. [Google Scholar] [CrossRef]
- Khan, Z.P.; Ferguson, C.N.; Jones, R.M. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999, 54, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Parra, R.; Tissier, R.; Alvarado, M.P.; Garde-Sanjuan, L.; Verwaerde, P.; Saponaro, V. Conventional and advanced echocardiographic assessment of systolic function in dogs sedated with dexmedetomidine or acepromazine. Res. Vet. Sci. 2021, 141, 129–137. [Google Scholar] [CrossRef]
- Riviere, J.E.; Papich, M.G. Veterinary Pharmacology and Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 308. [Google Scholar]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grape, S.; Kirkham, K.R.; Frauenknecht, J.; Albrecht, E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: A systematic review and meta-analysis with trial sequential analysis. Anaesthesia 2019, 74, 793–800. [Google Scholar] [CrossRef]
- Liu, X.; Xie, G.; Zhang, K.; Song, S.; Song, F.; Jin, Y.; Fang, X. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. J. Crit. Care 2017, 38, 190–196. [Google Scholar] [CrossRef]
- Constantin, J.M.; Momon, A.; Mantz, J.; Payen, J.F.; De Jonghe, B.; Perbet, S.; Cayot, S.; Chanques, G.; Perreira, B. Efficacy and safety of sedation with dexmedetomidine in critical care patients: A meta-analysis of randomized controlled trials. Anaesth. Crit. Care Pain Med. 2016, 35, 7–15. [Google Scholar] [CrossRef]
- Jun, J.H.; Kim, K.N.; Kim, J.Y.; Song, S.M. The effects of intranasal dexmedetomidine premedication in children: A systematic review and meta-analysis. Can. J. Anaesth. 2017, 64, 947–961. [Google Scholar] [CrossRef]
- Grint, N.J.; Burford, J.; Dugdale, A.H. Does pethidine affect the cardiovascular and sedative effects of dexmedetomidine in dogs? J. Small Anim. Pract. 2009, 50, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Holton, L.; Reid, J.; Scott, E.M.; Pawson, P.; Nolan, A. Development of a behaviour-based scale to measure acute pain in dogs. Vet. Rec. 2001, 148, 525–531. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- WebPlotDigitizer. Available online: https://automeris.io/WebPlotDigitizer (accessed on 20 March 2021).
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.T.; Lin, L.; Chu, H.; Tong, T. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth Methods 2020, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.W.; Lam, D.M. Heterogeneity in meta-analyses. Comparing apples and oranges? Anaesthesia 2017, 72, 532–534. [Google Scholar] [CrossRef] [Green Version]
- Herbert, G.L.; Bowlt, K.L.; Ford-Fennah, V.; Covey-Crump, G.L.; Murrell, J.C. Alfaxalone for total intravenous anaesthesia in dogs undergoing ovariohysterectomy: A comparison of premedication with acepromazine or dexmedetomidine. Vet. Anaesth. Analg. 2013, 40, 124–133. [Google Scholar] [CrossRef]
- Valtolina, C.; Robben, J.H.; Uilenreef, J.; Murrell, J.C.; Aspegren, J.; McKusick, B.C.; Hellebrekers, L.J. Clinical evaluation of the efficacy and safety of a constant rate infusion of dexmedetomidine for postoperative pain management in dogs. Vet. Anaesth. Analg. 2009, 36, 369–383. [Google Scholar] [CrossRef]
- Raszplewicz, J.; Macfarlane, P.; West, E. Comparison of sedation scores and propofol induction doses in dogs after intramuscular premedication with butorphanol and either dexmedetomidine or medetomidine. Vet. Anaesth. Analg. 2013, 40, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Pinelas, R.; Alibhai, H.I.; Mathis, A.; Jimenez Lozano, A.; Brodbelt, D.C. Effects of different doses of dexmedetomidine on anaesthetic induction with alfaxalone--a clinical trial. Vet. Anaesth. Analg. 2014, 41, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pohl, V.H.; Carregaro, A.B.; Lopes, C.; Gehrcke, M.I.; Muller, D.; Garlet, C.D. Epidural anesthesia and postoperatory analgesia with alpha-2 adrenergic agonists and lidocaine for ovariohysterectomy in bitches. Can. J. Vet. Res. 2012, 76, 215–220. [Google Scholar]
- Gutierrez-Blanco, E.; Victoria-Mora, J.M.; Ibancovichi-Camarillo, J.A.; Sauri-Arceo, C.H.; Bolio-Gonzalez, M.E.; Acevedo-Arcique, C.M.; Marin-Cano, G.; Steagall, P.V. Evaluation of the isoflurane-sparing effects of fentanyl, lidocaine, ketamine, dexmedetomidine, or the combination lidocaine-ketamine-dexmedetomidine during ovariohysterectomy in dogs. Vet. Anaesth. Analg. 2013, 40, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.d.C.; Hatschbach, E.; Carvalho, Y.K.d.; Minto, B.W.; Massone, F.; Nascimento Junior, P.d. Hemodynamics and bispectral index (BIS) of dogs anesthetized with midazolam and ketamine associated with medetomidine or dexmedetomidine and submitted to ovariohysterectomy. Acta Cir. Bras. 2010, 25, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigby, S.E.; Beths, T.; Bauquier, S.; Carter, J.E. Postinduction apnoea in dogs premedicated with acepromazine or dexmedetomidine and anaesthetized with alfaxalone or propofol. Vet. Anaesth. Analg. 2017, 44, 1007–1015. [Google Scholar] [CrossRef]
- Gutierrez-Blanco, E.; Victoria-Mora, J.M.; Ibancovichi-Camarillo, J.A.; Sauri-Arceo, C.H.; Bolio-Gonzalez, M.E.; Acevedo-Arcique, C.M.; Marin-Cano, G.; Steagall, P.V. Postoperative analgesic effects of either a constant rate infusion of fentanyl, lidocaine, ketamine, dexmedetomidine, or the combination lidocaine-ketamine-dexmedetomidine after ovariohysterectomy in dogs. Vet. Anaesth. Analg. 2015, 42, 309–318. [Google Scholar] [CrossRef]
- Hunt, J.R.; Grint, N.J.; Taylor, P.M.; Murrell, J.C. Sedative and analgesic effects of buprenorphine, combined with either acepromazine or dexmedetomidine, for premedication prior to elective surgery in cats and dogs. Vet. Anaesth. Analg. 2013, 40, 297–307. [Google Scholar] [CrossRef]
- Bell, A.M.; Auckburally, A.; Pawson, P.; Scott, E.M.; Flaherty, D. Two doses of dexmedetomidine in combination with buprenorphine for premedication in dogs; a comparison with acepromazine and buprenorphine. Vet. Anaesth. Analg. 2011, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Guzel, O.; Kaya, D.A.; Altunatmaz, K.; Sevim, G.; Sezer, D.; Erdikmen, D.O. Evaluation of the cardiorespiratory effects of the alpha-2 adrenoceptor agonists xylazine, medetomidine and dexmedetomidine in combination with ketamine in dogs. Vet. Med. (Praha) 2018, 63, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Karna, S.R.; Chambers, P.; Singh, P.; Lopez-Villalobos, N.; Kongara, K. Evaluation of analgesic interaction between morphine, maropitant and dexmedetomidine in dogs undergoing ovariohysterectomy. N. Z. Vet. J. 2021, 1–12. [Google Scholar] [CrossRef]
- Cattai, A.; Rabozzi, R.; Ferasin, H.; Isola, M.; Franci, P. Haemodynamic changes during propofol induction in dogs: New findings and approach of monitoring. BMC Vet. Res. 2018, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Anttila, M.; Penttilä, J.; Helminen, A.; Vuorilehto, L.; Scheinin, H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br. J. Clin. Pharmacol. 2003, 56, 691–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioeni, D.; Brioschi, F.A.; Di Cesare, F.; Rabbogliatti, V.; Amari, M.; Zanzani, S.; Cagnardi, P.; Ravasio, G. Oral Transmucosal or Intramuscular Administration of Dexmedetomidine–Methadone Combination in Dogs: Sedative and Physiological Effects. Animals 2020, 10, 2057. [Google Scholar] [CrossRef]
- Mason, K.P.; Zgleszewski, S.E.; Prescilla, R.; Fontaine, P.J.; Zurakowski, D. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr. Anaesth. 2008, 18, 393–402. [Google Scholar] [CrossRef]
- Siegenthaler, J.; Pleyers, T.; Raillard, M.; Spadavecchia, C.; Levionnois, O.L. Effect of Medetomidine, Dexmedetomidine, and Their Reversal with Atipamezole on the Nociceptive Withdrawal Reflex in Beagles. Animals 2020, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Martin-Flores, M.; Sakai, D.M.; Honkavaara, J.; Campoy, L. Hemodynamic effects of low-dose atipamezole in isoflurane-anesthetized cats receiving an infusion of dexmedetomidine. J. Feline Med. Surg. 2018, 20, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Akashi, N.; Murahata, Y.; Hosokawa, M.; Hikasa, Y.; Okamoto, Y.; Imagawa, T. Cardiovascular and renal effects of constant rate infusions of remifentanil, dexmedetomidine and their combination in dogs anesthetized with sevoflurane. J. Vet. Med. Sci. 2021, 83, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kjaergard, L.L.; Villumsen, J.; Gluud, C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann. Intern. Med. 2001, 135, 982–989. [Google Scholar] [CrossRef] [PubMed]

| Study | Year 1 | Country | Premedication | Dose 2 | Route | Group | n | Surgical Procedure | Induce/Maintain Anesthesia | Other Administration | Funding | Conflict of Interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Herbert et al. | 2012 | UK | dexmedetomidine | ≈10 μg·kg−1 | IM | DEX | 19 | OH | alfaxalone | buprenorphine | unknow | unknow |
| acepromazine | 0.05 mg·kg−1 | CON | 19 | |||||||||
| Valtolina et al. | 2009 | NL | dexmedetomidine | ≈1 μg·kg−1 | IV | DEX | 20 | exploratory laparotomy, thoracotomy or orthopedic surgery | isoflurane | sufentanil | Orion Pharma Animal Health | unknow |
| morphine | ≈0.1 mg·kg−1 | CON | 20 | |||||||||
| Pinelas et al. | 2014a | UK | dexmedetomidine | 1 μg·kg−1 | IM | DEX | 20 | diagnostic, orthopedic or elective soft tissue surgical procedures | alfaxalone | methadone | unknow | unknow |
| 2014b | dexmedetomidine | 3 μg·kg−1 | DEX | 18 | ||||||||
| methadone | 0.2 mg·kg−1 | CON | 23 | |||||||||
| Pohl et al. | 2012 | BRA | dexmedetomidine | 2 μg·kg−1 | EPI | DEX | 7 | OH | propofol | lidocaineand adrenalineand acepromazine | unknow | unknow |
| 2012a | xylazine | 0.25 mg·kg−1 | CON | 7 | and isoflurane | |||||||
| 2012b | detomidine | 30 μg·kg−1 | CON | 7 | ||||||||
| Gutierrez et al. | 2013 | MEX | dexmedetomidine | 1 μg·kg−1 | IV | DEX | 8 | OH | propofol | unknown | unknow | sponsored by a scholarship provided by PROMEP-SEP |
| 2013a | fentanil | 5 μg·kg−1 | CON | 10 | and isoflurane | |||||||
| 2013b | Ketamine | 1 mg·kg−1 | CON | 8 | ||||||||
| 2013c | lidocaine | 2 mg·kg−1 | CON | 9 | ||||||||
| 2013d | butorphanol | 0.4 mg·kg−1 | CON | 9 | ||||||||
| Silva et al. | 2010 | BRA | dexmedetomidine | 20 μg·kg−1·h−1 | CRI | DEX | 10 | OH | Midazolam and ketamine | levomepromazine and buprenorphine | unknow | unknow |
| medetomidine | 30 μg·kg−1·h−1 | CON | 10 | |||||||||
| Bigby et al. | 2017a | AU | dexmedetomidine | 5 μg·kg−1 | IM | DEX | 8 | elective neutering procedures | alfaxalone | methadone | unknow | no conflict of interest |
| acepromazine | 0.05 mg·kg−1 | CON | 8 | |||||||||
| 2017b | dexmedetomidine | 5 μg·kg−1 | IM | DEX | 8 | propofol | methadone | |||||
| acepromazine | 0.05 mg·kg−1 | CON | 8 | |||||||||
| Gutierrez et al. | 2015 | MEX | dexmedetomidine | 1 μg·kg−1 | IV | DEX | 8 | OH | propofol | unknown/none | Ministry of Public Education of Mexico PROMEP-SEP | unknow |
| 2015a | fentanil | 5 μg·kg−1 | CON | 10 | and isoflurane | |||||||
| 2015b | ketamine | 1 mg·kg−1 | CON | 8 | ||||||||
| 2015c | lidocaine | 2 mg·kg−1 | CON | 9 | ||||||||
| 2015d | butorphanol | 0.4 mg·kg−1 | CON | 9 | ||||||||
| Bell et al. | 2011a | UK | dexmedetomidine | ≈2.5 μg·kg−1 | IM | DEX | 20 | elective procedures | propofol | buprenorphine | unknow | unknow |
| 2011b | dexmedetomidine | ≈5 μg·kg−1 | DEX | 20 | ||||||||
| acepromazine | 30 μg·kg−1 | CON | 20 | |||||||||
| Guzel et al. | 2018 | TUR | dexmedetomidine | 3 μg·kg−1 | IV | DEX | 10 | surgical procedures due to miscellaneous conditions | none | ketamine | unknow | unknow |
| 2018a | medetomidine | 10 μg·kg−1 | CON | 10 | ||||||||
| 2018b | xylazine | 0.5 mg·kg−1 | CON | 10 | ||||||||
| Raszplewicz et al. | 2013 | UK | dexmedetomidine | 0.005 mg·kg−1 | IM | DEX | 25 | elective diagnostic imaging procedures | propofol | butorphanol | Janssen Animal Health | unknow |
| medetomidine | 0.01 mg·kg−1 | CON | 25 | |||||||||
| Grint et al. | 2009a | UK | dexmedetomidine | 5 μg·kg−1 | IM | DEX | 12 | routine OH or castration | propofol | none | Orion Pharma | unknow |
| 2009b | dexmedetomidine | 10 μg·kg−1 | DEX | 12 | and isoflurane | |||||||
| pethidine | 5 mg·kg−1 | CON | 12 | |||||||||
| Hunt et al. | 2013a | UK | dexmedetomidine | ≈10 μg·kg−1 | IM | DEX | 7 | elective surgeries | propofol | buprenorphine | Orion Pharma | unknow |
| acepromazine | 0.03 mg·kg−1 | CON | 9 | |||||||||
| 2013b | dexmedetomidine | ≈10 μg·kg−1 | IM | DEX | 9 | alfaxalone | buprenorphine | |||||
| acepromazine | 0.03 mg·kg−1 | CON | 10 |
| Certainty Assessment | No. of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Findings | No. of Studies | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Dexmedetomidine | Comparisons | Relative (95% CI) | Absolute (95% CI) | |
| Sedation score | 4 RCTs | serious 1 | serious 2 | not serious | not serious | none | 97 | 101 | - | MD 1.06 higher (1.44 higher to 3.57 higher) | ⨁⨁◯◯ LOW |
| Pain score–120 min after premedication | 3 RCTs | not serious | serious 2 | not serious | not serious | none | 60 | 66 | - | MD 0.34 higher (1.09 higher to 0.41 higher) | ⨁⨁⨁◯ MODERATE |
| HR–30 min after premedication | 8 RCTs | serious 1 | serious 2 | not serious | not serious | none | 177 | 188 | - | MD 13.24 lower (19.67 lower to 6.81 lower) | ⨁⨁◯◯ LOW |
| HR–60 min after premedication | 6 RCTs | serious 1 | serious 2 | not serious | not serious | none | 133 | 144 | - | MD 16.86 lower (26.47 lower to 7.24 lower) | ⨁⨁◯◯ LOW |
| SAP–30 min after premedication | 6 RCTs | not serious | serious 2 | not serious | not serious | none | 153 | 165 | - | MD 7.78 higher (1.83 higher to 13.74 higher) | ⨁⨁⨁◯ MODERATE |
| SAP–60 min after premedication | 5 RCTs | not serious | serious 2 | not serious | not serious | none | 113 | 124 | - | MD 3.59 higher (3.68 lower to 10.87 higher) | ⨁⨁⨁◯ MODERATE |
| MAP–30 min after premedication | 4 RCTs | not serious | not serious | not serious | not serious | none | 75 | 79 | - | MD 7.27 higher (1.61 higher to 12.93 higher) | ⨁⨁⨁⨁ HIGH |
| MAP–60 min after premedication | 4 RCTs | not serious | serious 2 | not serious | not serious | none | 75 | 78 | - | MD 8.06 higher (1.25 higher to 14.87 higher) | ⨁⨁⨁◯ MODERATE |
| Adverse effects | 3 RCTs | not serious | not serious | serious 3 | not serious | none | 20/44 (45.5%) | 33/70 (47.1%) | RR 0.86 (0.58 to 1.29) | 66 fewer per 1000 (from 198 fewer to 137 more) | ⨁⨁⨁◯ MODERATE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.-Y.; Liu, G.; Lin, J.-H.; Jin, Y.-P. Efficacy and Safety of Dexmedetomidine Premedication in Balanced Anesthesia: A Systematic Review and Meta-Analysis in Dogs. Animals 2021, 11, 3254. https://doi.org/10.3390/ani11113254
Pan S-Y, Liu G, Lin J-H, Jin Y-P. Efficacy and Safety of Dexmedetomidine Premedication in Balanced Anesthesia: A Systematic Review and Meta-Analysis in Dogs. Animals. 2021; 11(11):3254. https://doi.org/10.3390/ani11113254
Chicago/Turabian StylePan, Shi-Yue, Gang Liu, Jia-Hao Lin, and Yi-Peng Jin. 2021. "Efficacy and Safety of Dexmedetomidine Premedication in Balanced Anesthesia: A Systematic Review and Meta-Analysis in Dogs" Animals 11, no. 11: 3254. https://doi.org/10.3390/ani11113254
APA StylePan, S.-Y., Liu, G., Lin, J.-H., & Jin, Y.-P. (2021). Efficacy and Safety of Dexmedetomidine Premedication in Balanced Anesthesia: A Systematic Review and Meta-Analysis in Dogs. Animals, 11(11), 3254. https://doi.org/10.3390/ani11113254




