Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Metagenomic Sequencing and Assembly
2.3. Functional Annotation and Analysis of Proteins/Enzymes Involved in Lignocellulose Digestion in the Goats’ Rumen
2.4. Taxonomic Assignment
2.5. Mining Genes Coding for Enzymes/Proteins Related to Lignocellulose Degradation
2.6. Analysis of Prevotella Role in Digestion of Lignocellulose and Other Nutrients in the Goats’ Rumen
2.7. Endoxylanase Expression, Purification and Characterization
3. Results
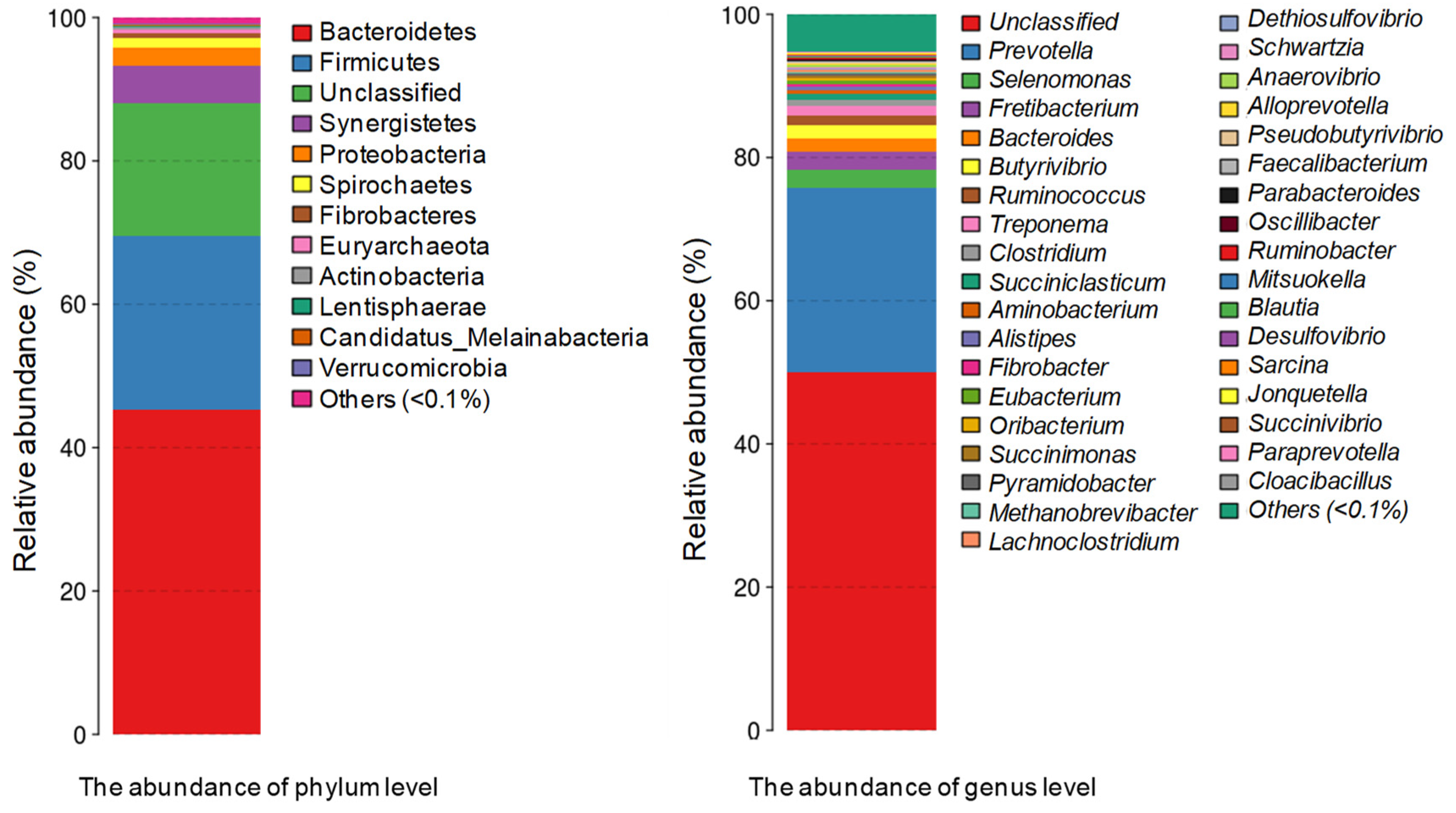
3.1. Metagenomic Deep Sequencing Analysis
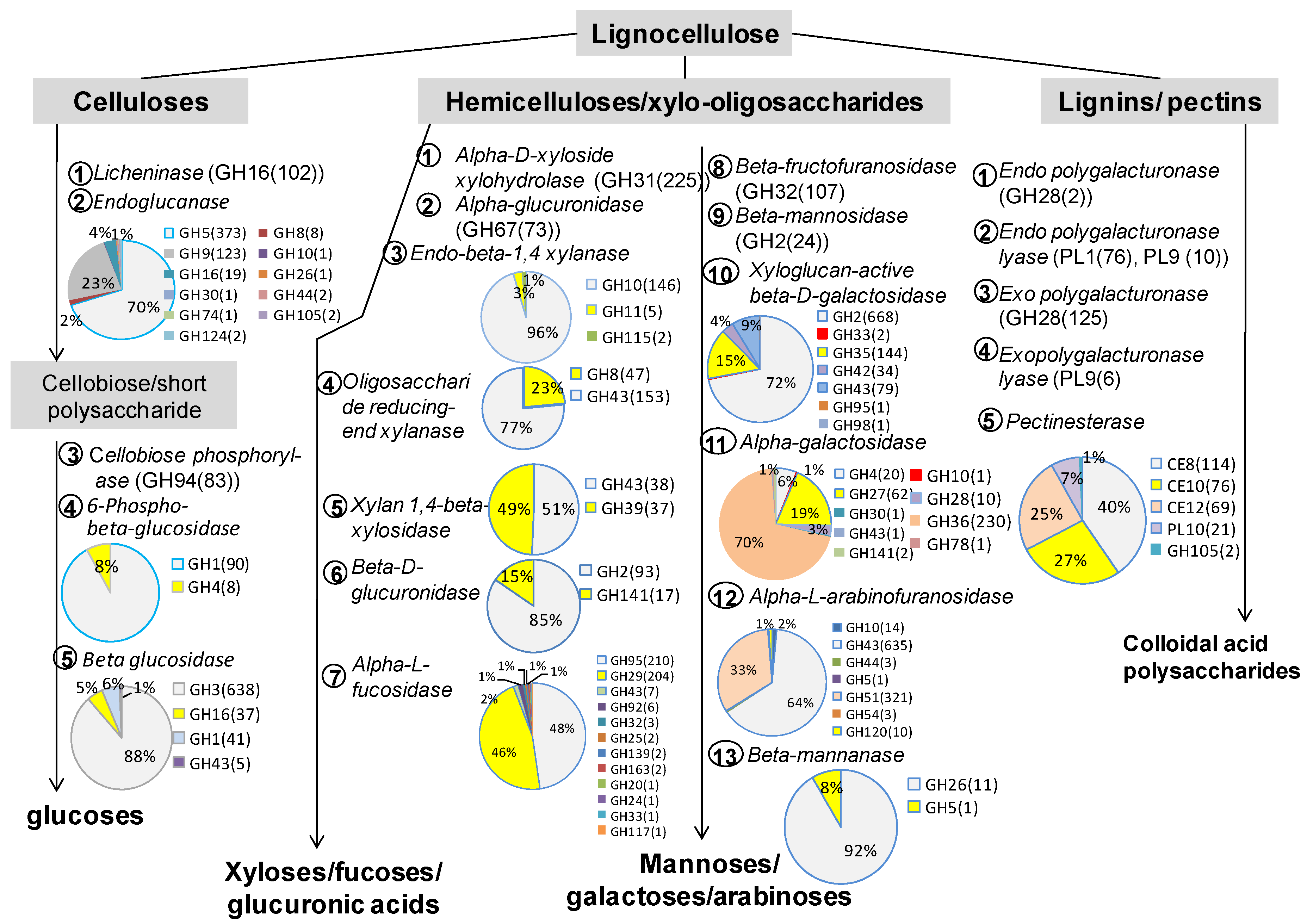
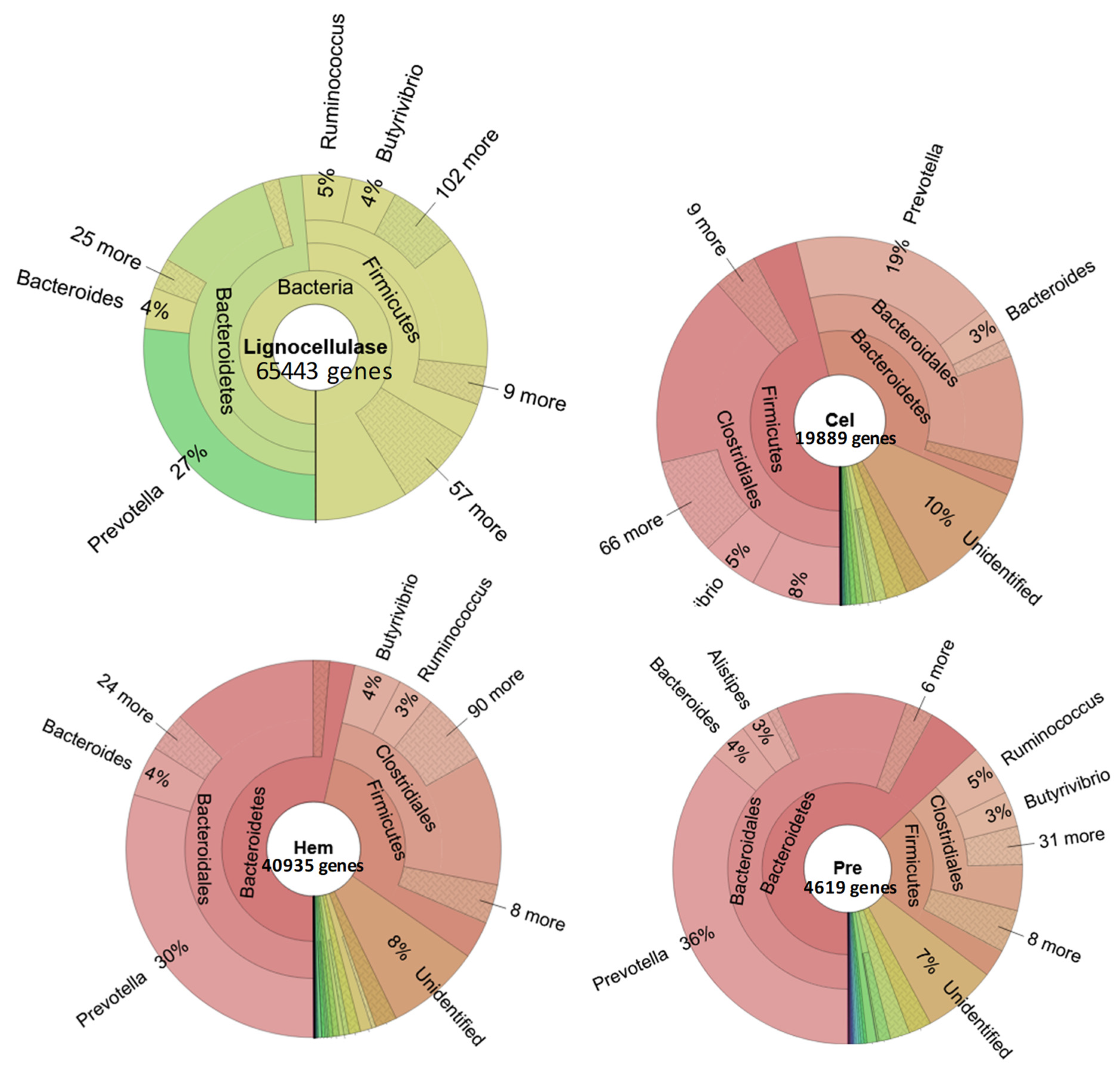
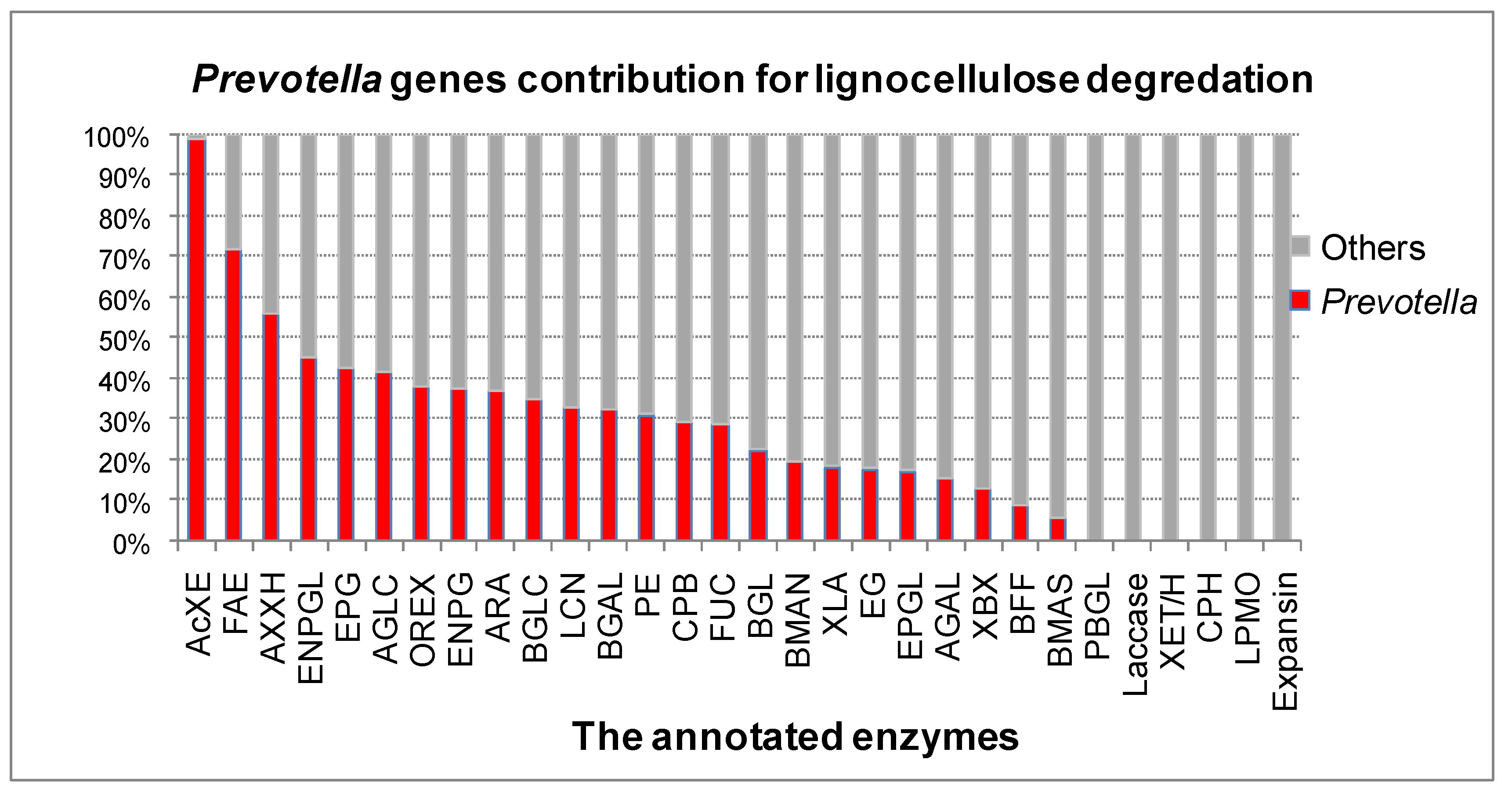
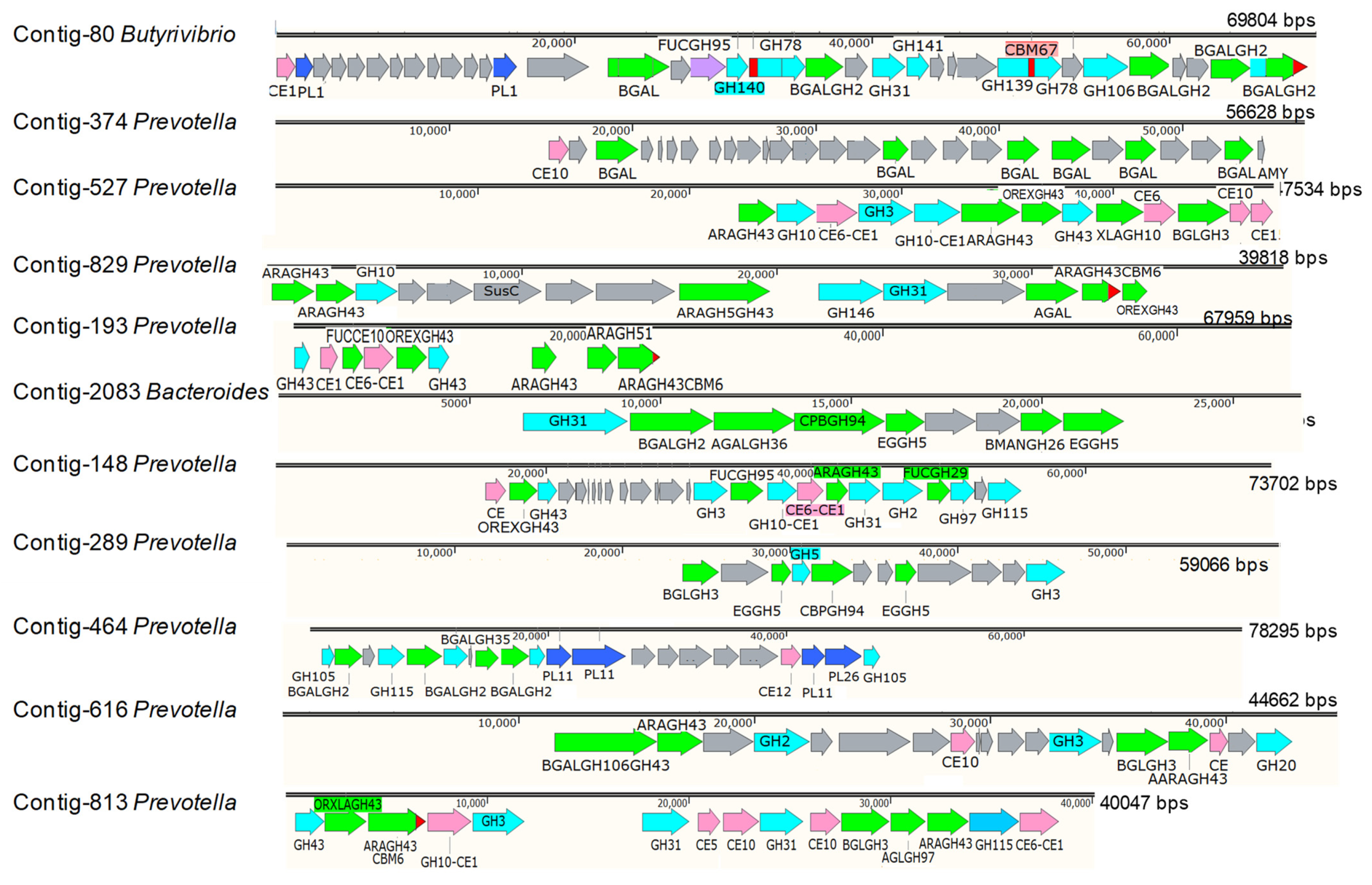
3.2. The Role of Prevotella in the Lignocellulose Digestion
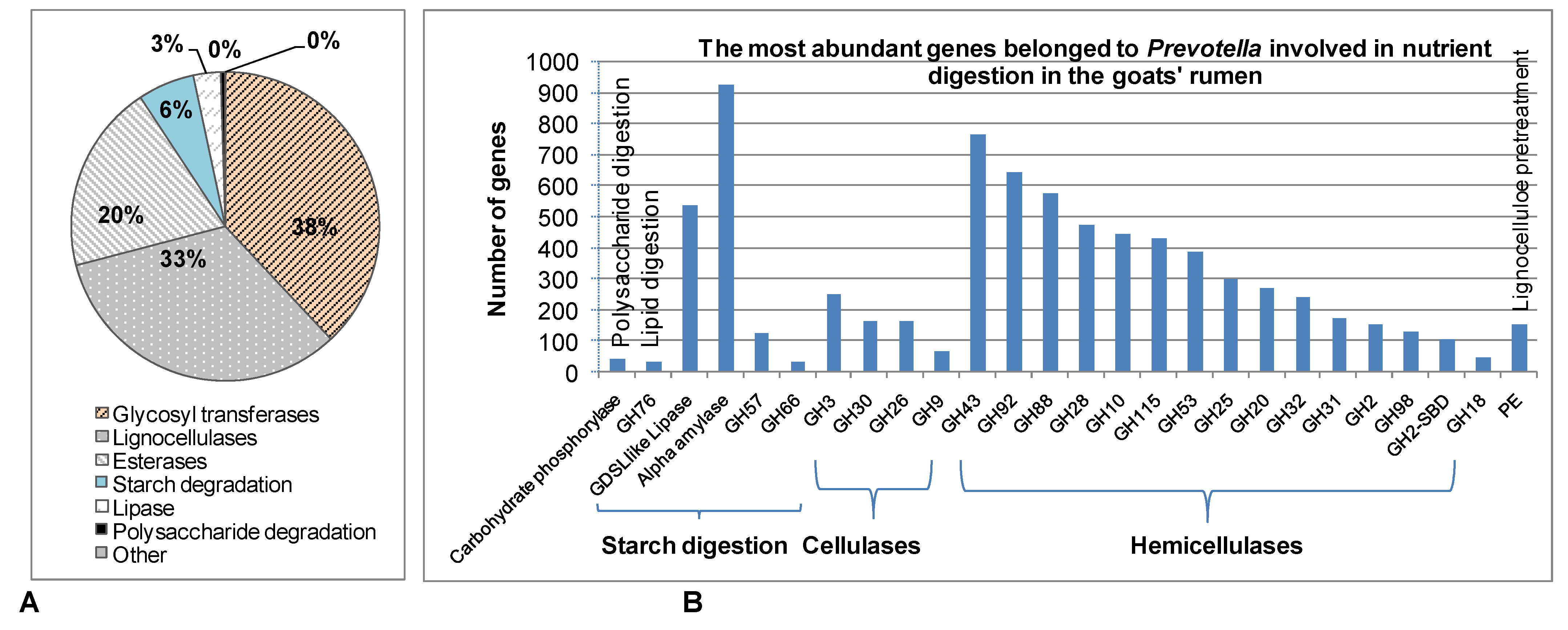
3.3. The Role of Prevotella in the Digestion of Other Nutrients
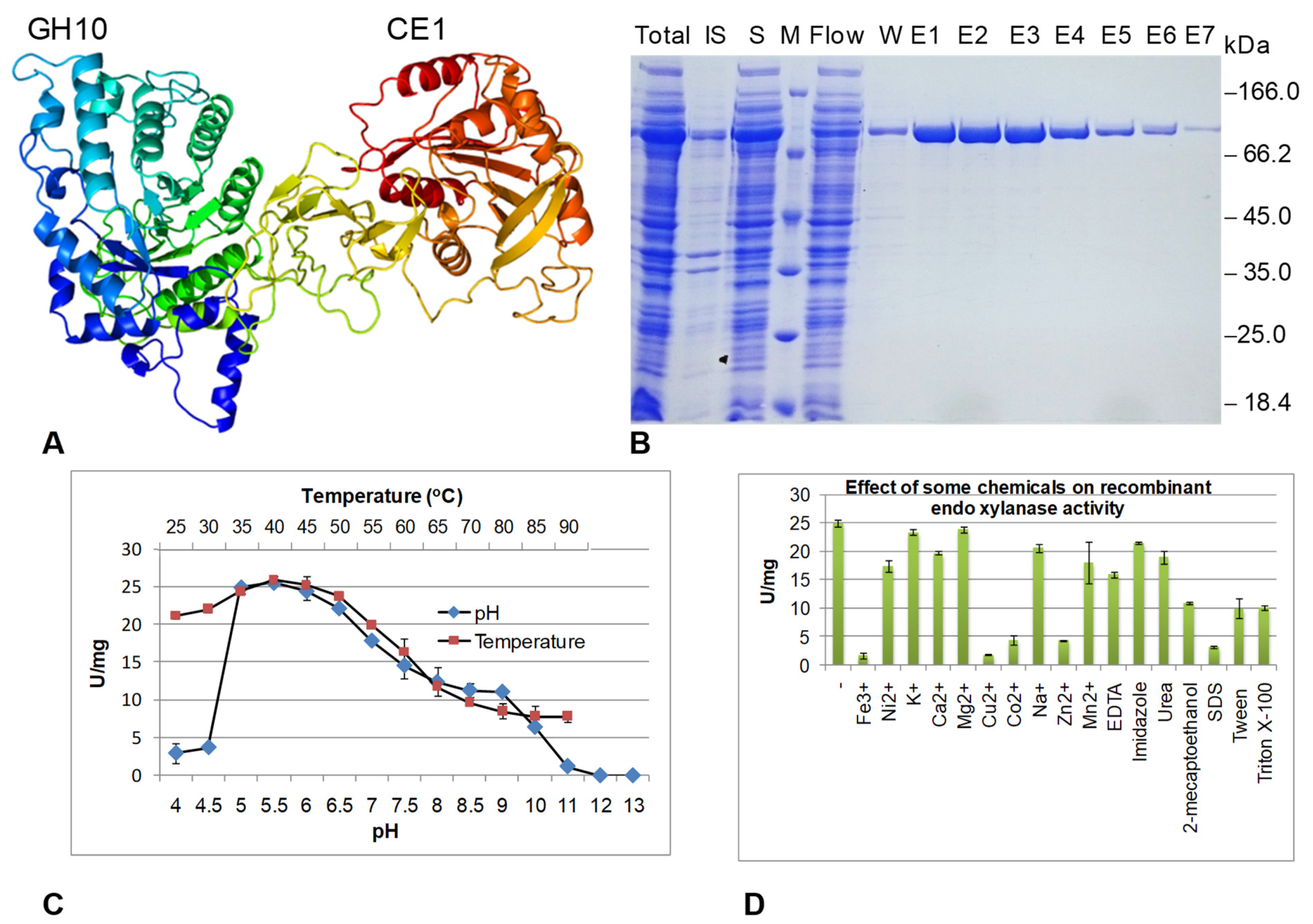
3.4. Expression and Characterization of Endoxylanase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, S.; Seo, J.; Choi, H.; Yoon, D.; Nam, J.; Kim, H.; Cho, S.; Chang, J. Metagenome Analysis of Protein Domain Collocation within Cellulase Genes of Goat Rumen Microbes. Asian-Australas J. Anim. Sci. 2013, 26, 1144–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mamuad, L.L.; Lee, S.S.; Lee, S.S. Recent Insight and Future Techniques to Enhance Rumen Fermentation in Dairy Goats. Asian-Australas J. Anim. Sci. 2019, 32, 1321–1330. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Connor, E.E. Rumen Function and Development. Vet. Clin. N. Am. Food Anim. Pr. 2017, 33, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Cellulose Degradation by Ruminal Microorganisms. Crit. Rev. Biotechnol. 1992, 12, 189–223. [Google Scholar] [CrossRef]
- Israeli-Ruimy, V.; Bule, P.; Jindou, S.; Dassa, B.; Moraïs, S.; Borovok, I.; Barak, Y.; Slutzki, M.; Hamberg, Y.; Cardoso, V.; et al. Complexity of the Ruminococcus flavefaciens FD-1 Cellulosome Reflects an Expansion of Family-Related Protein-Protein Interactions. Sci. Rep. 2017, 7, 42355. [Google Scholar] [CrossRef]
- Raut, M.P.; Couto, N.; Karunakaran, E.; Biggs, C.A.; Wright, P.C. Deciphering the Unique Cellulose Degradation Mechanism of the Ruminal Bacterium Fibrobacter succinogenes S85. Sci. Rep. 2019, 9, 16542. [Google Scholar] [CrossRef]
- Ekinci, M.S.; Ozcan, N.; Ozkose, E.; Flint, H.J. A Study on Cellulolytic and Hemicellulolytic Enzymes of Anaerobic Rumen Bacterium Ruminococcus flavefaciens Strain 17. Turk. J. Vet. Anim. Sci. 2001, 25, 703–709. [Google Scholar]
- Flint, H.J.; Bayer, E.A. Plant Cell Wall Breakdown by Anaerobic Microorganisms from the Mammalian Digestive Tract. Ann. N. Y. Acad. Sci. 2008, 1125, 280–288. [Google Scholar] [CrossRef]
- Chuang, S.T.; Ho, S.T.; Tu, P.W.; Li, K.Y.; Kuo, Y.L.; Shiu, J.S.; Wang, S.Y.; Chen, M.J. The Rumen Specific Bacteriome in Dry Dairy Cows and Its Possible Relationship with Phenotypes. Animals 2020, 10, 1791. [Google Scholar] [CrossRef]
- Wu, X.; Huang, S.; Huang, J.; Peng, P.; Liu, Y.; Han, B.; Sun, D. Identification of the Potential Role of the Rumen Microbiome in Milk Protein and Fat Synthesis in Dairy Cows Using Metagenomic Sequencing. Animals 2021, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Dao, T.K.; Nguyen, K.H.V.; Le, N.G.; Nguyen, T.M.P.; Le, T.L.; Phung, T.N.; van Straalen, N.M.; Roelofs, D.; Truong, N.H. Metagenomic Analysis of Bacterial Community Structure and Diversity of Lignocellulolytic Bacteria in Vietnamese Native Goat Rumen. Asian-Australas J. Anim. Sci. 2018, 31, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Y.; Yan, H.; Wang, X.; Qu, L.; Chen, Y. Rumen Bacterial Diversity of 80 to 110-Day-Old Goats Using 16S RRNA Sequencing. PLoS ONE 2015, 10, e0117811. [Google Scholar] [CrossRef]
- Lv, X.; Chai, J.; Diao, Q.; Huang, W.; Zhuang, Y.; Zhang, N. The Signature Microbiota Drive Rumen Function Shifts in Goat Kids Introduced to Solid Diet Regimes. Microorganisms 2019, 7, 516. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Skliros, D.; Flemetakis, E.; Tsiplakou, E. Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp. Microorganisms 2021, 9, 1528. [Google Scholar] [CrossRef] [PubMed]
- Potempa, M.; Potempa, J.; Kantyka, T.; Nguyen, K.-A.; Wawrzonek, K.; Manandhar, S.P.; Popadiak, K.; Riesbeck, K.; Eick, S.; Blom, A.M. Interpain A, a Cysteine Proteinase from Prevotella intermedia, Inhibits Complement by Degrading Complement Factor C3. PLoS Pathog. 2009, 5, e1000316. [Google Scholar] [CrossRef]
- Doust, R.H.; Mobarez, A.M. Collagenase Activity in Prevotella bivius Isolated from Patients with Premature Rupture of Membranes. Med. J. Islamic. Repub. Iran 2007, 18, 61–66. [Google Scholar]
- Bui, K.H.; Nguyen, B.M.; Dang, T.H.; Pham, K.D. Carcass Performance and Meat Quality of Co Goat, F1 (Boer x Bach Thao) and F2 (Boer x Bach Thao) Crossbred with Co Raised in Bac Kan Provine. Vietnam J. Sci. Dev. 2014, 12, 1223–1230. [Google Scholar]
- Pham, T.H.; Le, A.D.; Tran, Q.H.; Tran, Q.H. Yield and Quality of Meat of Co, Bach Thao and F1(Bach Thao X Co) Goat Raised in Dak Lak. Adv. Ecol. Environ. Res. 2019, 4, 231–240. [Google Scholar]
- Do, T.H.; Le, N.G.; Dao, T.K.; Nguyen, T.M.P.; Le, T.L.; Luu, H.L.; Nguyen, K.H.V.; Nguyen, V.L.; Le, L.A.; Phung, T.N.; et al. Metagenomic Insights into Lignocellulose-Degrading Genes through Illumina-Based de Novo Sequencing of the Microbiome in Vietnamese Native Goats’ Rumen. J. Gen. Appl. Microbiol. 2018, 64, 108–116. [Google Scholar] [CrossRef]
- Pavarina, G.C.; de Macedo Lemos, E.G.; Lima, N.S.M.; Pizauro, J.M., Jr. Characterization of a New Bifunctional Endo-1,4-β-Xylanase/Esterase Found in the Rumen Metagenome. Sci. Rep. 2021, 11, 10440. [Google Scholar] [CrossRef] [PubMed]
- AL-Darkazali, H.; Meevootisom, V.; Isarangkul, D.; Wiyakrutta, S. Gene Expression and Molecular Characterization of a Xylanase from Chicken Cecum Metagenome. Int. J. Microbiol. 2017, 2017, e4018398. [Google Scholar] [CrossRef]
- Linares-Pastén, J.A.; Hero, J.S.; Pisa, J.H.; Teixeira, C.; Nyman, M.; Adlercreutz, P.; Martinez, M.A.; Karlsson, E.N. Novel Xylan Degrading Enzymes from Polysaccharide Utilizing Loci of Prevotella copri DSM18205. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Shah, A.R. Integrated Lignocellulosic Biorefinery: Gateway for Production of Second Generation Ethanol and Value Added Products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Zhu, W.; Lomsadze, A.; Borodovsky, M. Ab Initio Gene Identification in Metagenomic Sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for Linking Genomes to Life and the Environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Szklarczyk, D.; Trachana, K.; Roth, A.; Kuhn, M.; Muller, J.; Arnold, R.; Rattei, T.; Letunic, I.; Doerks, T.; et al. EggNOG v3.0: Orthologous Groups Covering 1133 Organisms at 41 Different Taxonomic Ranges. Nucleic Acids Res. 2011, 40, D284–D289. [Google Scholar] [CrossRef]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN Analysis of Metagenomic Data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef]
- Krska, D.; Larsbrink, J. Investigation of a Thermostable Multi-Domain Xylanase-Glucuronoyl Esterase Enzyme from Caldicellulosiruptor kristjanssonii Incorporating Multiple Carbohydrate-Binding Modules. Biotechnol. Biofuels 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Silva, L.A.O.; Terrasan, C.R.F.; Carmona, E.C. Purification and Characterization of Xylanases from Trichoderma Inhamatum. Electron. J. Biotechnol. 2015, 18, 307–313. [Google Scholar] [CrossRef]
- Mattéotti, C.; Haubruge, E.; Thonart, P.; Francis, F.; De Pauw, E.; Portetelle, D.; Vandenbol, M. Characterization of a New β-Glucosidase/β-Xylosidase from the Gut Microbiota of the Termite (Reticulitermes santonensis). FEMS Microbiol. Lett. 2011, 314, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hang, S.; Mao, S.; Zhu, W. Diversity of Butyrivibrio Group Bacteria in the Rumen of Goats and Its Response to the Supplementation of Garlic Oil. Asian-Australas J. Anim. Sci. 2014, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, K.; Bhardwaj, A.; Gupta, N.; Lokanath, N.K.; Ghosh, A.; Reddy, V.S.; Ramakumar, S. Crystal Structures of Native and Xylosaccharide-Bound Alkali Thermostable Xylanase from an Alkalophilic Bacillus Sp. NG-27: Structural Insights into Alkalophilicity and Implications for Adaptation to Polyextreme Conditions. Protein Sci. 2006, 15, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Holck, J.; Fredslund, F.; Møller, M.S.; Brask, J.; Krogh, K.B.R.M.; Lange, L.; Welner, D.H.; Svensson, B.; Meyer, A.S.; Wilkens, C. A Carbohydrate-Binding Family 48 Module Enables Feruloyl Esterase Action on Polymeric Arabinoxylan. J. Biol. Chem. 2019, 294, 17339–17353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, B.; Guo, M.; Liu, G.; Yang, Y.; Wang, X.; Chen, Y.; Zhang, E. Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization. Animals 2019, 9, 1028. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Koike, S.; Kobayashi, Y. Genetic Diversity and Diet Specificity of Ruminal Prevotella Revealed by 16S RRNA Gene-Based Analysis. FEMS Microbiol. Lett. 2010, 305, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and Low Abundance of Classical Ruminal Bacterial Species in the Bovine Rumen Revealed by Relative Quantification Real-Time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Nguyen, K.H.V.; Dao, T.K.; Nguyen, H.D.; Nguyen, K.H.; Nguyen, T.Q.; Nguyen, T.T.; Nguyen, T.M.P.; Truong, N.H.; Do, T.H. Some Characters of Bacterial Cellulases in Goats’ Rumen Elucidated by Metagenomic DNA Analysis and the Role of Fibronectin 3 Module for Endoglucanase Function. Anim. Biosci. 2021, 34, 867–879. [Google Scholar] [CrossRef]
- Dodd, D.; Moon, Y.-H.; Swaminathan, K.; Mackie, R.I.; Cann, I.K.O. Transcriptomic Analyses of Xylan Degradation by Prevotella bryantii and Insights into Energy Acquisition by Xylanolytic Bacteroidetes. J. Biol. Chem. 2010, 285, 30261–30273. [Google Scholar] [CrossRef]
- Underlin, E.N.; Frommhagen, M.; Dilokpimol, A.; van Erven, G.; de Vries, R.P.; Kabel, M.A. Feruloyl Esterases for Biorefineries: Subfamily Classified Specificity for Natural Substrates. Front. Bioeng. Biotechnol. 2020, 8, 332. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Yao, Q.; Zong, W.; Dai, S.; Deng, Z.; Liu, S.; Yun, J.; Yang, X.; Li, H. Cloning, Characterization of a Novel Acetyl Xylan Esterase, and Its Potential Application on Wheat Straw Utilization. All Life 2021, 14, 622–635. [Google Scholar] [CrossRef]
- Sheridan, P.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.B.; Bernalier-Donadille, A.; Duncan, S.H.; O’Toole, P.W.; P Scott, K.; J Flint, H. Polysaccharide Utilization Loci and Nutritional Specialization in a Dominant Group of Butyrate-Producing Human Colonic Firmicutes. Microb. Genom. 2016, 2, e000043. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.K.; Naas, A.E.; Kracun, S.K.; Schückel, J.; Fangel, J.U.; Agger, J.W.; Willats, W.G.T.; Eijsink, V.G.H.; Pope, P.B. A Polysaccharide Utilization Locus from an Uncultured Bacteroidetes Phylotype Suggests Ecological Adaptation and Substrate Versatility. Appl. Environ. Microbiol. 2015, 81, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ilmberger, N.; Güllert, S.; Dannenberg, J.; Rabausch, U.; Torres, J.; Wemheuer, B.; Alawi, M.; Poehlein, A.; Chow, J.; Turaev, D.; et al. A Comparative Metagenome Survey of the Fecal Microbiota of a Breast- and a Plant-Fed Asian Elephant Reveals an Unexpectedly High Diversity of Glycoside Hydrolase Family Enzymes. PLoS ONE 2014, 9, e106707. [Google Scholar] [CrossRef]
- Li, J.; Gálvez, E.J.C.; Amend, L.; Almasi, É.; Iljazovic, A.; Lesker, T.R.; Bielecka, A.A.; Strowig, T. A Versatile Genetic Toolbox for Prevotella copri Enables Studying Polysaccharide Utilization Systems. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rodríguez, F. Control of Lactate Accumulation in Ruminants Using Prevotella bryantii; UMI Microform: Ann Arbor, MI, USA, 2003. [Google Scholar] [CrossRef]
- Cremonesi, P.; Conte, G.; Severgnini, M.; Turri, F.; Monni, A.; Capra, E.; Rapetti, L.; Colombini, S.; Chessa, S.; Battelli, G.; et al. Evaluation of the Effects of Different Diets on Microbiome Diversity and Fatty Acid Composition of Rumen Liquor in Dairy Goat. Animal 2018, 12, 1856–1866. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liu, T.; Chen, S.; Liu, H.; Wang, H.; Li, K.; Song, Y.; Luo, X.; Zhao, J.; et al. The Critical Roles of Exposed Surface Residues for the Thermostability and Halotolerance of a Novel GH11 Xylanase from the Metagenomic Library of a Saline-Alkaline Soil. Int. J. Biol. Macromol. 2019, 133, 316–323. [Google Scholar] [CrossRef]
- Ghadikolaei, K.K.; Sangachini, E.D.; Vahdatirad, V.; Noghabi, K.A.; Zahiri, H.S. An Extreme Halophilic Xylanase from Camel Rumen Metagenome with Elevated Catalytic Activity in High Salt Concentrations. AMB Express 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Bashir, S.; Tabassum, R. An Update on Carbohydrases: Growth Performance and Intestinal Health of Poultry. Heliyon 2019, 5, e01437. [Google Scholar] [CrossRef] [PubMed]
| Enzymes | EC Number | Abbr. | Normal Sequencing (8.4 G) | Deep Sequencing of 45 G | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total Genes | Lack 3’-End | Lack 5’-End | Lack Both Ends | Complete | % Complete Genes | ||||
| Cellulases | 448 | 21,029 | 5786 | 3816 | 9091 | 2336 | 11.1 | ||
| Licheninase | 3.2.1.73 | LCN | 1 | 281 | 49 | 62 | 68 | 102 | 36.3 |
| Endoglucanase | 3.2.1.4 | EG | 66 | 7368 | 1871 | 1406 | 3081 | 1010 | 13.7 |
| Cellobiose phosphorylase | 2.4.1.20 | CPB | 27 | 1439 | 424 | 268 | 649 | 98 | 6.8 |
| Beta glucosidase | 3.2.1.21 | BGL | 341 | 10,444 | 3001 | 1780 | 4702 | 961 | 9.2 |
| 6-Phospho-beta-glucosidase | 3.2.1.86 | PBGL | 13 | 1281 | 393 | 274 | 501 | 113 | 8.8 |
| Cellobiose dehydrogenase | 1.1.99.18 | 0 | 0 | ||||||
| Cellobiohydrolase | 3.2.1.91 | CPH | 0 | 216 | 48 | 26 | 90 | 52 | 24.1 |
| Hemicellulases | 815 | 41,756 | 12,007 | 6658 | 17,199 | 5892 | 14.1 | ||
| Acetyl mannan esterase | 3.1.1.6 | AcME | 0 | 0 | |||||
| Acetylxylan esterase | 3.1.1.72 | AcXE | 0 | 4 | 0 | 0 | 4 | 0 | 0.0 |
| Alpha-D-xyloside xylohydrolase | 3.2.1.177 | AXXH | 0 | 2833 | 840 | 460 | 1277 | 256 | 9.0 |
| Alpha-glucuronidase | 3.2.1.139 | AGLC | 37 | 561 | 155 | 109 | 224 | 73 | 13.0 |
| Beta-D-glucuronidase | 3.2.1.31 | BGLC | 30 | 888 | 292 | 127 | 343 | 126 | 14.2 |
| Beta-fructofuranosidase | 3.2.1.26 | BFF | 0 | 1106 | 368 | 197 | 427 | 114 | 10.3 |
| Beta-mannosidase | 3.2.1.25 | BMAS | 62 | 659 | 189 | 90 | 329 | 51 | 7.7 |
| Endo-transglycosylase/ hydrolase | 2.4.1.207 | XET/H | 0 | 2 | 0 | 1 | 1 | 0 | 0.0 |
| Endo-β-1,4 xylanase | 3.2.1.8 | XLA | 67 | 3400 | 938 | 634 | 1121 | 707 | 20.8 |
| Oligosaccharide reducing-end xylanase | 3.2.1.156 | OREX | 0 | 1213 | 422 | 191 | 363 | 237 | 19.5 |
| Xylan 1,4-beta-xylosidase | 3.2.1.37 | XBX | 10 | 1018 | 262 | 197 | 337 | 222 | 21.8 |
| Xyloglucan-active β-D-galactosidase | 3.2.1.23 | BGAL | 290 | 11,690 | 3326 | 1705 | 5490 | 1169 | 10.0 |
| Alpha-galactosidase | 3.2.1.22 | AGAL | 63 | 4020 | 1174 | 693 | 1638 | 515 | 12.8 |
| Alpha-L- arabinofuranosidase | 3.2.1.55 | ARA | 138 | 6229 | 1882 | 891 | 2282 | 1174 | 18.8 |
| Alpha-L-fucosidase | 3.2.1.51 | FUC | 55 | 6896 | 1890 | 1136 | 2663 | 1207 | 17.5 |
| Beta-mannanase | 3.2.1.78 | BMAN | 63 | 1237 | 269 | 227 | 700 | 41 | 3.3 |
| Pretreatments | 167 | 4769 | 1382 | 782 | 1845 | 760 | 15.9 | ||
| Expansin | 1 | 33 | 5 | 13 | 9 | 6 | 18.2 | ||
| Feruloyl esterase | 3.1.1.73 | FAE | 0 | 92 | 35 | 9 | 17 | 31 | 33.7 |
| Laccases | 1.10.3.2 | 0 | 9 | 2 | 2 | 4 | 1 | 11.1 | |
| Lignin peroxidase | 1.11.1.14 | LiP | 0 | 0 | |||||
| Lytic polysaccharide monooxygenase | 1.14.99.56 | LPMO | 0 | 11 | 3 | 0 | 7 | 1 | 9.1 |
| Manganese peroxidase | 1.11.1.13 | MnP | 0 | 0 | |||||
| Pectinesterase | 3.1.1.11 | PE | 86 | 2525 | 704 | 483 | 919 | 419 | 16.6 |
| Exopolygalacturonase | 3.2.1.67 | EPG | 38 | 635 | 216 | 66 | 223 | 130 | 20.5 |
| Exopolygalacturonase lyase | 4.2.2.9 | EPGL | 0 | 59 | 8 | 8 | 37 | 6 | 10.2 |
| Endopolygalacturonase | 3.2.1.15 | ENPG | 0 | 27 | 4 | 7 | 14 | 2 | 7.4 |
| Endopolygalacturonase lyase | 4.2.2.2 | ENPGL | 42 | 1378 | 405 | 194 | 615 | 164 | 11.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, T.-K.; Do, T.-H.; Le, N.-G.; Nguyen, H.-D.; Nguyen, T.-Q.; Le, T.-T.-H.; Truong, N.-H. Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing. Animals 2021, 11, 3257. https://doi.org/10.3390/ani11113257
Dao T-K, Do T-H, Le N-G, Nguyen H-D, Nguyen T-Q, Le T-T-H, Truong N-H. Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing. Animals. 2021; 11(11):3257. https://doi.org/10.3390/ani11113257
Chicago/Turabian StyleDao, Trong-Khoa, Thi-Huyen Do, Ngoc-Giang Le, Hong-Duong Nguyen, Thi-Quy Nguyen, Thi-Thu-Hong Le, and Nam-Hai Truong. 2021. "Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing" Animals 11, no. 11: 3257. https://doi.org/10.3390/ani11113257
APA StyleDao, T.-K., Do, T.-H., Le, N.-G., Nguyen, H.-D., Nguyen, T.-Q., Le, T.-T.-H., & Truong, N.-H. (2021). Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing. Animals, 11(11), 3257. https://doi.org/10.3390/ani11113257







