Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Extraction
2.3. Calculations and Statistical Analysis
2.4. Heterogeneity
2.5. Publication Bias
2.6. Meta-Regression
3. Results
3.1. Study Attributes and Excluded Studies
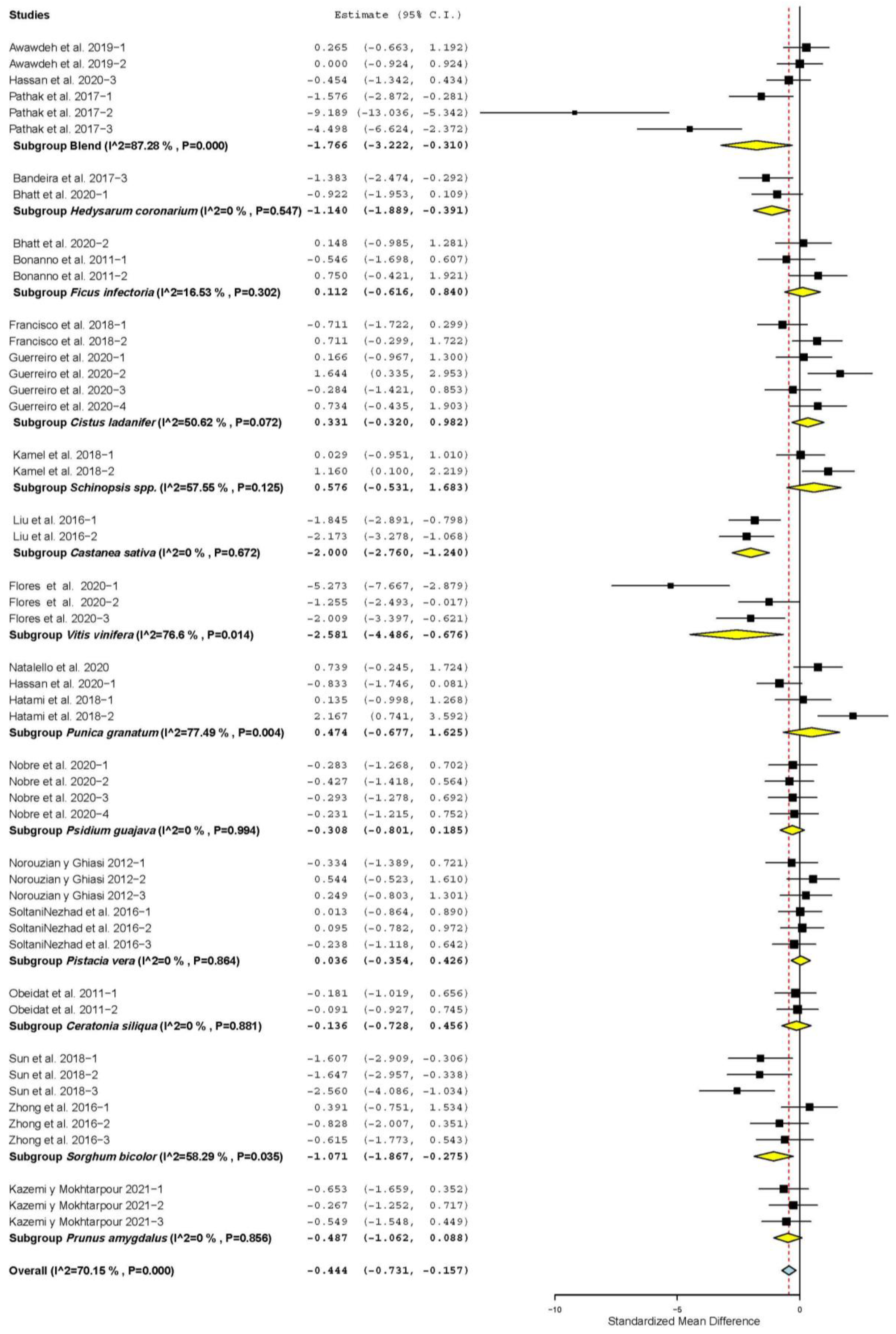
3.2. Growth Performance and Carcass Characteristics
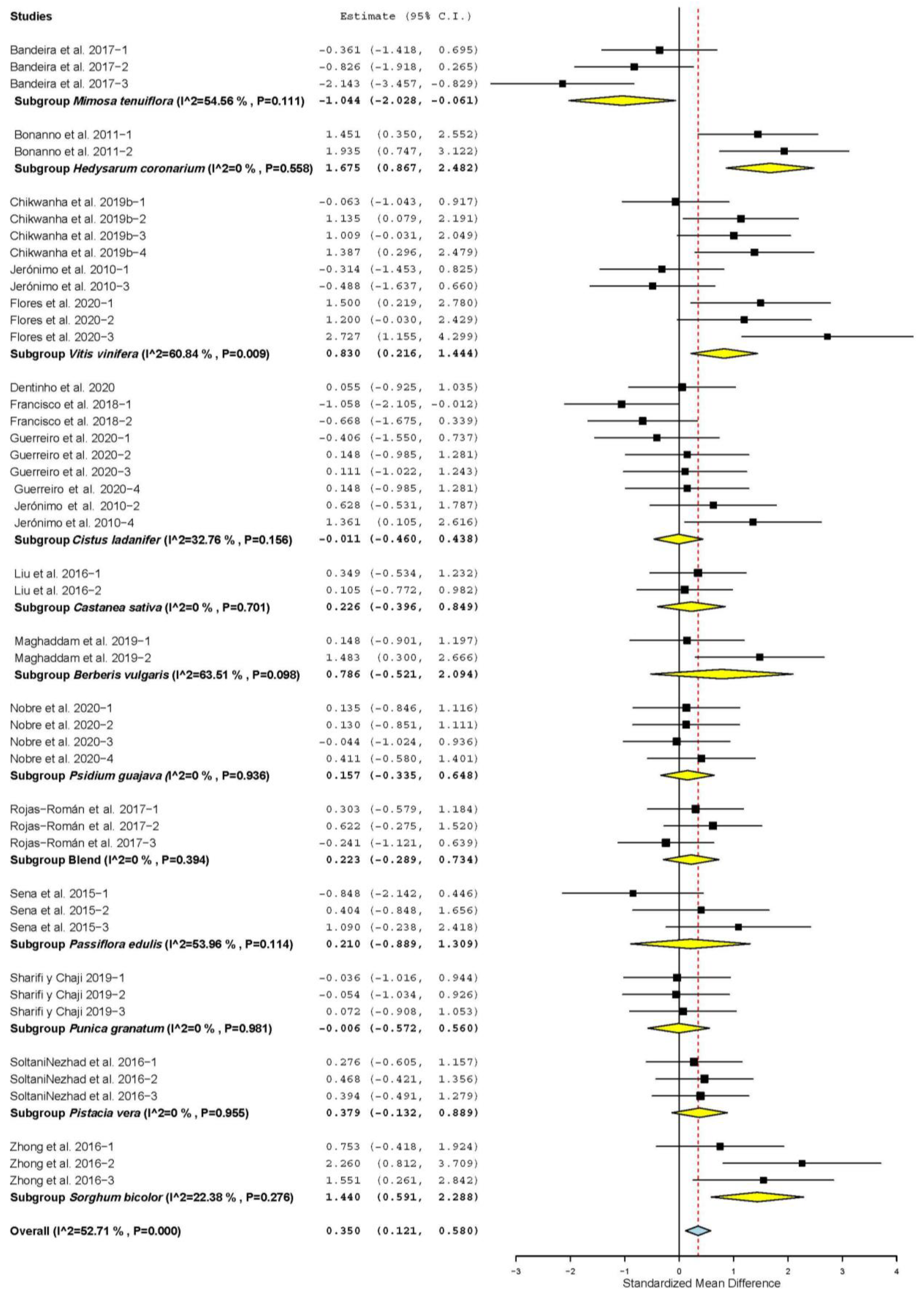
3.3. Meat Quality Characteristics
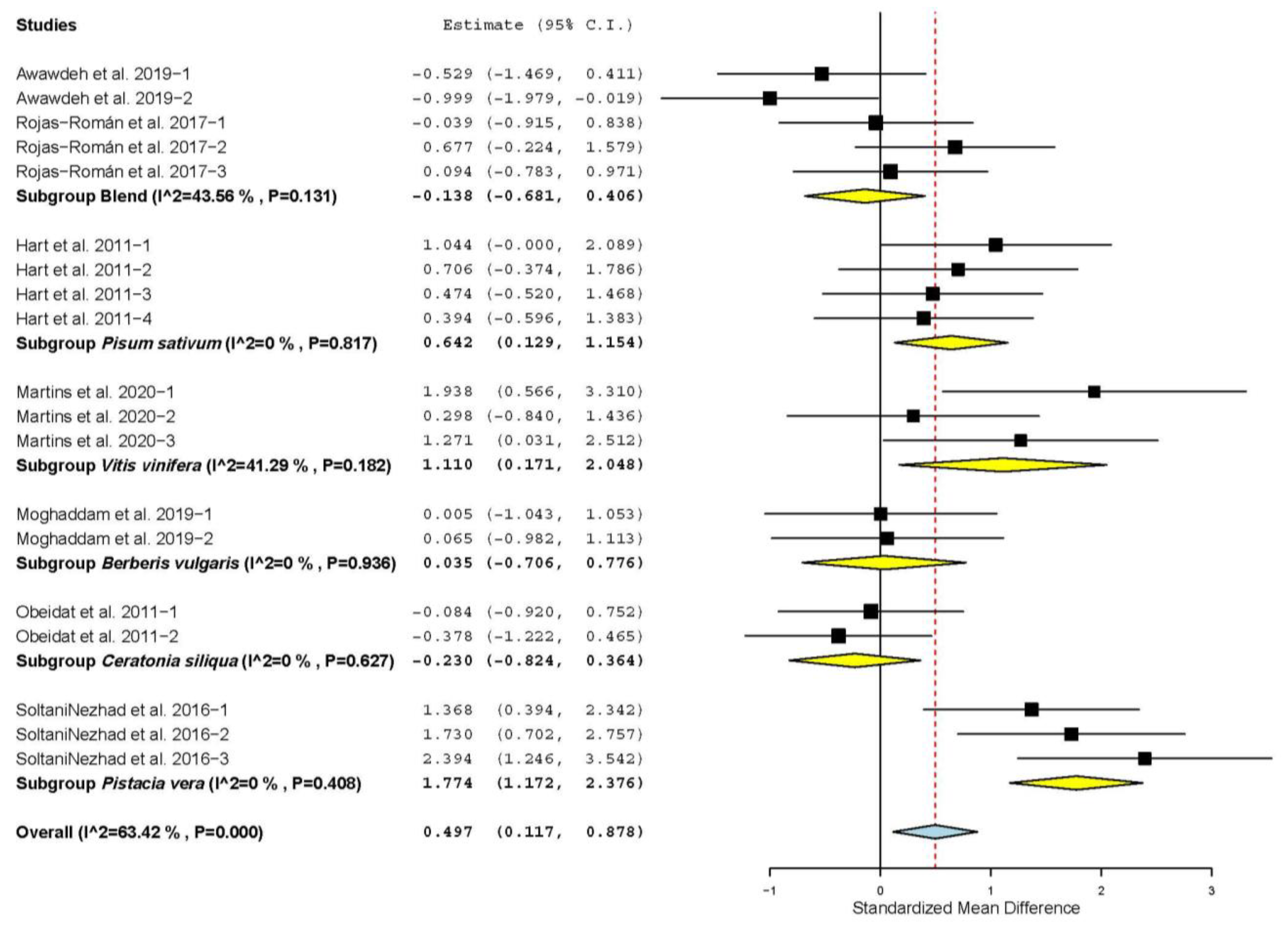
3.4. Antioxidant Status
3.5. Analysis of Publication Bias
3.6. Meta-Regression
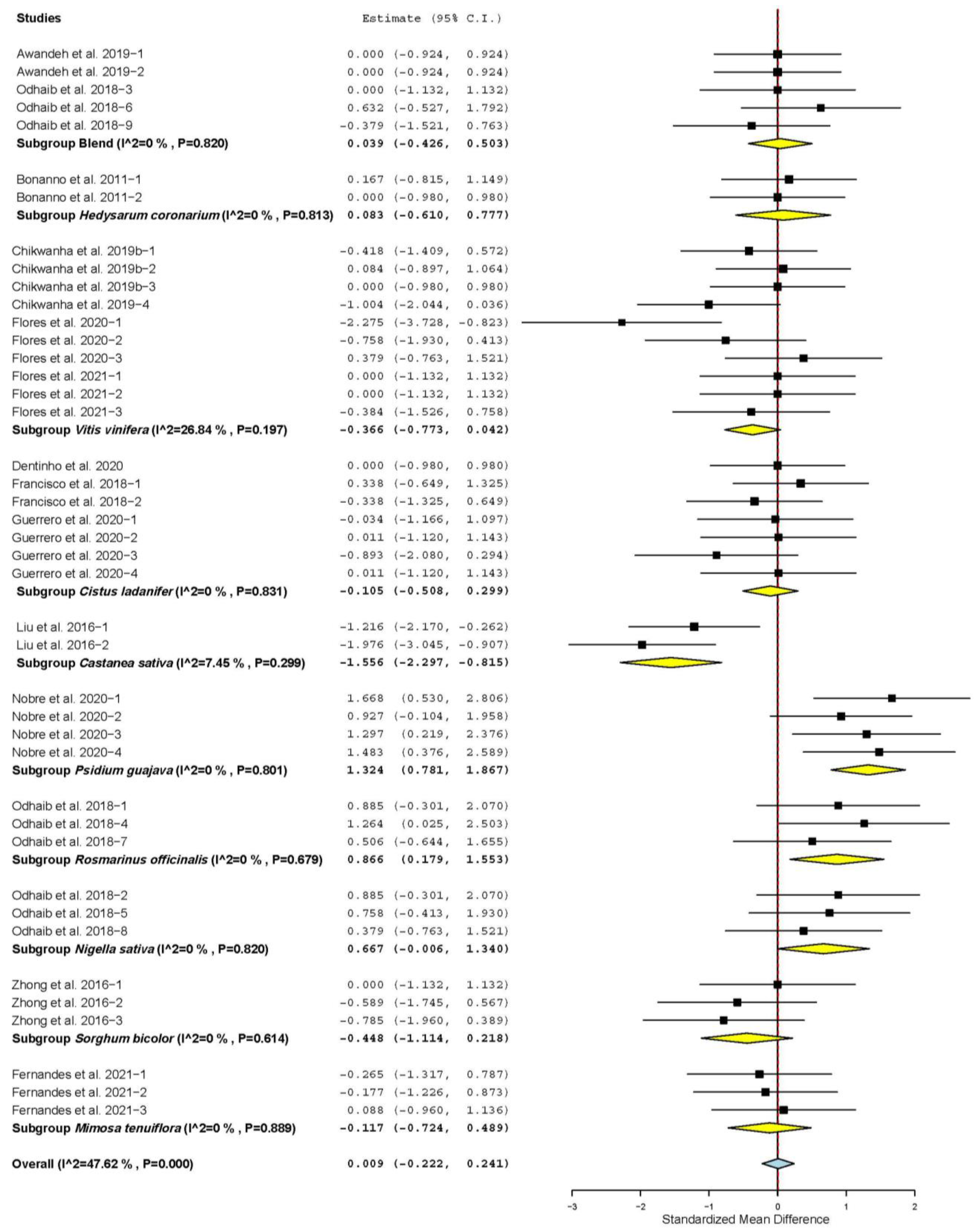
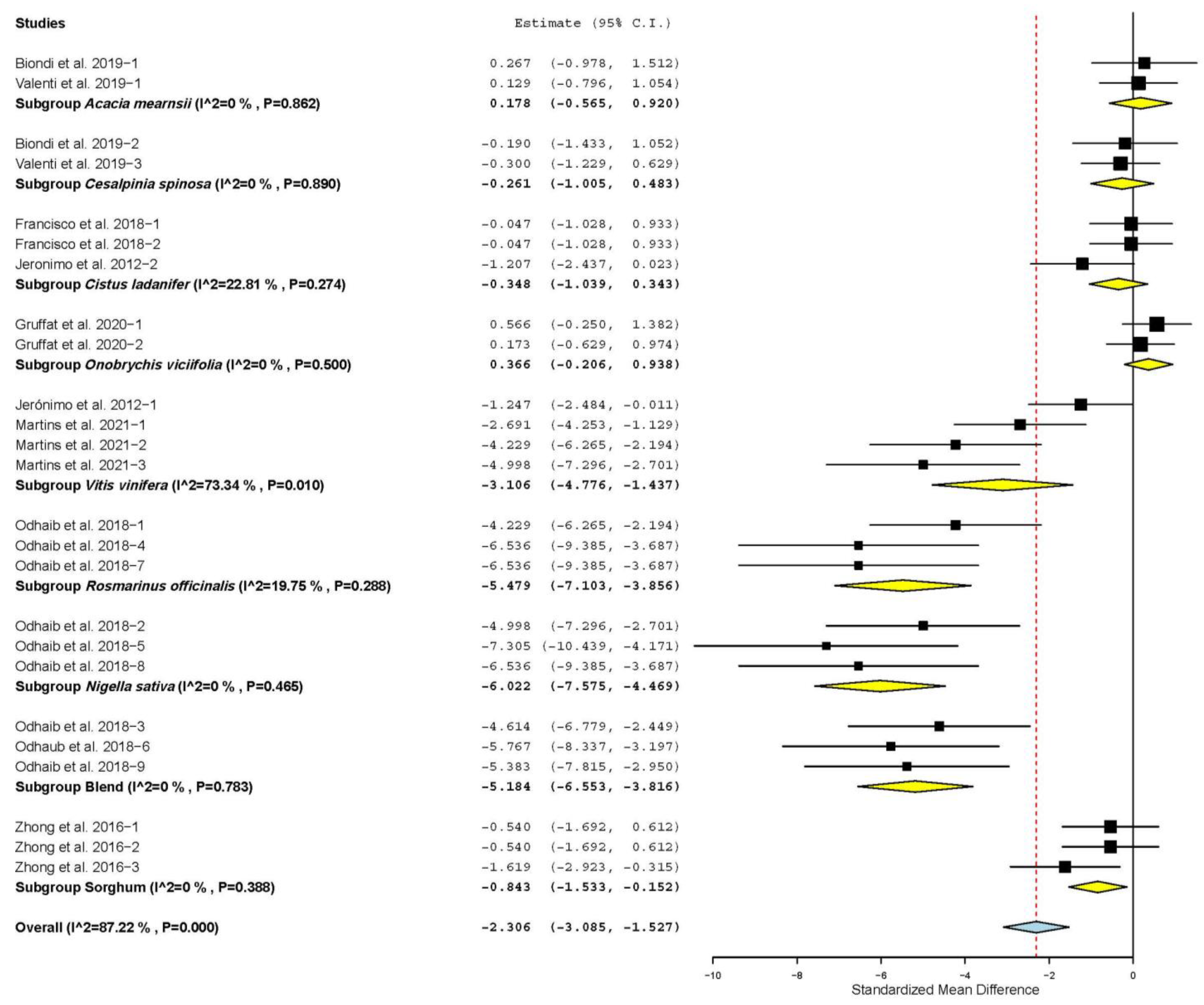
3.7. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Author | Country | Tannin Source | Tannin Type | Method of Inclusion |
|---|---|---|---|---|
| Awawdeh et al. [98] | Jordan | B, B | B, B | N, N |
| Bandeira et al. [99] | Brazil | Mimosa tenuiflora (n = 3) | CT, CT, CT | N, N, N |
| Bhatt et al. [100] | India | PA, ER | B, B | N, N |
| Biondi et al. [68] | Spain | AM, CS | B, B | E, E |
| Bonanno et al. [101] | Italy | Hedysarum coronarium | CT, CT | N, N |
| Buccioni et al. [102] | Italy | CH, QU | HT, CT | E, E |
| Chikwanha et al. [103] | South Africa | VV (n = 4) | B, B, B, B, B | N, N, N, N, N |
| Chikwanha et al. [104] | South Africa | VV (n = 4) | B, B, B, B, B | N, N, N, N, N |
| Costa et al. [105] | Brazil | AM (n = 4) | CT, CT, CT, CT, CT | E, E, E, E, E |
| Dentinho et al. [20] | Brazil | CL | CT | E |
| Dey et al. [106] | India | Ficus infectoria (n = 3) | B, B, B | N, N, N |
| Abdalla et al. [107] | Brazil | OP, Clep | B, B | N, N |
| Fernandes et al. [14] | Brazil | Mimosa tenuiflora (n = 3) | B, B, B | N, N, N |
| Francisco et al. [108] | Portugal | CL (n = 2) | CT, CT | N, N |
| Girard et al. [109] | Switzerland | LC and OV | B, B | N, N |
| Guerreiro et al. [110] | Portugal | CL (n = 4) | CT, CT, CT, CT, CT | N, N, N, N, N |
| Gruffat et al. [111] | France | OV (n = 2) | CT, CT | N, N |
| Hart et al. [112] | United Kingdom | Pisum sativum (n = 4) | CT, CT, CT, CT, CT | N, N, N, N, N |
| Hassan et al. [113] | Egypt | Punica granatum, MI, B | B, B, B | N, N, N |
| Hatami et al. [114] | Iran | Punica granatum (n = 3) | B, B | N, N |
| Jerónimo et al. [115] | Portugal | VV (n = 2), CL (n = 2) | CT, CT, CT, CT, CT | E, N, E, N |
| Jerónimo et al. [116] | Portugal | VV (n = 2), CL (n = 2) | CT, CT, CT, CT, CT | E, N, E, N |
| Kamel et al. [117] | Saudi Arabia | QU (n = 2) | CT, CT | E, E |
| Kazemi and Mokhtarpour [118] | Iran | Prunus amygdalus (n = 3) | B, B, B | N, N, N |
| Leparmarai et al. [119] | Switzerland | VV | B | E |
| Lima et al. [120] | Brazil | Macrotyloma axillare | B | N |
| Liu et al. [21] | China | CH (n = 2) | HT, HT | E, E |
| López-Andrés et al. [91] | Italy | QU | CT | E |
| Majewska and Kowalik [121] | Poland | VAC, Quercus sp. | B, B | N, N |
| Flores et al. [122] | Brazil | VV (n = 3) | B, B, B | N, N, N |
| Flores et al. [123] | Brazil | VV (n = 3) | B, B, B | N, N, N |
| Moghaddam et al. [124] | Iran | Berberis vulgaris (n = 2) | B, B | N, N |
| Natalello et al. [18] | Italy | Punica granatum | B | N |
| Nobre et al. [125] | Brazil | Psidium guajava (n = 4) | B, B, B, B, B | N, N, N, N, N |
| Norouzian and Ghiasi [126] | Iran | Pistacia vera (n = 3) | B, B, B | N, N, N |
| Obeidat et al. [127] | Jordan | Ceratonia siliqua (n = 2) | CT, CT | N, N |
| Odhaib et al. [128] | Malaysia | RO (n = 3), NS (n = 3), B (n = 3) | B (n = 9) | N (n = 9) |
| Pathak et al. [13] | India | B, B | CT, CT | N, N |
| Peng et al. [95] | Canada | Dalea purpurea | CT | N |
| Po et al. [129] | Australia | Ilex paraguarensis | CT | N |
| Priolo et al. [16] | Italy | Corylus avellana | B | N |
| Rajabi et al. [130] | Iran | Punica granatum (n = 3) | B, B, B | N, N, N |
| Rojas-Román et al. [15] | Mexico | B, B, B | B, B, B | E, E, E |
| Sanchez et al. [131] | Mexico | Caesalpinia coriaria | B | N |
| Sena et al. [132] | Brazil | Passiflora edulis (n = 3) | CT, CT, CT | N, N, N |
| Sharifi and Chaji [133] | Iran | Punica granatum (n = 3) | B, B, B | N, N, N |
| SoltaniNezhad et al. [134] | Iran | Pistacia vera (n = 3) | B, B, B | N, N, N |
| Sun et al. [135] | China | Sorghum bicolor (n = 3) | CT, CT, CT | N, N, N |
| Valenti et al. [17] | Italy | AM, QU, CS | CT, HT, HT | E, E, E |
| Wang et al. [22] | Ireland | Corylus avellana (n = 2) | B, B | N, N |
| Yisehak et al. [136] | Ethiopia | Albizia gummifera | CT | N |
| Zhao et al. [137] | China | No reported | B, B | E, E |
| Zhong et al. [19] | China | Sorghum bicolor (n = 3) | CT, CT, CT | N, N, N |
References
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Mund, M.D.; Khan, U.H.; Tahir, U.; Mustafa, B.E.; Fayyaz, A. Antimicrobial drug residues in poultry products and implications on public health: A review. Int. J. Food Prop. 2017, 20, 1433–1446. [Google Scholar] [CrossRef]
- Wang, H.; Ren, L.; Yu, X.; Hu, J.; Chen, Y.; He, G.; Jiang, Q. Antibiotic residues in meat, milk and aquatic products in Shanghai and human exposure assessment. Food Control 2017, 80, 217–225. [Google Scholar] [CrossRef]
- Callaway, T.R.; Lillehoj, H.; Chuanchuen, R.; Gay, C.G. Alternatives to Antibiotics: A Symposium on the Challenges and Solutions for Animal Health and Production. Antibiotics 2021, 10, 471. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef] [Green Version]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Jerónimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Sales-Baptista, E.; Lopes, O.; Capela e Silva, F. Tannins in ruminant nutrition: Impact on animal performance and quality of edible products. In Tannins: Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2016; pp. 121–168. [Google Scholar]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Pimentel, P.R.S.; Pelelgrini, C.B.; Lanna, D.P.D.; Brant, L.M.S.; Ribeiro, C.V.D.M.; Silva, T.M.; Barbosa, A.M.; da Silva, J.J.M.; Bezerra, L.R.; Oliveira, R.L. Effects of Acacia mearnsii extract as a condensed-tannin source on animal performance, carcass yield and meat quality in goats. Anim. Feed Sci. Technol. 2021, 271, 114733. [Google Scholar] [CrossRef]
- Pathak, A.K.; Dutta, N.; Pattanaik, A.K.; Chaturvedi, V.B.; Sharma, K. Effect of condensed tannins from Ficus infectoria and Psidium guajava leaf meal mixture on nutrient metabolism, methane emission and performance of lambs. Asian-Australas. J. Anim. Sci. 2017, 30, 1702–1710. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.; Pereira, F.J.; Menezes, D.; Caldas, A.C.; Cavalcante, I.; Oliveira, J.; Silva, J.J.; Cézar, M.; Bezerra, L. Carcass and meat quality in lambs receiving natural tannins from Mimosa tenuiflora hay. Small Rumin. Res. 2021, 198, 106362. [Google Scholar] [CrossRef]
- Rojas-Román, L.; Castro-Pérez, B.; Estrada-Angulo, A.; Angulo-Montoya, C.; Yocupicio-Rocha, J.; López-Soto, M.; Barreras, A.; Zinn, R.A.; Plascencia, A. Influence of long-term supplementation of tannins on growth performance, dietary net energy and carcass characteristics: Finishing lambs. Small Rumin. Res. 2017, 153, 137–141. [Google Scholar] [CrossRef]
- Priolo, A.; Valenti, B.; Natalello, A.; Bella, M.; Luciano, G.; Pauselli, M. Fatty acid metabolism in lambs fed hazelnut skin as a partial replacer of maize. Anim. Feed Sci. Technol. 2021, 272, 114794. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of different dietary tannin extracts on lamb growth performances and meat oxidative stability: Comparison between mimosa, chestnut and tara. Animal 2019, 13, 435–443. [Google Scholar] [CrossRef]
- Natalello, A.; Priolo, A.; Valenti, B.; Codini, M.; Mattioli, S.; Pauselli, M.; Puccio, M.; Lanza, M.; Stergiadis, S.; Luciano, G. Dietary pomegranate by-product improves oxidative stability of lamb meat. Meat Sci. 2020, 162, 108037. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Fang, Y.; Wang, Y.; Sun, H.; Zhou, D. Effects of substituting finely ground sorghum for finely ground corn on feed digestion and meat quality in lambs infected with Haemonchus contortus. Anim. Feed Sci. Technol. 2015, 211, 31–40. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Paulos, K.; Francisco, A.; Belo, A.T.; Jerónimo, E.; Almeida, J.; Bessa, R.J.B.; Santos-Silva, J. Effect of soybean meal treatment with Cistus ladanifer condensed tannins in growth performance, carcass and meat quality of lambs. Livest. Sci. 2020, 236, 104021. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Lv, M.; Zhao, J.; Xiong, B. Effects of chestnut tannins on the meat quality, welfare, and antioxidant status of heat-stressed lambs. Meat Sci. 2016, 116, 236–242. [Google Scholar] [CrossRef]
- Wang, S.; Giller, K.; Hillmann, E.; Marquardt, S.; Schwarm, A. Effect of supplementation of pelleted hazel (Corylus avellana) leaves on blood antioxidant activity, cellular immune response and heart beat parameters in sheep. J. Anim. Sci. 2019, 97, 4496–4502. [Google Scholar] [CrossRef] [PubMed]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: A review of the effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef] [PubMed]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Sauvant, D.; Schmidely, P.; Daudin, J.J.; St-Pierre, N.R. Meta-analyses of experimental data in animal nutrition. Animal 2008, 2, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons: Chichester, UK, 2009; p. 413. [Google Scholar]
- Doré, T.; Makowski, D.; Malézieux, E.; Munier-Jolain, N.; Tchamitchian, M.; Tittonell, P. Facing up to the paradigm of ecological intensification in agronomy: Revisiting methods, concepts and knowledge. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Abhijith, A.; Dunshea, F.R.; Warner, R.D.; Leury, B.J.; Ha, M.; Chauhan, S.S. A Meta-Analysis of the Effectiveness of High, Medium, and Low Voltage Electrical Stimulation on the Meat Quality of Small Ruminants. Foods 2020, 9, 1587. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herremans, S.; Vanwindekens, F.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Effect of dietary tannins on milk yield and composition, nitrogen partitioning and nitrogen use efficiency of lactating dairy cows: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1209–1218. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Hernández-García, P.A. Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis. Sustainability 2021, 13, 7410. [Google Scholar] [CrossRef]
- Lean, I.J.; Thompson, J.M.; Dunshea, F.R. A Meta-Analysis of Zilpaterol and Ractopamine Effects on Feedlot Performance, Carcass Traits and Shear Strength of Meat in Cattle. PLoS ONE 2014, 9, e115904. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A Meta-analysis Describing the Effects of the Essential oils Blend Agolin Ruminant on Performance, Rumen Fermentation and Methane Emissions in Dairy Cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons Ltd: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, open-source software for metaanalysis in ecology and evolutionary biology. Methods Ecol. Evol. 2016, 8, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Invited review: Use of meta-analysis in animal health and reproduction: Methods and applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS (Statistical Analysis System). SAS/STAT User’s Guide (Release 6.4); SAS Inst.: Cary, NC, USA, 2017. [Google Scholar]
- Torres, R.N.S.; Moura, D.C.; Ghedini, C.P.; Ezequiel, J.M.B.; Almeida, M.T.C. Meta-analysis of the effects of essential oils on ruminal fermentation and performance of sheep. Small Rumin. Res. 2020, 189, 106148. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care, 2nd ed.; MBJ Publishing Group: London, UK, 2001; pp. 109–121. [Google Scholar]
- Littell, J.H.; Corcoran, J.; Pillai, V. Systematic Reviews and Meta-Analysis, 1st ed.; Oxford University Press: Oxford, UK, 2008; pp. 111–132. [Google Scholar]
- Sterne, J.A.C.; Harbord, R.M. Funnel plots in meta-analysis. Stata J. 2004, 4, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.A.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ 2007, 176, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Amer. Statist. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Toldrá, F. Lawrie’s Meat Science, 8th ed.; Woodhead Publishing Limited: Cambridge, UK, 2017; 713p. [Google Scholar]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.; Xu, Z.; Lin, S. Dietary supplementation of tannic acid modulates nitrogen excretion pattern and urinary nitrogenous constituents of beef cattle. Livest. Sci. 2016, 191, 148–152. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Dong, S.; Li, H.; Gasco, L.; Xiong, Y.; Guo, K.J.; Zoccarato, I. Antioxidative activity of the polyphenols from the involucres of Castanea mollissima Blume and their mitigating effects on heat stress. Poult. Sci. 2015, 94, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramirez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [Green Version]
- Glendinning, J.I. Is the bitter rejection response always adaptive? Physiol. Behav. 1994, 56, 1217–1227. [Google Scholar] [CrossRef]
- Lamy, E.; Da Costa, G.; Santos, R.; Capela e Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Baptista, E.S. Effect of condensed tannin ingestion in sheep and goat parotid saliva proteome. J. Anim. Physiol. Anim. Nutr. 2011, 95, 304–312. [Google Scholar] [CrossRef]
- Lamy, E.; Rodrigues, L.; Guerreiro, O.; Soldado, D.; Francisco, A.; Lima, M.; Silva, F.C.E.; Lopes, O.; Santos-Silva, J.; Jerónimo, E. Changes in salivary protein composition of lambs supplemented with aerial parts and condensed tannins: Extract from Cistus ladanifer L.-A preliminary study. Agrofor. Syst. 2020, 94, 1501–1559. [Google Scholar] [CrossRef]
- Austin, P.J.; Suchar, L.A.; Robbins, C.T.; Hagerman, A.E. Tannin-binding proteins in saliva of deer and their absence in saliva of sheep and cattle. J. Chem. Ecol. 1989, 15, 1335–1347. [Google Scholar] [CrossRef]
- McArthur, C.; Sanson, G.D.; Beal, A.M. Salivary proline-rich proteins in mammals—Roles in oral homeostasis and counteracting dietary tannin. J. Chem. Ecol. 1995, 21, 663–691. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Rawel, H.; Schweigert, F.J.; Capela e Silva, F.; Ferreira, A.; Costa, A.R.; Antunes, C.; Almeida, A.M.; Coelho, A.V.; Sales-Baptista, E. The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity. Molecules 2011, 16, 2766–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corazzin, M.; Del Bianco, S.; Bovolenta, S.; Piasentier, E. Carcass Characteristics and Meat Quality of Sheep and Goat. In More than Beef, Pork and Chicken-The Production, Processing, and Quality Traits of Other Sources of Meat for Human Diet; Lorenzo, J.M., Munekata, P.E.S., Barba, F., Toldrá, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 119–165. ISBN 978-3-030-05483-0. [Google Scholar]
- Liang, H.; Xu, L.; Zhao, X.; Pan, K.; Yi, Z.; Bai, J.; Qi, X.; Xin, J.; Li, M.; Ouyang, K.; et al. RNA-Seq analysis reveals the potential molecular mechanisms of daidzein on adipogenesis in subcutaneous adipose tissue of finishing Xianan beef cattle. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, T.; Cao, Y.; Deng, B.; Zhang, J.; Zhao, J. Effects of dietary sea buckthorn pomace supplementation on skeletal muscle mass and meat quality in lambs. Meat Sci. 2020, 166, 108141. [Google Scholar] [CrossRef]
- Biondi, L.; Rabdazzo, C.L.; Russo, N.; Pino, A.; Natalello, A.; Van Hoorde, K.; Caggia, C. Dietary Supplementation of Tannin-Extracts to Lambs: Effects on Meat Fatty Acids Composition and Stability and on Microbial Characteristics. Foods 2019, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, R.; Barone, C.M.A.; Claps, S.; Varricchio, E.; Rufrano, D.; Caroprese, M.; Albenzio, M.; De Palo, P.; Campanile, G.; Neglia, G. Effects of dietary supplementation with polyphenols on meat quality in Saanen goat kids. BMC Vet. Res. 2018, 14, 181. [Google Scholar] [CrossRef]
- Calnan, H.; Jacob, R.; Pethick, D.; Gardner, G. Factors affecting the colour of lamb meat from the longissimus muscle during display: The influence of muscle weight and muscle oxidative capacity. Meat Sci. 2014, 96, 1049–1057. [Google Scholar] [CrossRef]
- Komprda, T.; Kuchtík, J.; Jarošová, A.; Dračková, E.; Zemánek, L.; Filipčík, R. Meat quality characteristics of lambs of three organically raised breeds. Meat Sci. 2012, 91, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Jimenez-Badillo, M.R.; Rodriguez, S. Effect of sex and carcass weight on carcass traits and meat quality in goat kids of cabrito transmontano. Span. J. Agric. Res. 2011, 9, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Fruet, A.P.B.; Giotto, F.M.; Fonseca, M.A.; Nörnberg, J.L.; de Mello, A.S. Effects of the incorporation of tannin extract from quebracho colorado wood on color parameters, lipid oxidation, and sensory attributes of beef patties. Foods 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Alvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.O.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; GarciaOliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Malheiros, J.M.; Braga, C.P.; Grove, R.A.; Ribeiro, F.A.; Calkins, C.R.; Adamec, J.; Chardulo, L.A.L. Influence of oxidative damage to proteins on meat tenderness using a proteomics approach. Meat Sci. 2019, 148, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.Z.; Zhou, D.W.; Tan, C.Y.; Tan, Z.L.; Han, X.F.; Zhou, C.S.; Tang, S.X. Effect of tea catechins on regulation of antioxidant enzyme expression in H2O2-induced skeletal muscle cells of goat in vitro. J. Agric. Food Chem. 2011, 59, 11338–11343. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Giannetto, C.; Bazzano, M.; Giudice, E.; Fazio, F. Oxidative stress associated with road transportation in ewes. Small Rumin. Res. 2013, 112, 235–238. [Google Scholar] [CrossRef]
- Ablikim, B.; Liu, Y.; Kerim, A.; Shen, P.; Abdurerim, P.; Zhou, G.H. Effects of breed, muscle type, and frozen storage on physico-chemical characteristics of lamb meat and its relationship with tenderness. CyTA J. Food 2016, 14, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Q.; Liu, G.; Tan, X.; Zhang, X.; Liu, X.; Wei, C. Gallic acid as a key substance to inhibit proliferation and adipogenesis in bovine subcutaneous adipocyte. Anim. Biotechnol. 2020, 1–7. [Google Scholar] [CrossRef]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Liu, X.; Kim, J.K.; Li, Y.; Li, J.; Liu, F.; Chen, X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J. Nutr. 2005, 135, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Barlocco, N.; Vadell, A.; Ballesteros, F.; Galietta, G.; Cozzolino, D. Predicting intramuscular fat, moisture and Warner-Bratzler shear force in pork muscle using near infrared reflectance spectroscopy. Anim. Sci. 2006, 82, 111–116. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, D.; Li, K. Effects of chestnut tannins on performance and antioxidative status of transition dairy cows. J. Dairy Sci. 2013, 96, 5901–5907. [Google Scholar] [CrossRef] [Green Version]
- Chivandi, E.; Dangarembizi, R.; Nyakudya, T.T.; Erlwanger, K.H. Use of Essential Oils as a Preservative of Meat. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and Antioxidant Capacity of Plant Extracts Rich in Polyphenols, given as a Single Acute Dose, in Sheep Made Highly Susceptible to Lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Celi, P. Oxidative stress in ruminants. In Studies on Veterinary Medicine. Oxidative Stress in Applied Basic Research and Clinical Practice; Mandelker, L., Vajdovich, P., Eds.; Humana Press: Totowa, NJ, USA; New York, NY, USA, 2011; pp. 191–231. ISBN 978-1-61779-070-6. [Google Scholar]
- Cuervo, W.; Sordillo, L.M.; Abuelo, A. Oxidative Stress Compromises Lymphocyte Function in Neonatal Dairy Calves. Antioxidants 2021, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; Mcallister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.L.; Wang, S.X.; Wang, Y.X. Effect of purple prairie clover (Dalea purpurea vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Awawdeh, M.S.; Dager, H.K.; Obeidat, B.S. Effects of alternative feedstuffs on growth performance, carcass characteristics, and meat quality of growing Awassi lambs. Ital. J. Anim. Sci. 2019, 18, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, P.A.V.; Pereira Filho, J.M.; Silva, A.M.A.; Cezar, M.F.; Bakke, A.O.; Silva, U.L.; Borburema, J.B.; Bezerra, L.R. Performance and carcass characteristics of lambs fed diets with increasing levels of Mimosa tenuiflora (Willd.) hay replacing Buffel grass hay. Trop. Anim. Health Prod. 2017, 49, 1001–1007. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Sahoo, A.; Sarkar, S.; Soni, L.; Sharma, P.; Gadekar, Y.P. Dietary supplementation of plant bioactive-enriched aniseed straw and eucalyptus leaves modulates tissue fatty acid profile and nuggets quality of lambs. Animal 2020, 14, 2642–2651. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Miceli, G.; Di Grigoli, A.; Frenda, A.S.; Tornambè, G.; Giambalvo, D.; Amato, G. Effects of feeding green forage of sulla (Hedysarum coronarium L.) on lamb growth, gastrointestinal nematode infection, and carcass and meat quality. Animal 2011, 5, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Buccioni, A.; Pauselli, M.; Minieri, S.; Roscini, V.; Mannelli, F.; Rapaccini, S.; Lupi, P.; Conte, G.; Serra, A.; Cappucci, A. Chestnut or quebracho tannins in the diet of grazing ewes supplemented with soybean oil: Effects on animal performances, blood parameters and fatty acid composition of plasma and milk lipids. Small Rumin. Res. 2017, 153, 23–30. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Moelich, E.; Gouws, P.; Muchenje, V.; Nolte, J.V.E.; Dugan, M.E.R.; Mapiye, C. Effects of feeding increasing levels of grape (Vitis vinifera cv. Pinotage) pomace on lamb shelf-life and eating quality. Meat Sci. 2019, 157, 107887. [Google Scholar] [CrossRef] [PubMed]
- Chikwanha, O.C.; Muchenje, V.; Nolte, J.E.; Dugan, M.E.R.; Mapiye, C. Grape pomace (Vitis vinifera L. cv. Pinotage) supplementation in lamb diets: Effects on growth performance, carcass and meat quality. Meat Sci. 2019, 147, 6–12. [Google Scholar] [CrossRef]
- Costa, E.d.S.; Ribiero, C.; Silva, T.; Ribeiro, R.; Vieira, J.; Lima, A.d.O.; Barbosa, A.; da Silva, J.J.M.; Bezerra, L.R.; Oliveira, R.L. Intake, nutrient digestibility, nitrogen balance, serum metabolites and growth performance of lambs supplemented with Acacia mearnsii condensed tannin extract. Anim. Feed Sci. Technol. 2021, 272, 114744. [Google Scholar] [CrossRef]
- Dey, A.; Dutta, N.; Pattanaik, A.K.; Sharma, K. Antioxidant status, metabolic profile and immune response of lambs supplemented with tannin rich Ficus infectoria leaf meal. Jpn. J. Vet. Res. 2015, 63, 15–24. [Google Scholar] [CrossRef]
- Abdalla Filho, A.L.; Corrêa, P.S.; Lemos, L.N.; Dineshkumar, D.; Issakowicz, J.; Ieda, E.H.; Lima, P.M.T.; Barreal, M.; McManus, C.; Mui, T.S.; et al. Diets based on plants from Brazilian Caatinga altering ruminal parameters, microbial community and meat fatty acids of Santa Inês lambs. Small Rumin. Res. 2017, 154, 70–77. [Google Scholar] [CrossRef]
- Francisco, A.; Alves, S.P.; Portugal, P.V.; Dentinho, M.T.; Jerónimo, E.; Sengo, S.; Almeida, J.; Bressan, M.C.; Pires, V.M.R.; Alfaia, C.M.; et al. Effects of dietary inclusion of citrus pulp and rockrose soft stems and leaves on meat quality and fatty acid composition. Animal 2017, 12, 872–881. [Google Scholar] [CrossRef]
- Girard, M.; Dohme-Meier, F.; Silacci, P.; Ampuero Kragten, S.; Kreuzer, M.; Bee, G. Forage legumes rich in condensed tannins may increase n-3 fatty acid levels and sensory quality of lamb meat. J. Sci. Food Agric. 2016, 96, 1923–1933. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Soldado, D.; Cachucho, L.; Almeida, J.M.; Francisco, A.; Jerónimo, E. Inclusion of the aerial part and condensed tannin extract from Cistus ladanifer L. in lamb diets-Effects on growth performance, carcass and meat quality and fatty acid composition of intramuscular and subcutaneous fat. Meat Sci. 2020, 160, 107945. [Google Scholar] [CrossRef]
- Gruffat, D.; Durand, D.; Rivaroli, D.; do Prado, I.N.; Prache, S. Comparison of muscle fatty acid composition and lipid stability in lambs stall-fed or pasture-fed alfalfa with or without sainfoin pellet supplementation. Animal 2020, 14, 1093–1101. [Google Scholar] [CrossRef]
- Hart, K.J.; Sinclair, L.A.; Wilkinson, R.G.; Huntington, J.A. Effect of Whole-Crop Pea (Pisum Sativum L.) Silages Differing in Condensed Tannin Content as a Substitute for Grass Silage and Soybean Meal on the Performance, Metabolism, and Carcass Characteristics of Lambs. J. Anim. Sci. 2011, 89, 3663–3676. [Google Scholar] [CrossRef] [Green Version]
- Hassan, T.M.M.; Ahmed-Farid, O.A.; Abdel-Fattah, F.A.I. Effects of different sources and levels of tannins on live performance and antioxidant response of Ossimi lambs. J. Agric. Sci. 2020, 158, 339–348. [Google Scholar] [CrossRef]
- Hatami, A.; Alipour, D.; Hozhabri, F.; Tabatabaei, M. Effect of different levels of pomegranate marc with or without polyethylene glycol on performance, nutrients digestibility and protozoal population in growing lambs. Anim. Feed Sci. Tech. 2018, 235, 15–22. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alves, S.P.; Dentinho, M.T.P.; Martins, S.V.; Prates, J.A.M.; Vasta, V.; Santos-Silva, J.; Bessa, R.J.B. Effect of grape seed extract, Cistus ladanifer L. and vegetable oil supplementation on fatty acid composition of abomasal digesta and intramuscular fat of lambs. J. Agric. Food Chem. 2010, 58, 10710–10721. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, E.; Alfaia, C.M.M.; Alves, S.P.; Dentinho, M.T.P.; Prates, J.A.M.; Vasta, V.; Santos-Silva, J.; Bessa, R.J.B. Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.E.M.; Al-Dobaib, S.N.; Salem, A.Z.; Lopez, S.; Alaba, P.A. Influence of dietary supplementation with sunflower oil and quebracho tannins on growth performance and meat fatty acid profile of Awassi lambs. Anim. Feed Sci. Technol. 2018, 235, 97–104. [Google Scholar] [CrossRef]
- Kazemi, M.; Mokhtarpour, A. In vitro and in vivo evaluation of some tree leaves as forage sources in the diet of Baluchi male lambs. Small Rumin. Res. 2021, 201, 106416. [Google Scholar] [CrossRef]
- Leparmarai, P.T.; Sinz, S.; Kunz, C.; Liesegang, A.; Ortmann, S.; Kreuzer, M.; Marquardt, S. Transfer of total phenols from a grapeseed-supplemented diet to dairy sheep and goat milk, and effects on performance and milk quality. J. Anim. Sci. 2019, 97, 1840–1851. [Google Scholar] [CrossRef]
- Lima, P.M.T.; Filho, A.L.A.; Issakowicz, J.; Ieda, E.H.; Corrêa, P.S.; Mattos, W.T.; Gerdes, L.; McManus, C.; Abdalla, A.L.; Louvandini, H. Methane emission, ruminal fermentation parameters and fatty acid profile of meat in Santa Inês lambs fed the legume macrotiloma. Anim. Prod. Sci. 2020, 60, 665–673. [Google Scholar] [CrossRef]
- Majewska, M.P.; Kowalik, B. Growth Performance, Carcass Characteristics, Fatty Acid Composition, and Blood Biochemical Parameters of Lamb Fed Diet with the Addition of Lingonberry Leaves and Oak Bark. Eur. J. Lipid Sci. Technol. 2020, 122, 1900273. [Google Scholar] [CrossRef]
- Flores, D.R.M.; da Fonseca, P.A.F.; Schmitt, J.; Tonetto, C.J.; Junior, A.G.R.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nörnberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on productive performance, carcass characteristics, and blood parameters. Livest. Sci. 2020, 240, 104169. [Google Scholar] [CrossRef]
- Flores, D.R.M.; da Fonseca, P.A.F.; Schmitt, J.; Tonetto, C.J.; Junior, A.G.R.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nörnberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on meat quality. Small Rumin. Res. 2021, 195, 106234. [Google Scholar] [CrossRef]
- Moghaddam, V.K.; Elahi, M.Y.; Nasri, M.H.F.; Elghandour, M.M.M.Y.; Monroy, J.C.; Salem, A.Z.M.; Karami, M.; Mlambo, V. Growth performance and carcass characteristics of finishing male lambs fed barberry pomace-containing diets. Anim. Biotechnol. 2019, 32, 178–184. [Google Scholar] [CrossRef]
- Nobre, P.T.; Munekata, P.E.; Costa, R.G.; Carvalho, F.R.; Ribeiro, N.L.; Queiroga, R.C.; Sousa, S.; Silva, A.C.R.; Lorenzo, J.M. The impact of dietary supplementation with guava (Psidium guajava L.) agroindustrial waste on growth performance and meat quality of lambs. Meat Sci. 2020, 164, 108105. [Google Scholar] [CrossRef] [PubMed]
- Norouzian, M.A.; Ghiasi, S.E. Carcass performance and mineral content in Balouchi lamb fed pistachio byproduct. Meat Sci. 2012, 92, 157–159. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Alrababah, M.A.; Abdullah, A.Y.; Alhamad, M.N.; Gharaibeh, M.A.; Rababah, T.M.; Ishmais, M.A. Growth performance and carcass characteristics of Awassi lambs fed diets containing carob pods (Ceratonia siliqua L.). Small Rumin. Res. 2011, 96, 149–154. [Google Scholar] [CrossRef]
- Odhaib, K.J.; Adeyemi, K.D.; Sazili, A.Q. Carcass traits, fatty acid composition, gene expression, oxidative stability and quality attributes of different muscles in Dorper lambs fed Nigella sativa seeds, Rosmarinus officinalis leaves and their combination. Asian-Australas. J. Anim. Sci. 2018, 31, 1345–1357. [Google Scholar] [CrossRef]
- Po, E.; Horsburgh, K.; Raadsma, H.W.; Celi, P. Yerba Mate (Ilex paraguarensis) as a novel feed supplement for growing lambs. Small Rumin. Res. 2012, 106, 131–136. [Google Scholar] [CrossRef]
- Rajabi, M.; Rouzbehan, Y.; Rezaei, J. A strategy to improve nitrogen utilization, reduce environmental impact, and increase performance and antioxidant capacity of fattening lambs using pomegranate peel extract. J. Anim. Sci. 2017, 95, 499–510. [Google Scholar] [CrossRef]
- Sánchez, N.; Mendoza, G.D.; Martínez, J.A.; Hernández, P.A.; Camacho, L.M.; Lee-Rangel, H.A.; Vázquez, A.; Flores, R. Effect of Caesalpinia coriaria fruits and soybean oil on finishing lamb performance and meat characteristics. Biomed Res. Int. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sena, J.A.B.; Villela, S.D.J.; Santos, R.A.; Pereira, I.G.; Castro, G.H.F.; Mourthe, M.H.F.; Bonfá, C.S.; Martins, P.G.M.A. Intake, digestibility, performance, and carcass traits of rams provided with dehydrated passion fruit (Passiflora edulis f. flavicarpa) peel, as a substitute of Tifton 85 (Cynodon spp.). Small Rumin. Res. 2015, 129, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, A.; Chaji, M. Effects of processed recycled poultry bedding with tannins extracted from pomegranate peel on the nutrient digestibility and growth performance of lambs. S. Afr. J. Anim. Sci. 2019, 49, 290–300. [Google Scholar] [CrossRef]
- SoltaniNezhad, B.; Dayani, O.; Khezri, A.; Tahmasbi, R. Performance and carcass characteristics in fattening lambs fed diets with different levels of pistachio byproducts silage with wasted date. Small Rumin. Res. 2016, 137, 177–182. [Google Scholar] [CrossRef]
- Sun, H.X.; Gao, T.S.; Zhong, R.Z.; Fang, Y.; Di, G.L.; Zhou, D.W. Effects of corn replacement by sorghum in diets on performance, nutrient utilization, blood parameters, antioxidant status and meat color stability in lambs. Can. J. Anim. Sci. 2018, 98, 723–731. [Google Scholar] [CrossRef]
- Yisehak, K.; Biruk, K.; Abegaze, B.; Janssens, G.P.J. Growth of sheep fed tanninrich Albizia gummifera with or without polyethylene glycol. Trop. Anim. Health Prod. 2014, 46, 1113–1118. [Google Scholar] [CrossRef]
- Zhao, M.D.; Di, L.F.; Tang, Z.Y.; Jiang, W.; Li, C.Y. Effect of tannins and cellulase on growth performance, nutrients digestibility, blood profiles, intestinal morphology and carcass characteristics in Hu sheep. Asian-Australas. J. Anim. Sci. 2019, 32, 1540–1547. [Google Scholar] [CrossRef]

| Parameter | Mean | Median | Minimum | Maximum | SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Features | NC | Control | Tannin | Control | Tannin | Control | Tannin | Control | Tannin | Control | Tannin |
| Forage, g kg−1 DM | 122 | 428.5 | 432.2 | 400.0 | 400.0 | 0 | 0 | 1000 | 1000 | 265.6 | 261.3 |
| DM, g kg−1 | 100 | 874.8 | 871.7 | 900.0 | 903.4 | 160.0 | 156.0 | 953.9 | 947.5 | 122.8 | 130.8 |
| OM, g kg−1 DM | 46 | 857.5 b | 866.8 a | 910.0 | 922.0 | 148.0 | 146.0 | 957.2 | 984.5 | 193.7 | 188.7 |
| CP, g kg−1 DM | 124 | 156.3 | 156.9 | 157.0 | 156.5 | 84.0 | 89.0 | 251.0 | 255.0 | 26.9 | 26.5 |
| EE, g kg−1 DM | 106 | 35.5 b | 40.7 a | 29.3 | 36.3 | 13.0 | 12.8 | 81.0 | 98.3 | 16.6 | 18.3 |
| NDF, g kg−1 DM | 122 | 388.8 a | 375.9 b | 385.7 | 365.5 | 156.0 | 152.0 | 731.1 | 704.9 | 179.6 | 108.7 |
| ADF, g kg−1 DM | 94 | 211.2 | 212.1 | 190.0 | 179.5 | 81.0 | 69.8 | 516.0 | 496.5 | 94.1 | 94.2 |
| Ca, g kg−1 DM | 24 | 7.0 | 7.2 | 6.1 | 6.7 | 3.4 | 3.6 | 17.0 | 18.0 | 3.1 | 3.4 |
| P, g kg−1 DM | 22 | 4.0 | 4.0 | 4.4 | 4.4 | 2.0 | 2.0 | 5.8 | 6.2 | 1.2 | 1.3 |
| ME, MJ kg−1 DM | 56 | 10.6 | 10.6 | 10.5 | 10.7 | 9.2 | 8.8 | 12.5 | 12.6 | 0.8 | 0.9 |
| Tannin, g kg−1 DM | 135 | - | 20.2 | - | 15.5 | - | 0.02 | - | 132.0 | - | 20.6 |
| Duration, days | 135 | 70.0 | 70.0 | 28.0 | 180.0 | 30.0 | |||||
| 95% CI | Heterogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | N | NC | SMD | SE | Lower | Upper | p-Value | Q | p-Value | I2 (%) |
| Daily weight gain (DWG) | 42 | 104 | 0.274 | 0.116 | 0.046 | 0.501 | 0.018 | 472.57 | <0.001 | 78.20 |
| Dry matter intake (DMI) | 42 | 104 | 0.090 | 0.124 | −0.152 | 0.333 | 0.466 | 524.508 | <0.001 | 80.36 |
| Feed conversion ratio (FCR) | 27 | 60 | −0.308 | 0.127 | −0.556 | −0.060 | 0.015 | 197.05 | <0.001 | 70.06 |
| Hot carcass yield (HCY) | 26 | 59 | 0.234 | 0.108 | 0.023 | 0.445 | 0.030 | 142.03 | <0.001 | 59.16 |
| Cold carcass yield (CCY) | 9 | 23 | 0.510 | 0.228 | 0.063 | 0.957 | 0.025 | 86.09 | <0.001 | 74.45 |
| Backfat thickness (BFT) | 9 | 24 | 0.565 | 0.193 | 0.188 | 0.943 | 0.003 | 77.94 | <0.001 | 70.49 |
| Longissimus dorsi muscle area (LMA) | 10 | 22 | 0.413 | 0.170 | 0.080 | 0.747 | 0.015 | 52.40 | <0.001 | 59.92 |
| 95% CI | Heterogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | N | NC | SMD | SE | Lower | Upper | p-Value | Q | p-Value | I2 (%) |
| Meat pH | 19 | 52 | 0.037 | 0.098 | −0.156 | 0.230 | 0.706 | 89.29 | <0.001 | 42.88 |
| Lightness (L*) | 20 | 54 | 0.008 | 0.128 | −0.243 | 0.260 | 0.950 | 151.88 | <0.001 | 65.10 |
| Redness (a*) | 20 | 54 | 0.365 | 0.120 | 0.129 | 0.601 | 0.002 | 133.39 | <0.001 | 62.27 |
| Yellowness (b*) | 20 | 54 | 0.048 | 0.145 | −0.236 | 0.332 | 0.742 | 186.70 | <0.001 | 71.61 |
| WBSF | 15 | 42 | −0.027 | 0.093 | −0.210 | 0.155 | 0.769 | 53.74 | 0.088 | 23.71 |
| Drip loss (DL) | 4 | 17 | −2.828 | 0.516 | −3.839 | −1.817 | <0.001 | 149.57 | <0.001 | 89.30 |
| Cooking loss (CL) | 14 | 42 | 0.105 | 0.216 | −0.317 | 0.528 | 0.626 | 243.00 | <0.001 | 83.13 |
| Moisture | 5 | 16 | −0.693 | 0.333 | −1.345 | −0.041 | 0.037 | 77.25 | < 0.001 | 80.58 |
| Protein | 8 | 23 | 0.249 | 0.282 | −0.304 | 0.802 | 0.378 | 114.45 | <0.001 | 80.78 |
| Intramuscular fat (IMF) | 16 | 40 | −0.168 | 0.186 | −0.532 | 0.196 | 0.366 | 172.04 | <0.001 | 77.33 |
| Ash | 6 | 20 | 0.507 | 0.332 | −0.144 | 1.158 | 0.127 | 108.41 | <0.001 | 82.47 |
| Malondialdehyde (MDAc) | 10 | 29 | −2.020 | 0.326 | −2.659 | −1.380 | <0.001 | 195.96 | <0.001 | 85.65 |
| Metmyoglobin (MetMb) | 3 | 6 | −0.482 | 0.222 | −0.916 | −0.047 | 0.030 | 5.25 | 0.387 | 4.68 |
| 95% CI | Heterogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | N | NC | SMD | SE | Lower | Upper | p-Value | Q | p-Value | I2 (%) |
| Total antioxidant capacity (TAC) | 9 | 17 | 1.120 | 0.222 | 0.686 | 1.555 | <0.001 | 43.661 | <0.001 | 63.35 |
| Superoxide dismutase (SOD) | 6 | 14 | −0.122 | 0.328 | −0.766 | 0.521 | 0.709 | 61.306 | <0.001 | 78.79 |
| Catalase (CAT) | 5 | 12 | 0.848 | 0.239 | 0.380 | 1.315 | <0.001 | 22.963 | 0.018 | 52.10 |
| Glutathione peroxidase (GPx) | 3 | 6 | 0.801 | 0.209 | 0.392 | 1.211 | <0.001 | 2.267 | 0.811 | 0 |
| Malondialdehyde (MDAs) | 7 | 17 | −0.535 | 0.244 | −1.014 | -0.056 | 0.029 | 54.824 | <0.001 | 70.81 |
| Parameter | Tannins Dose | Supplementation Period | Lamb’s Age | Tannins Type | Tannins Source | Method of Inclusion | EED | NDFD | |
|---|---|---|---|---|---|---|---|---|---|
| DWG | QM | 6.943 | 8.263 | 0.378 | 1.070 | 43.329 | 5.786 | 1.092 | 0.240 |
| df | 1 | 1 | 1 | 2 | 32 | 1 | 1 | 1 | |
| p-Value | 0.008 | 0.004 | 0.989 | 0.586 | 0.087 | 0.016 | 0.296 | 0.624 | |
| R2 (%) | 0.54 | 2.85 | 0 | 0 | 0 | 1.17 | 0.92 | 0 | |
| DMI | QM | 4.800 | 10.206 | 0.927 | 2.503 | 113.649 | 0.892 | 0.033 | 2.752 |
| df | 1 | 1 | 1 | 2 | 32 | 1 | 1 | 1 | |
| p-Value | 0.028 | 0.001 | 0.336 | 0.286 | <0.001 | 0.345 | 0.856 | 0.097 | |
| R2 (%) | 3.02 | 2.49 | 0 | 0 | 14.37 | 0 | 0 | 0 | |
| FCR | QM | 7.711 | 3.716 | 5.348 | 9.193 | 48.362 | 0.129 | 0.006 | 0.335 |
| df | 1 | 1 | 1 | 2 | 22 | 1 | 1 | 1 | |
| p-Value | 0.005 | 0.050 | 0.021 | 0.010 | <0.001 | 0.720 | 0.940 | 0.563 | |
| R2 (%) | 8.84 | 3.35 | 6.57 | 13.11 | 29.30 | 0 | 0 | 0 | |
| HCY | QM | 7.401 | 2.618 | 1.168 | 0.017 | 65.118 | 0.226 | 3.348 | 4.094 |
| df | 1 | 1 | 1 | 2 | 22 | 1 | 1 | 1 | |
| p-Value | 0.007 | 0.106 | 0.280 | 0.992 | <0.001 | 0.634 | 0.067 | 0.043 | |
| R2 (%) | 14.48 | 4.97 | 1.39 | 0 | 68.77 | 0 | 7.78 | 6.27 | |
| LMA | QM | 0.004 | 5.968 | 13.647 | 0.100 | 36.514 | 0.625 | 2.658 | 2.251 |
| df | 1 | 1 | 1 | 1 | 6 | 1 | 1 | 1 | |
| p-Value | 0.947 | 0.015 | <0.001 | 0.751 | <0.001 | 0.429 | 0.103 | 0.134 | |
| R2 (%) | 0 | 26.30 | 66.18 | 0 | 97.92 | 0 | 8.86 | 8.61 | |
| Meat pH | QM | 0.453 | 1.354 | 0.076 | 17.572 | 68.102 | 6.236 | 0.852 | 0.016 |
| df | 1 | 1 | 1 | 2 | 18 | 1 | 1 | 1 | |
| p-Value | 0.501 | 0.245 | 0.783 | <0.001 | <0.001 | 0.013 | 0.356 | 0.899 | |
| R2 (%) | 0 | 2.62 | 0 | 54.54 | 100 | 23.06 | 0 | 0 | |
| L* | QM | 0.132 | 1.913 | 0.728 | 9.171 | 38.080 | 2.731 | 3.146 | 0.126 |
| df | 1 | 1 | 1 | 2 | 19 | 1 | 1 | 1 | |
| p-Value | 0.716 | 0.167 | 0.393 | 0.010 | 0.006 | 0.098 | 0.076 | 0.723 | |
| R2 (%) | 0 | 0 | 0 | 11.65 | 26.61 | 1.34 | 0 | 0 | |
| a* | QM | 0.003 | 13.834 | 16.603 | 4.698 | 29.143 | 0.435 | 12.199 | 10.968 |
| df | 1 | 1 | 1 | 2 | 19 | 1 | 1 | 1 | |
| p-Value | 0.956 | <0.001 | <0.001 | 0.095 | 0.064 | 0.509 | <0.001 | <0.001 | |
| R2 (%) | 0 | 18.51 | 28.53 | 2.86 | 13.73 | 0 | 19.28 | 21.86 | |
| b* | QM | 1.982 | 0.257 | 5.999 | 19.021 | 37.939 | 1.091 | 0.590 | 0.014 |
| df | 1 | 1 | 1 | 2 | 19 | 1 | 1 | 1 | |
| p-Value | 0.159 | 0.612 | 0.014 | <0.001 | 0.009 | 0.296 | 0.442 | 0.906 | |
| R2 (%) | 0 | 0 | 2.80 | 12.91 | 1.12 | 0 | 0 | 0 | |
| CL | QM | 4.339 | 0.121 | 0.471 | 4.947 | 52.306 | 2.199 | 0.121 | 0.036 |
| df | 1 | 1 | 1 | 2 | 14 | 1 | 1 | 1 | |
| p-Value | 0.037 | 0.728 | 0.492 | 0.084 | <0.001 | 0.138 | 0.728 | 0.849 | |
| R2 (%) | 1.26 | 0 | 0 | 8.34 | 16.48 | 3.11 | 0 | 0 | |
| IMF | QM | 3.967 | 0.419 | 7.676 | 0.866 | 80.997 | 2.191 | 1.462 | 3.435 |
| df | 1 | 1 | 1 | 2 | 18 | 1 | 1 | 1 | |
| p-Value | 0.047 | 0.517 | 0.006 | 0.649 | <0.001 | 0.139 | 0.227 | 0.064 | |
| R2 (%) | 9.56 | 0.38 | 14.55 | 0 | 54.24 | 0.33 | 1.96 | 2.56 | |
| MDAc | QM | 9.33 | 11.05 | 56.60 | 29.38 | 143.390 | 9.83 | 13.23 | 0.31 |
| df | 1 | 1 | 1 | 2 | 12 | 1 | 1 | 1 | |
| p-Value | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | 0.574 | |
| R2 (%) | 17.00 | 1.15 | 56.60 | 32.50 | 93.81 | 5.11 | 4.60 | 4.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Lee-Rangel, H.A. Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis. Animals 2021, 11, 3184. https://doi.org/10.3390/ani11113184
Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, Lee-Rangel HA. Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis. Animals. 2021; 11(11):3184. https://doi.org/10.3390/ani11113184
Chicago/Turabian StyleOrzuna-Orzuna, José Felipe, Griselda Dorantes-Iturbide, Alejandro Lara-Bueno, Germán David Mendoza-Martínez, Luis Alberto Miranda-Romero, and Héctor Aarón Lee-Rangel. 2021. "Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis" Animals 11, no. 11: 3184. https://doi.org/10.3390/ani11113184
APA StyleOrzuna-Orzuna, J. F., Dorantes-Iturbide, G., Lara-Bueno, A., Mendoza-Martínez, G. D., Miranda-Romero, L. A., & Lee-Rangel, H. A. (2021). Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis. Animals, 11(11), 3184. https://doi.org/10.3390/ani11113184






