The Uptake and Deconjugation of Androstenone Sulfate in the Adipose Tissue of the Boar
Abstract
:Simple Summary
Abstract
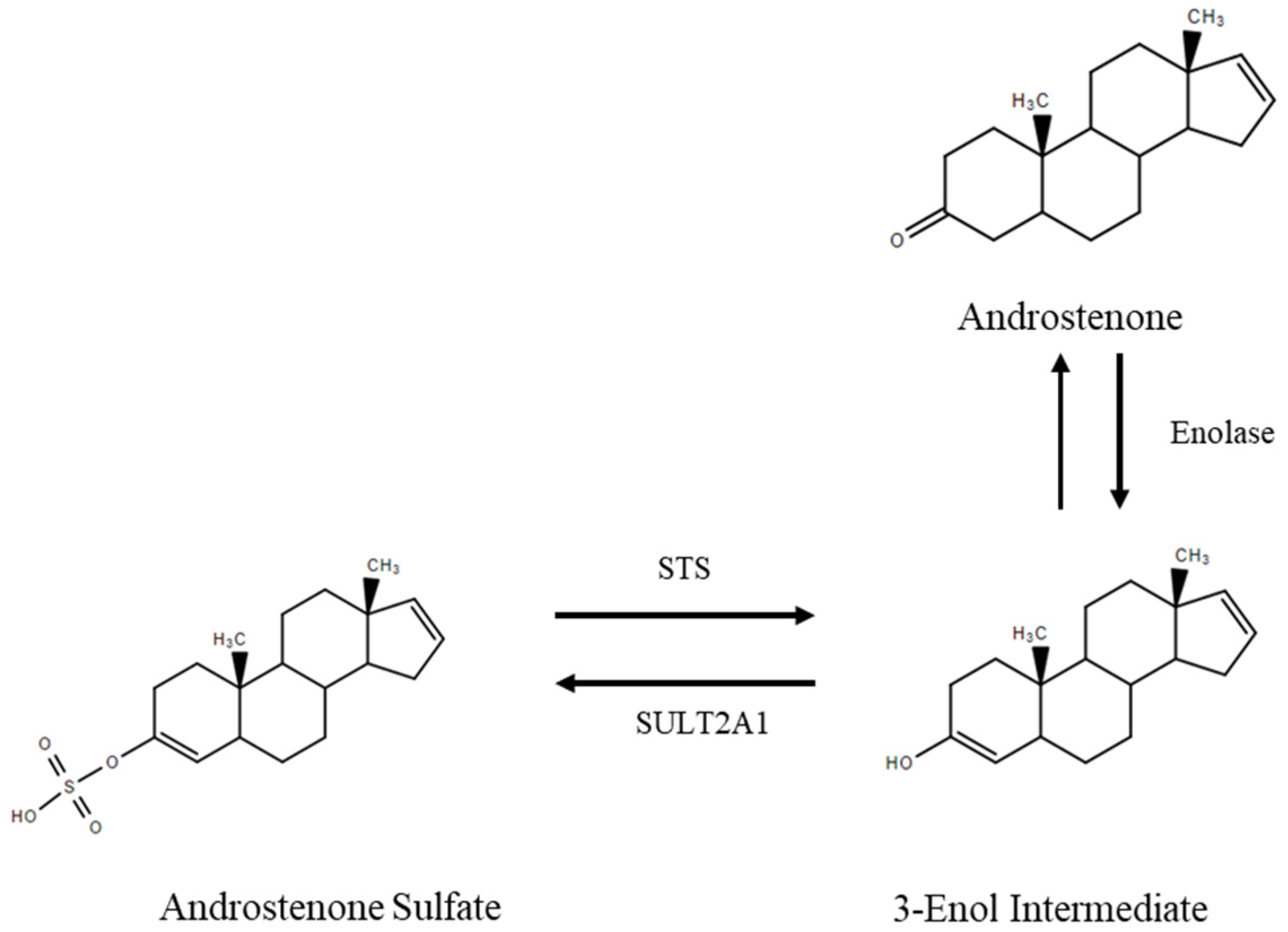
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Extraction and Gene Expression Analysis
2.3. Radiolabeled Androstenone Sulfate Synthesis and Purification
2.4. Porcine Adipocyte Isolation
2.5. Steroid Transport Studies using Mature Adipocytes
2.6. Sulfatase Assay
2.7. Statistical Analysis
3. Results
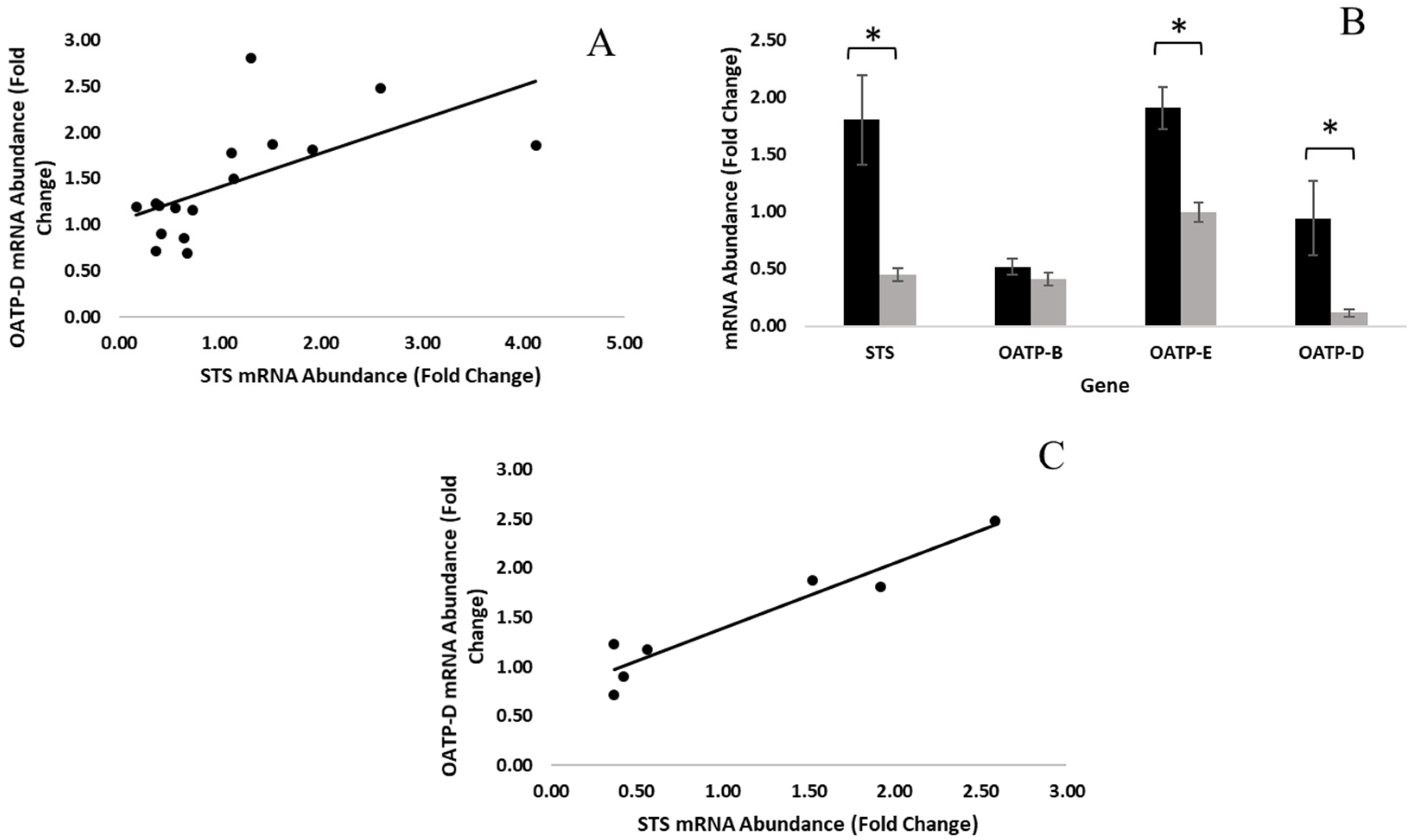
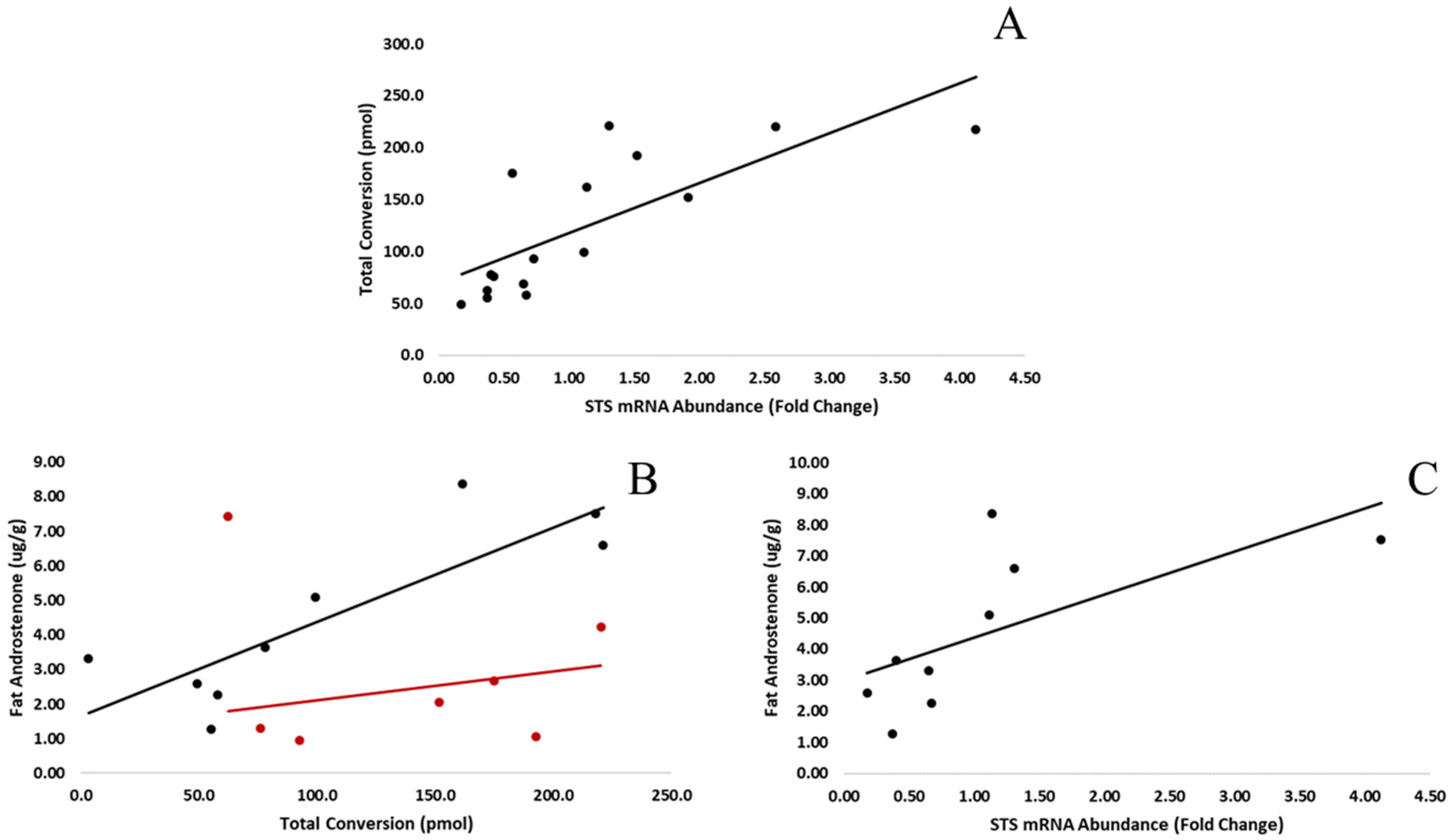
3.1. Gene Expression Differences
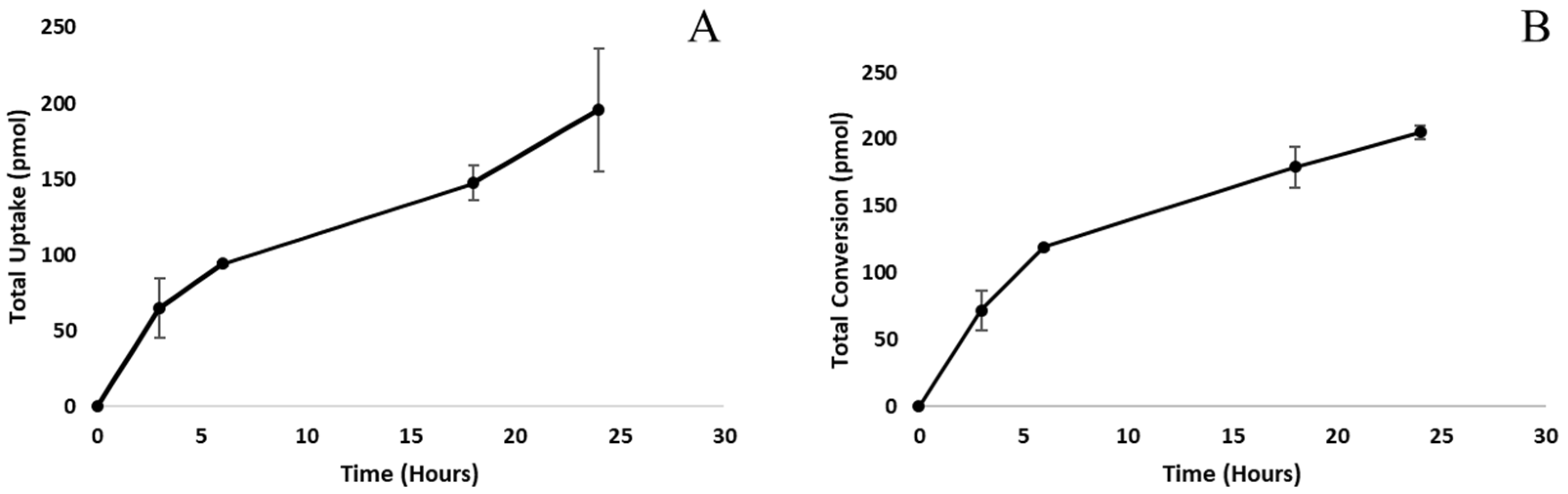
3.2. Time Course Analysis of Steroid Uptake and Conversion
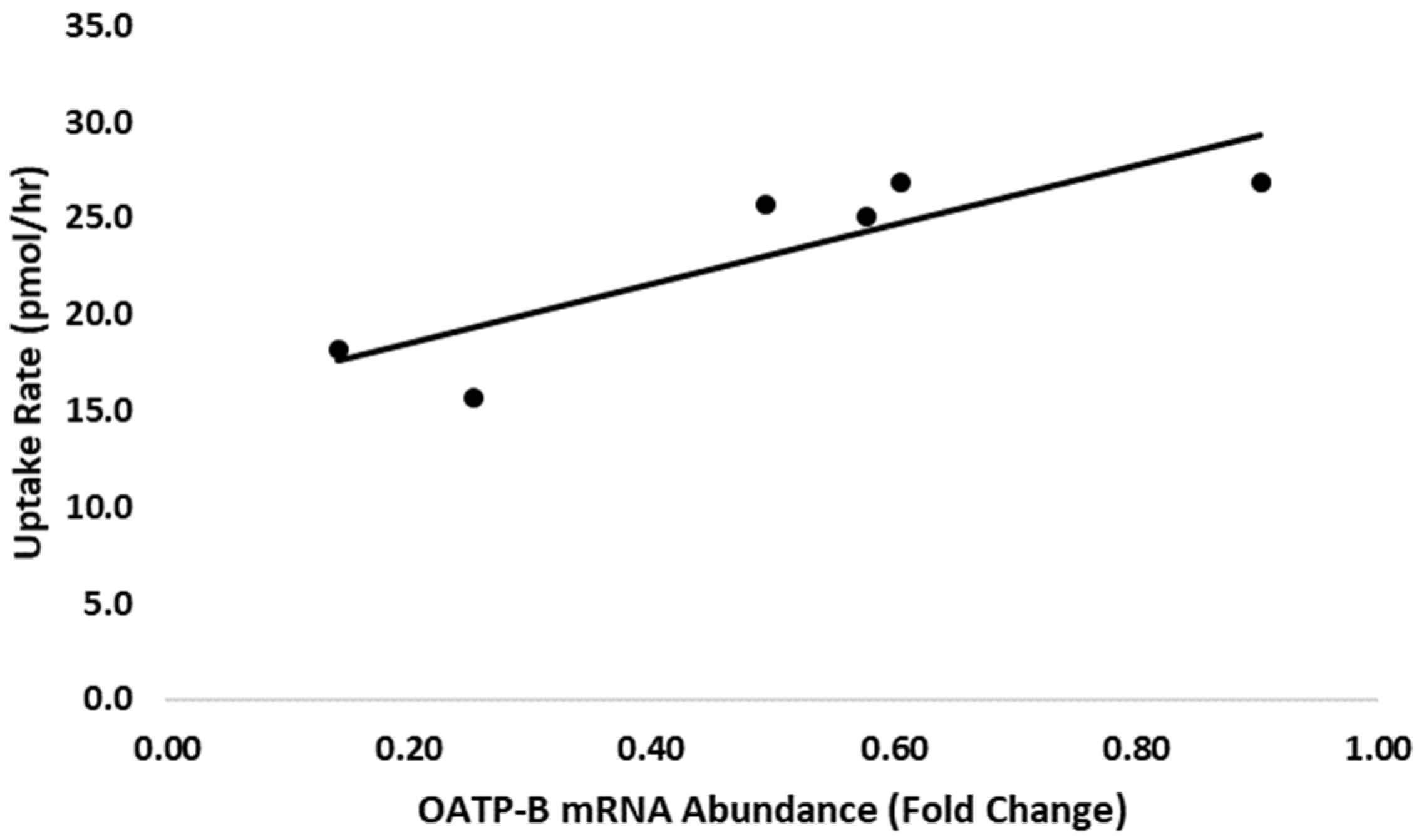
3.3. Uptake of Androstenone Sulfate by Adipocytes
3.4. Conversion of Androstenone Sulfate to Free Androstenone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bonneau, M. Use of entire male pig meat in the European Union. Meat Sci. 1998, 49, 257–272. [Google Scholar] [CrossRef]
- Laderoute, H.; Bone, C.; Squires, E.J. The sulfoconjugation of androstenone and dehydroepiandrosterone by human and porcine sulfotransferase enzymes. Steroids 2018, 136, 8–16. [Google Scholar] [CrossRef]
- Sinclair, P.A.; Squires, E.J. Testicular sulfoconjugation of the 16-androstene steroids by hydroxysteroid sulfotransferase: Its effect on the concentrations of 5α-androstenone in plasma and fat of the mature domestic boar. J. Anim. Sci. 2005, 83, 358–365. [Google Scholar] [CrossRef]
- Sinclair, P.A.; Squires, E.J.; Raeside, J.I.; Renaud, R. Synthesis of free and sulphoconjugated 16-androstene steroids by the Leydig cells of the mature domestic boar. J. Steroid Biochem. Mol. Biol. 2005, 96, 217–228. [Google Scholar] [CrossRef]
- Strott, C.A. Sulfonation and molecular action. Endocr. Rev. 2002, 23, 703–732. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Purohit, A.; Woo, L.W.L.; Newman, S.P.; Potter, B.V.L. Steroid sulfatase: Molecular biology, regulation and inhibition. Endocr. Rev. 2005, 26, 171–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalla Valle, L.; Toffolo, V.; Nardi, A.; Fiore, C.; Bernante, P.; Di Liddo, R.; Parnigotto, P.P.; Colombo, L. Tissue-specific transcriptional initiation and activity of steroid sulfatase complementing dehydroepiandrosterone sulfate uptake and intracrine steroid activations in human adipose tissue. J. Endocrinol. 2006, 190, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Hagenbuch, B.; Meier, P.J. The superfamily of organic anion transporting polypeptides. Biochem. Biophys. Acta 2003, 1609, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Laderoute, H.; Bone, C.; Brewer, D.; Squires, E.J. The synthesis of 16-androstene sulfoconjugates from primary porcine Leydig cell culture. Steroids 2019, 146, 14–20. [Google Scholar] [CrossRef]
- Bone, C.; Anderson, C.; Lou, Y.; Squires, E.J. The characterization of androstenone transport in boar plasma. J. Steroid Biochem. Mol. Biol. 2018, 185, 218–224. [Google Scholar] [CrossRef]
- Bone, C.; Squires, E.J. The binding of free and sulfated androstenone in the plasma of the boar. Animals 2021, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ruder, H.J.; Loriaux, L.; Lipsett, M.B. Estrone sulfate: Production rate and metabolism in man. J. Clin. Investig. 1972, 51, 1020–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeside, J.I.; Renaud, R.L. Estrogen and androgen production by purified Leydig cells of mature boars. Biol. Reprod. 1983, 28, 727–733. [Google Scholar] [CrossRef]
- Hansen-Møller, J. Rapid high-performance liquid chromatographic method for simultaneous determination of androstenone, skatole and indole in back fat from pigs. J. Chromatogr. B Biomed. Appl. 1994, 661, 219–230. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]. Accession No. DQ139314, Sus Scrofa Steroid Sulfatase (Sts) mRNA, Partial cds. Available online: https://www.ncbi.nlm.nih.gov/nuccore/DQ139314.1 (accessed on 25 July 2021).
- Mutembei, H.M.; Kowalewski, M.P.; Ugele, B.; Schuler, G.; Hoffmann, B. Expression and activity of steroid sulphatase in the boar testis. Reprod. Domest. Anim. 2009, 44, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]. Accession No. XM_003357928, Sus Scrofa Actin Gamma 1 (ACTG1) mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/XM_003357928 (accessed on 25 July 2021).
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]. Accession No. XM_021062511, PREDICTED: Sus Scrofa Solute Carrier Organic Anion Transporter Family Member 2B1 (SLCO2B1) mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/XM_021062511 (accessed on 25 July 2021).
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]. Accession No. XM_021078225, PREDICTED: Sus Scrofa Solute Carrier Organic Anion Transporter Family Member 4A1 (SLCO4A1) Transcript Variant X1, mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/XM_021078225 (accessed on 25 July 2021).
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]. Accession No. XM_005666296, Predicted: Sus Scrofa Solute Carrier Organic Anion Transporter Family Member 3A1 (SLCO3A1), Transcript Variant X1, mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/XM_005666296 (accessed on 25 July 2021).
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]. Accession No. NM_001256595, Sus Scrofa Solute Carrier Organic Anion Transporter Family Member 1A2 (SLCO1A2) mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NM_001256595 (accessed on 25 July 2021).
- Alexandersson, I.; Harms, M.J.; Boucher, J. Isolation and culture of human mature adipocytes using membrane mature aggregate cultures. J. Vis. Exp. 2020, 156. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Rydhmer, L.; Chen, G.; Madej, A.; Andersson, H.K.; Lundström, K. Boar taint is related to endocrine and anatomical changes at puberty but not to aggressive behavior in entire male pigs. Reprod. Dom. Anim. 2005, 40, 500–506. [Google Scholar] [CrossRef]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OAYs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeside, J.I.; Christie, H.L.; Renaud, R.L. Androgen and estrogen metabolism in the reproductive tract and accessory sex glands of the domestic boar (Sus scrofa). Biol. Reprod. 1999, 61, 1242–1248. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, P.A.; Squires, E.J.; Raeside, J.I. Early postnatal plasma concentrations of testicular steroid hormones, pubertal development, and carcass leanness as potential indicators of boar taint in market weight intact male pigs. J. Anim. Sci. 2001, 79, 1868–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzenberger, F.; Toole, G.S.; Christie, H.L.; Raeside, J.I. Plasma levels of several androgens and estrogens from birth to puberty in male domestic pigs. Acta Endocrinol. 1993, 128, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.A.; Gilmore, W.J.; Lin, Z.; Lou, Y.; Squires, E.J. Molecular cloning and regulation of porcine SULT2A1: Relationship between SULT2A1 expression and sulfoconjugation of androstenone. J. Mol. Endocrinol. 2006, 36, 301–311. [Google Scholar] [CrossRef]
- Squires, E.J.; Bone, C.; Cameron, J. Pork production with entire males: Directions for control of boar taint. Animals 2020, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
| Primer | Forward Sequence | Reverse Sequence | Tm (°C) | Reference |
|---|---|---|---|---|
| STS | 5′-GAAGACAGGATCATTGACG-3′ | 5′-AGAACTTGGGTGTGAAGAAG-3′ | 85.7 | [16,17] |
| β-Actin | 5′-CGTGGACATCAGGAAGGAC-3′ | 5′-TCTGCTGGAAGGTGGACAG-3′ | 90.2 | [17,18] |
| OATP-B | 5′-TCAGCACACCCCTCTTCTTC-3′ | 5′-GACAAGGCGTGAGGTACTCC-3′ | 89.9 | [19] |
| OATP-E | 5′-TCGGGAAAACCATCAGAGAC-3′ | 5′-CCAGGTACCCGAACAAGGT-3′ | 90.8 | [20] |
| OATP-D | 5′-ATGTGCCTGTCTGGGAATCT-3′ | 5′-AGGTGCTGAGGTGTTTCCAT-3′ | 88.8 | [21] |
| OATP-A | 5′-TGCATTCAAACACCAGGAAA-3′ | 5′-GCATGTAATCCCACACCAAGA-3′ | 81.8 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bone, C.; Squires, E.J. The Uptake and Deconjugation of Androstenone Sulfate in the Adipose Tissue of the Boar. Animals 2021, 11, 3158. https://doi.org/10.3390/ani11113158
Bone C, Squires EJ. The Uptake and Deconjugation of Androstenone Sulfate in the Adipose Tissue of the Boar. Animals. 2021; 11(11):3158. https://doi.org/10.3390/ani11113158
Chicago/Turabian StyleBone, Christine, and E. James Squires. 2021. "The Uptake and Deconjugation of Androstenone Sulfate in the Adipose Tissue of the Boar" Animals 11, no. 11: 3158. https://doi.org/10.3390/ani11113158






