Pigment and Fatty Acid Heterogeneity in the Sea Slug Elysia crispata Is Not Shaped by Habitat Depth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Photosynthetic Pigment Analysis
2.3. Lipid Analysis
2.4. Statistical Analysis
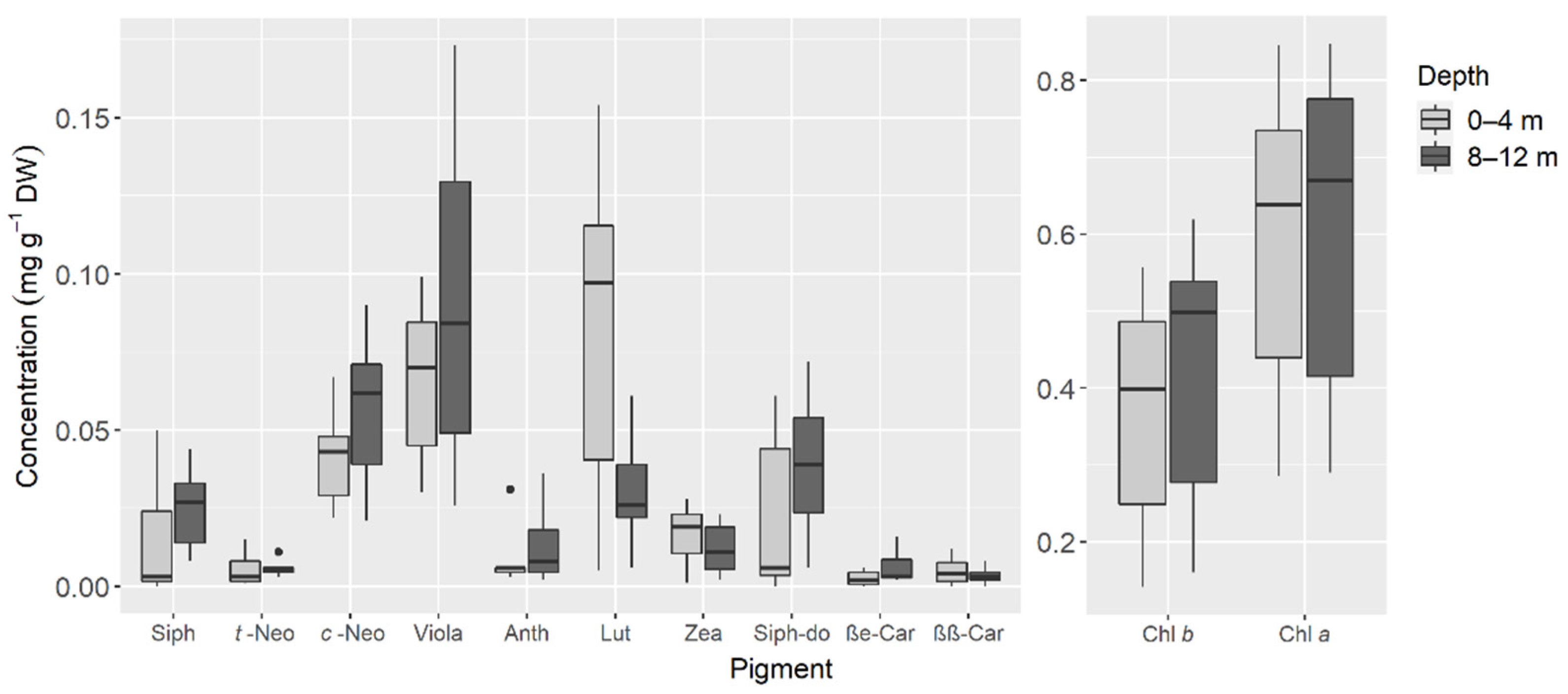
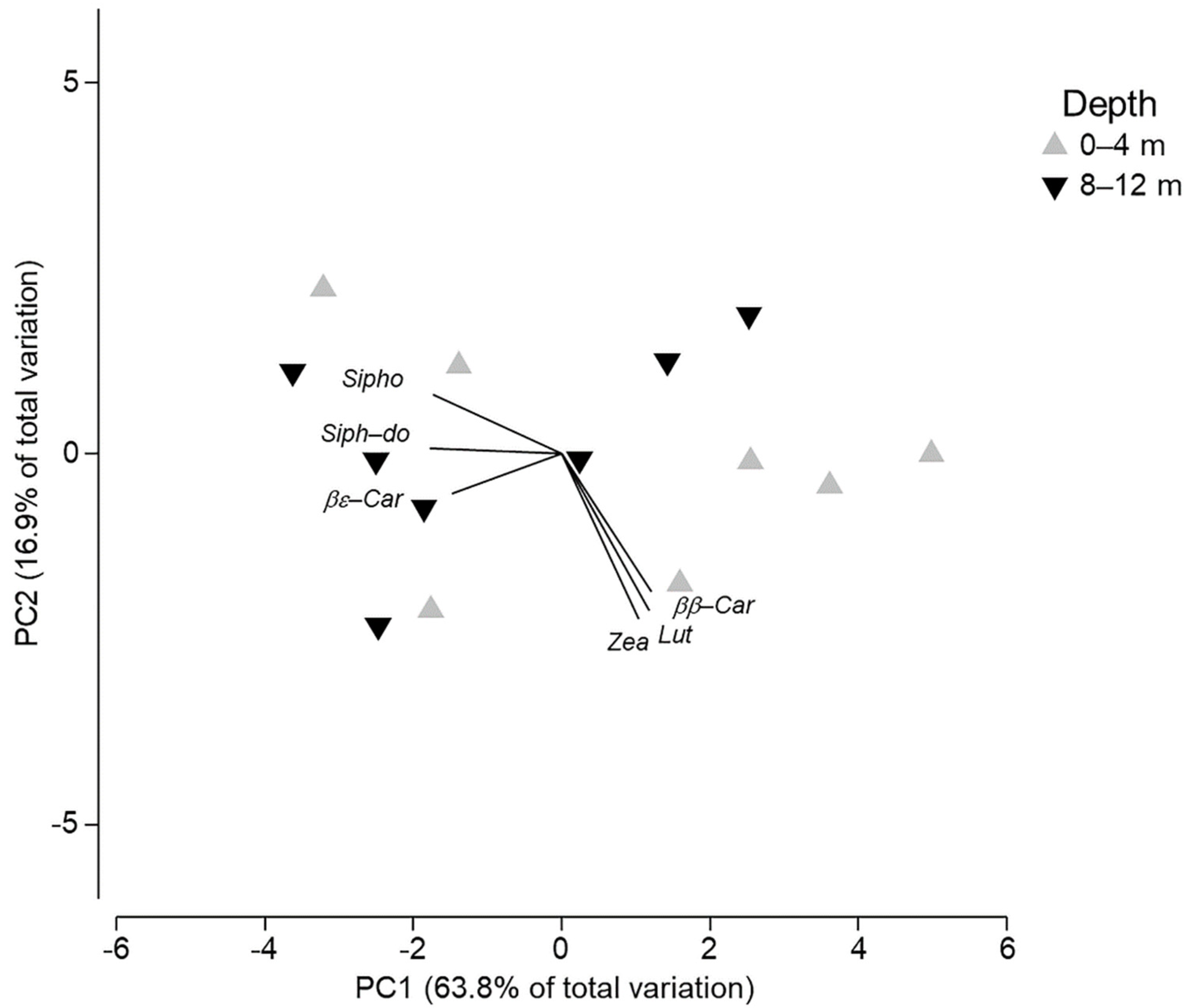
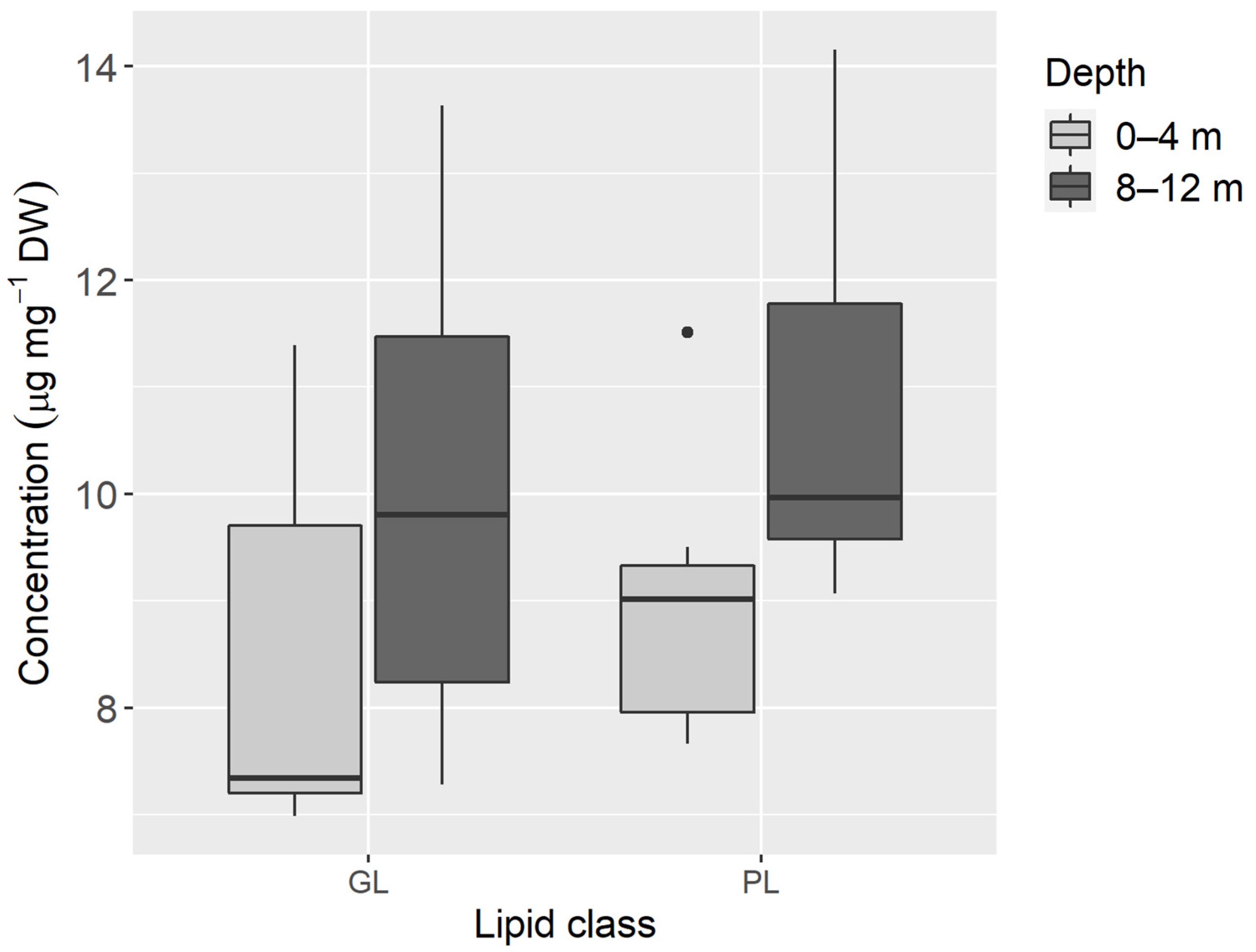
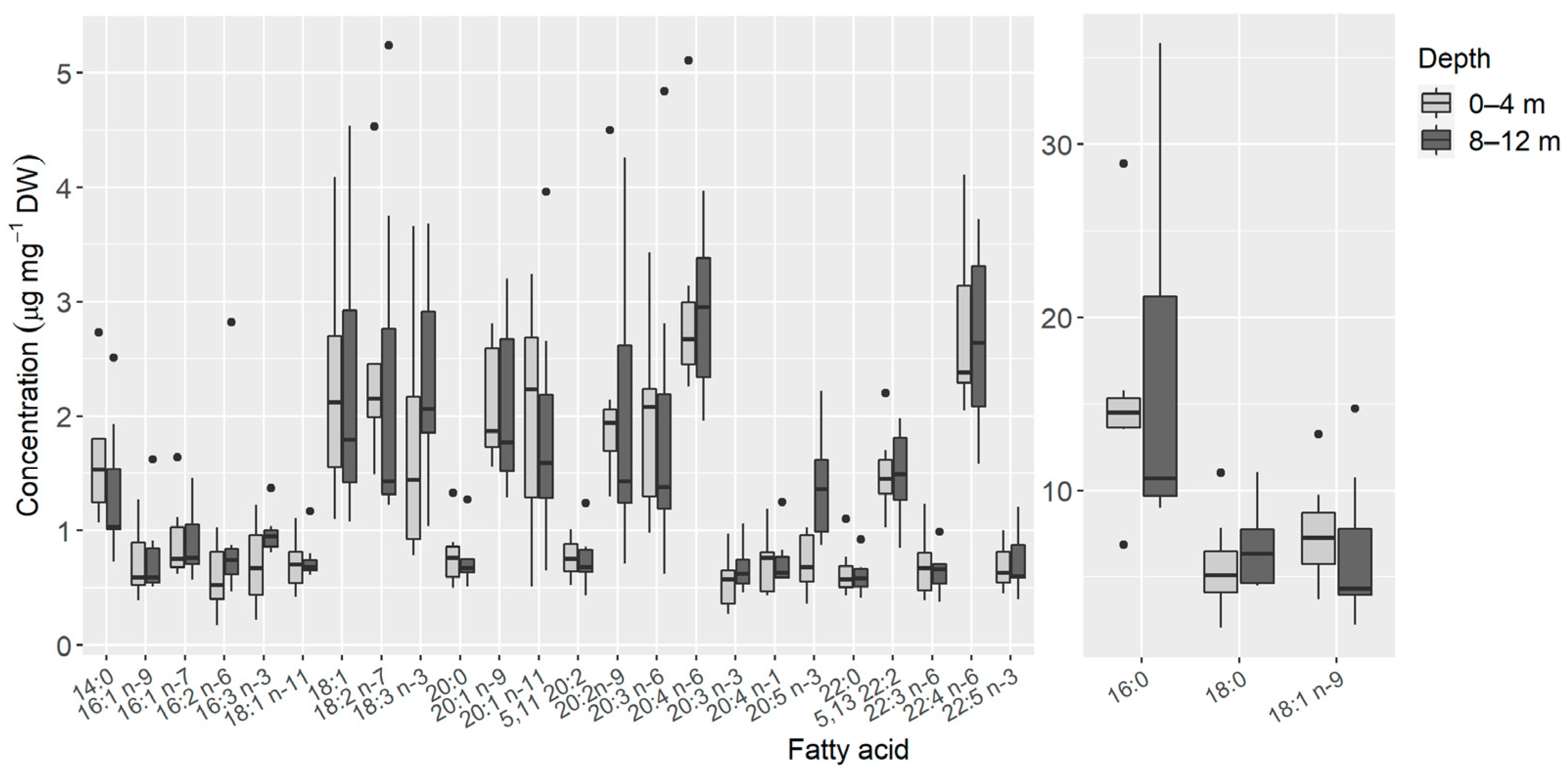
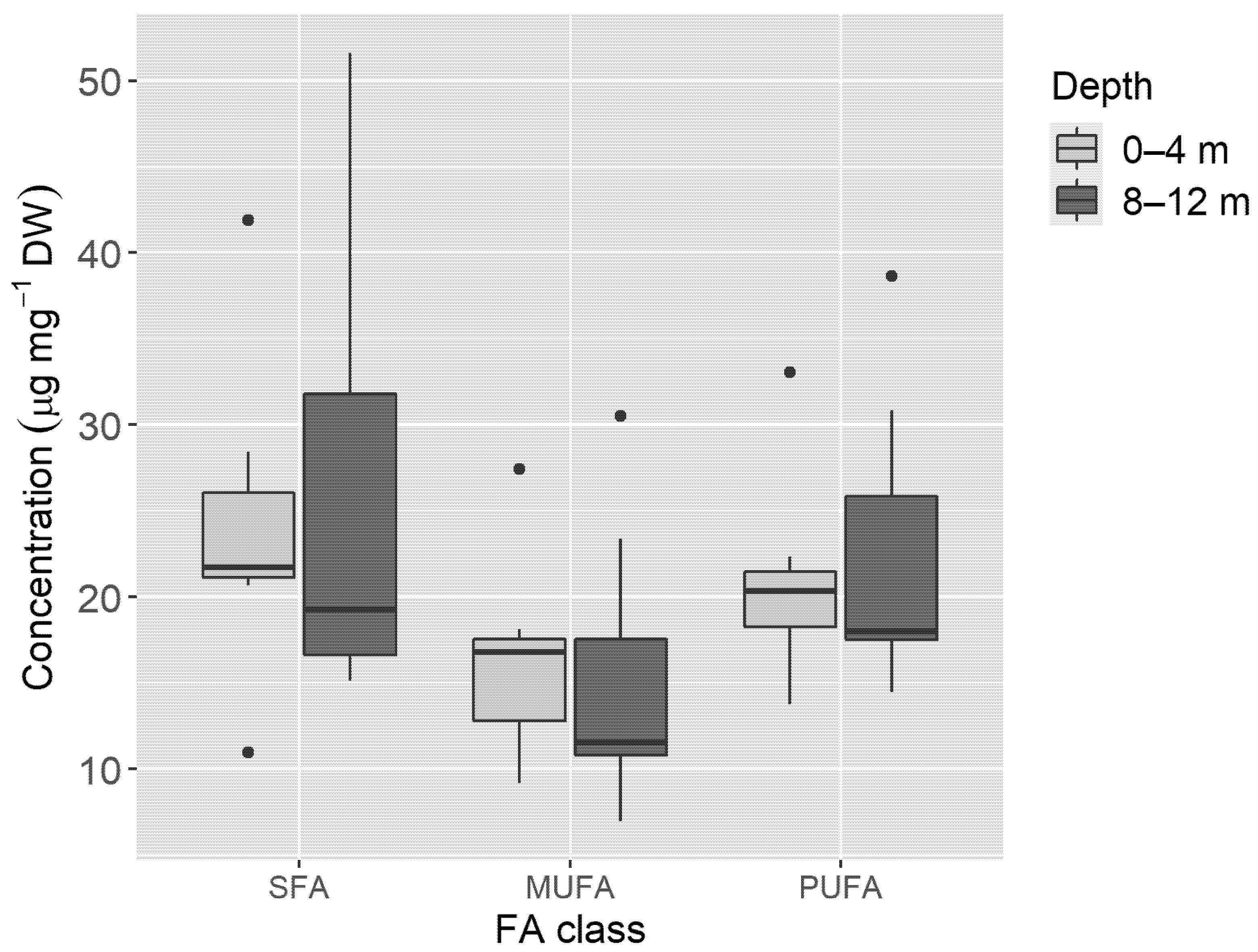
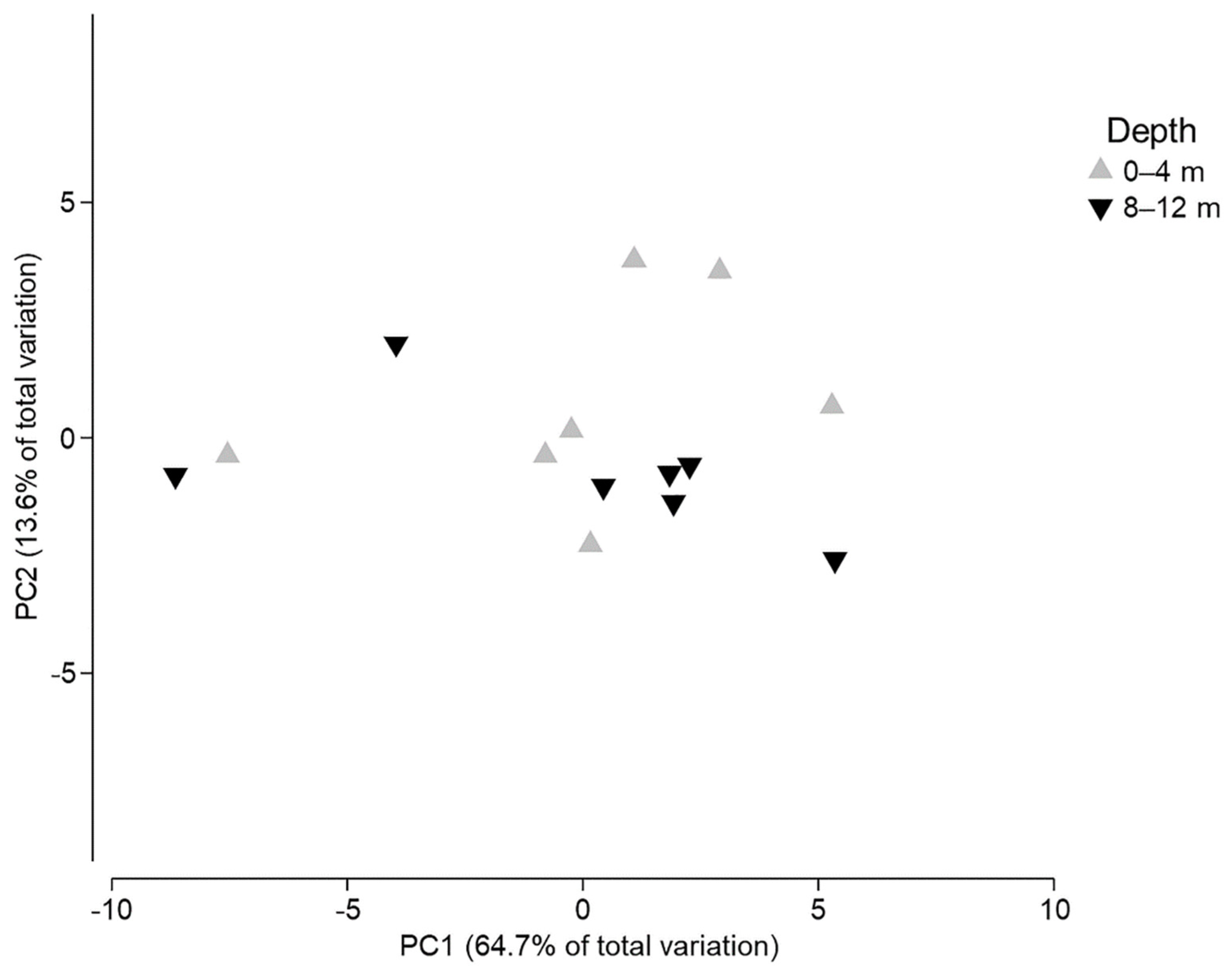
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Händeler, K.; Grzymbowski, Y.P.; Krug, P.J.; Wägele, H. Functional chloroplasts in metazoan cells—A unique evolutionary strategy in animal life. Front. Zool. 2009, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, N.W.L.; Stephenson, I.; Herranz, M.; Husnik, F.; Keeling, P.J.; Leander, B.S. A new case of kleptoplasty in animals: Marine flatworms steal functional plastids from diatoms. Sci. Adv. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Baumgartner, F.A.; Pavia, H.; Toth, G.B. Individual specialization to non-optimal hosts in a polyphagous marine invertebrate herbivore. PLoS ONE 2014, 9. [Google Scholar] [CrossRef][Green Version]
- de Vries, J.; Rauch, C.; Christa, G.; Gould, S.B. A sea slug’s guide to plastid symbiosis. Acta Soc. Bot. Pol. 2014, 83, 415–421. [Google Scholar] [CrossRef]
- Rauch, C.; Tielens, A.G.M.; Serôdio, J.; Gould, S.B.; Christa, G. The ability to incorporate functional plastids by the sea slug Elysia viridis is governed by its food source. Mar. Biol. 2018, 165, 1–13. [Google Scholar] [CrossRef]
- Christa, G.; Händeler, K.; Kück, P.; Vleugels, M.; Franken, J.; Karmeinski, D.; Wägele, H. Phylogenetic evidence for multiple independent origins of functional kleptoplasty in Sacoglossa (Heterobranchia, Gastropoda). Org. Divers. Evol. 2015, 15, 23–36. [Google Scholar] [CrossRef]
- Vieira, S.; Calado, R.; Coelho, H.; Serôdio, J. Effects of light exposure on the retention of kleptoplastic photosynthetic activity in the sacoglossan mollusc Elysia viridis. Mar. Biol. 2009, 156, 1007–1020. [Google Scholar] [CrossRef]
- Laetz, E.M.J.; Wägele, H. How does temperature affect functional kleptoplasty? Comparing populations of the solar-powered sister-species Elysia timida Risso, 1818 and Elysia cornigera Nuttall, 1989 (Gastropoda: Sacoglossa). Front. Zool. 2018, 15, 1–13. [Google Scholar] [CrossRef]
- Dionísio, G.; Faleiro, F.; Bispo, R.; Lopes, A.R.; Cruz, S.; Paula, J.R.; Repolho, T.; Calado, R.; Rosa, R. Distinct bleaching resilience of photosynthetic plastid-bearing mollusks under thermal stress and high CO2 conditions. Front. Physiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Green, B.J.; Li, W.-Y.; Manhart, J.R.; Fox, T.C.; Summer, E.J.; Kennedy, R.A.; Pierce, S.K.; Rumpho, M.E. Mollusc-algal chloroplast endosymbiosis. Photosynthesis, thylakoid protein maintenance, and chloroplast gene expression continue for many months in the absence of the algal nucleus. Plant Physiol. 2000, 124, 331–342. [Google Scholar] [CrossRef]
- Costa, J.; Giménez-Casalduero, F.; Melo, R.; Jesus, B. Colour morphotypes of Elysia timida (Sacoglossa, Gastropoda) are determined by light acclimation in food algae. Aquat. Biol. 2012, 17, 81–89. [Google Scholar] [CrossRef][Green Version]
- Middlebrooks, M.L.; Curtis, N.E.; Pierce, S.K. Algal sources of sequestered chloroplasts in the sacoglossan sea slug Elysia crispata vary by location and ecotype. Biol. Bull. 2019, 236, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.E.; Massey, S.E.; Pierce, S.K. The symbiotic chloroplasts in the sacoglossan Elysia clarki are from several algal species. Invertebr. Biol. 2006, 125, 336–345. [Google Scholar] [CrossRef]
- Clark, K.B.; Busacca, M. Feeding specificity and chloroplast retention in four tropical Ascoglossa, with a discussion of the extent of chloroplast symbiosis and the evolution of the order. J. Molluscan Stud. 1978, 44, 272–282. [Google Scholar] [CrossRef]
- Jensen, K.R. A review of sacoglossan diets with comparative notes on radular and buccal anatomy. Malacol. Rev. 1980, 13, 55–77. [Google Scholar]
- Middlebrooks, M.L.; Pierce, S.K.; Bell, S.S. Foraging behavior under starvation conditions is altered via photosynthesis by the marine gastropod, Elysia clarki. PLoS ONE 2011, 6, e22162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curtis, N.E.; Middlebrooks, M.L.; Schwartz, J.A.; Pierce, S.K. Kleptoplastic sacoglossan species have very different capacities for plastid maintenance despite utilizing the same algal donors. Symbiosis 2015, 65, 23–31. [Google Scholar] [CrossRef]
- Curtis, N.E.; Schwartz, J.A.; Pierce, S.K. Ultrastructure of sequestered chloroplasts in sacoglossan gastropods with differing abilities for plastid uptake and maintenance. Invertebr. Biol. 2010, 129, 297–308. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Wilhelm, C.; Jungandreas, A.; Jakob, T.; Goss, R. Light acclimation in diatoms: From phenomenology to mechanisms. Mar. Genom. 2014, 16, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Cartaxana, P.; Newcomer, R.; Dionísio, G.; Calado, R.; Serôdio, J.; Pelletreau, K.N.; Rumpho, M.E. Photoprotection in sequestered plastids of sea slugs and respective algal sources. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cartaxana, P.; Morelli, L.; Quintaneiro, C.; Calado, G.; Calado, R.; Cruz, S. Kleptoplasts photoacclimation state modulates the photobehaviour of the solar- powered sea slug Elysia viridis. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Cartaxana, P.; Morelli, L.; Jesus, B.; Calado, G.; Calado, R.; Cruz, S. The photon menace: Kleptoplast protection in the photosynthetic sea slug Elysia timida. J. Exp. Biol. 2019, 222, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Jesus, B.; Ventura, P.; Calado, G. Behaviour and a functional xanthophyll cycle enhance photo-regulation mechanisms in the solar-powered sea slug Elysia timida (Risso, 1818). J. Exp. Mar. Bio. Ecol. 2010, 395, 98–105. [Google Scholar] [CrossRef]
- Pierce, S.K.; Curtis, N.E.; Schwartz, J.A. Chlorophyll a synthesis by an animal using transferred algal nucleargenes. Symbiosis 2009, 49, 121–131. [Google Scholar] [CrossRef]
- Middlebrooks, M.L.; Bell, S.S.; Pierce, S.K. The kleptoplastic sea slug Elysia clarki prolongs photosynthesis by synthesizing chlorophyll a and b. Symbiosis 2012, 57, 127–132. [Google Scholar] [CrossRef]
- Trench, R.K.; Smith, D.C. Synthesis of pigment in symbiotic chloroplasts. Nature 1970, 227, 196–197. [Google Scholar] [CrossRef]
- Ventura, P.; Calado, G.; Jesus, B. Photosynthetic efficiency and kleptoplast pigment diversity in the sea slug Thuridilla hopei (Vérany, 1853). J. Exp. Mar. Bio. Ecol. 2013, 441, 105–109. [Google Scholar] [CrossRef]
- Cruz, S.; Calado, R.; Serôdio, J.; Jesus, B.; Cartaxana, P. Pigment profile in the photosynthetic sea slug Elysia viridis (Montagu, 1804). J. Molluscan Stud. 2014, 80, 475–481. [Google Scholar] [CrossRef][Green Version]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Voogt, P.A. Lipids: Their Distribution and Metabolism. In The Mollusca. Metabolic Biochemistry and Molecular Biomechanics; Hochachka, P.W., Ed.; Academic Press, Inc.: New York, NY, USA, 1983; Volume 1, pp. 329–370. ISBN 0127514015. [Google Scholar]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates. Part II: Mollusca. Prog. Lipid Res. 1982, 21, 109–153. [Google Scholar] [CrossRef]
- Pelletreau, K.N.; Weber, A.P.M.; Weber, K.L.; Rumpho, M.E. Lipid accumulation during the establishment of kleptoplasty in Elysia chlorotica. PLoS ONE 2014, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Trench, R.K.; Boyle, J.E.; Smith, D.C. The association between chloroplasts of Codium fragile and the mollusc Elysia viridis. II. Chloroplast ultrastructure and photosynthetic carbon fixation in E. viridis. Proc. R. Soc. Lond.-Biol. Sci. 1973, 184, 63–81. [Google Scholar] [CrossRef]
- Rey, F.; Da Costa, E.; Campos, A.M.; Cartaxana, P.; MacIel, E.; Domingues, P.; Domingues, M.R.M.; Calado, R.; Cruz, S. Kleptoplasty does not promote major shifts in the lipidome of macroalgal chloroplasts sequestered by the sacoglossan sea slug Elysia viridis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Rey, F.; Melo, T.; Cartaxana, P.; Calado, R.; Domingues, P.; Cruz, S.; Domingues, M.R.M. Coping with starvation: Contrasting lipidomic dynamics in the cells of two sacoglossan sea slugs incorporating stolen plastids from the same macroalga. Integr. Comp. Biol. 2020, 60, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B. Ascoglossan (=Sacoglossa) molluscs in the Florida Keys: Rare marine invertebrates at special risk. Bull. Mar. Sci. 1994, 54, 900–916. [Google Scholar]
- Camacho-García, Y.E.; Pola, M.; Carmona, L.; Padula, V.; Villani, G.; Cervera, J.L. Diversity and distribution of the heterobranch sea slug fauna on the Caribbean of Costa Rica. Cah. Biol. Mar. 2014, 55, 109–127. [Google Scholar]
- Lalli, C.M.; Parsons, T.R. Biological Oceanography, An Introduction, 2nd ed.; The Open University: Burlington, MA, USA, 1997. [Google Scholar]
- Krug, P.J.; Vendetti, J.E.; Valdés, Á. Molecular and morphological systematics of Elysia Risso, 1818 (Heterobranchia: Sacoglossa) from the Caribbean region. Zootaxa 2016, 4148, 1–137. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Cartaxana, P.; Brotas, V. HPLC determination of phytoplankton and microphytobenthos pigments: Comparing resolution and sensitivity of a C18 and a C8 method. Limnol. Oceanogr. Methods 2007, 5, 363–370. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Leray, C. CyberLipid. Available online: http://cyberlipid.gerli.com/techniques-of-analysis/analysis-of-complex-lipids/glycoglycerolipid-analysis/quantitative-estimation/ (accessed on 13 May 2021).
- Bell, B.M.; Daniels, D.G.H.; Fearn, T.; Stewart, B.A. Lipid compositions, baking qualities and other characteristics of wheat varieties grown in the U.K. J. Cereal Sci. 1987, 5, 277–286. [Google Scholar] [CrossRef]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. The Lipid Web. Available online: www.lipidmaps.org/resources/lipidweb (accessed on 4 December 2019).
- Cartaxana, P.; Rey, F.; Ribeiro, M.; Moreira, A.S.P.; Rosário, M.; Domingues, M.; Calado, R.; Cruz, S. Nutritional state determines reproductive investment in the mixotrophic sea slug Elysia viridis. Mar. Ecol. Prog. Ser. 2019, 611, 167–177. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2009; ISBN 0131008463. [Google Scholar]
- Clarke, K.R.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK; Ivybridge, UK, 2014; ISBN 8003098955. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2021; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Software PRIMER v7; PRIMER-E Ltd.: Devon, UK, 2015. [Google Scholar]
- Roller, R.A.; Bianchi, T.S. HPLC analysis of chloroplast pigments from the marine ascoglossan Tridachia crispata (Mörch, 1863) (Mollusca: Opisthobranchia). Am. Malacol. Bull. 1995, 11, 139–143. [Google Scholar]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Casalduero, F.; Muniain, C. The role of kleptoplasts in the survival rates of Elysia timida (Risso, 1818): (Sacoglossa: Opisthobranchia) during periods of food shortage. J. Exp. Mar. Bio. Ecol. 2008, 357, 181–187. [Google Scholar] [CrossRef]
- Evertsen, J.; Johnsen, G. In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis. Mar. Biol. 2009, 156, 847–859. [Google Scholar] [CrossRef]
- Murata, N.; Siegenthaler, P.-A. Lipids in photosynthesis: An overview. In Lipids in Photosynthesis: Structure, Function and Genetics; Siegenthaler, P.-A., Murata, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 1–20. [Google Scholar]
- Cartaxana, P.; Rey, F.; LeKieffre, C.; Lopes, D.; Hubas, C.; Spangenberg, J.E.; Escrig, S.; Jesus, B.; Calado, G.; Domingues, M.R.M.; et al. Photosynthesis from stolen chloroplasts can support sea slug reproductive fitness. Proc. R. Soc. B 2021, 288. [Google Scholar] [CrossRef]
- Salas-Perez, J.J.; Granados-Barba, A. Oceanographic characterization of the Veracruz reefs system. Atmosfera 2008, 21, 281–301. [Google Scholar]
- Mateos-Jasso, A.; Zavala-Hidalgo, J.; Romero-Centeno, R.; Allende-Arandía, M.E. Variability of the thermohaline structure in the northern Veracruz Coral Reef System, Mexico. Cont. Shelf Res. 2012, 50–51, 30–40. [Google Scholar] [CrossRef]
- Monroig, Ó.; Kabeya, N. Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: A comprehensive review. Fish. Sci. 2018, 84, 911–928. [Google Scholar] [CrossRef]
- Parrish, C.C. Essential fatty acids in aquatic food webs. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 309–326. ISBN 978-0-387-89366-2. [Google Scholar]
- Rey, F.; Cartaxana, P.; Melo, T.; Calado, R.; Pereira, R.; Abreu, H.; Domingues, P.; Cruz, S.; Domingues, R.M. Domesticated populations of Codium tomentosum display lipid extracts with lower seasonal shifts than conspecifics from the wild-relevance for biotechnological applications of this green seaweed. Mar. Drugs 2020, 18, 188. [Google Scholar] [CrossRef]
- Zhukova, N.V. Lipids and fatty acids of nudibranch mollusks: Potential sources of bioactive compounds. Mar. Drugs 2014, 12, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Fernandes, F.; Madureira-Carvalho, Á.; Valentão, P.; Lobo-da-Cunha, A.; Calado, G.; Andrade, P.B. Profiling of Heterobranchia sea slugs from portuguese coastal waters as producers of anti-cancer and anti-inflammatory agents. Molecules 2018, 23, 1027. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V. Lipid classes and fatty acid composition of the tropical nudibranch mollusks Chromodoris sp. and Phyllidia coelestis. Lipids 2007, 42, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Middlebrooks, M.L.; Bell, S.S.; Curtis, N.E.; Pierce, S.K. Atypical plant-herbivore association of algal food and a kleptoplastic sea slug (Elysia clarki) revealed by DNA barcoding and field surveys. Mar. Biol. 2014, 161, 1429–1440. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Medel-Alvarado, T. Estructura de la comunidad de corales y peces en profundidades someras y mesofóticas en el suroeste del golfo de México. Master’s Thesis, Universidad Veracruzana, Boca del Río, México, 2019. [Google Scholar]
- Liaño-Carrera, F.; Camarena-Luhrs, T.; Gómez-Barrero, A.; Martos-Fernández, F.J.; Ramírez-Macias, J.I.; Salas-Monreal, D. New coral reef structures in a tropical coral reef system. Lat. Am. J. Aquat. Res. 2019, 47, 270–281. [Google Scholar] [CrossRef]
- Thimijan, R.W.; Heins, R.D. Photometric, radiometric, and quantum light units of measure: A review of procedures for interconversion. HortScience 1983, 18, 818–822. [Google Scholar] [CrossRef]
- Galicia-García, C.; Morales-García, A. Investigaciones sobre macroalgas realizadas en el Sistema Arrecifal Veracruzano. In Investigaciones Científicas en el Sistema Arrecifal Veracruzano; Universidad Autónoma de Campeche: Campeche, México, 2007; pp. 141–160. [Google Scholar]
- Curtis, N.E.; Pierce, S.K.; Massey, S.E.; Schwartz, J.A.; Maugel, T.K. Newly metamorphosed Elysia clarki juveniles feed on and sequester chloroplasts from algal species different from those utilized by adult slugs. Mar. Biol. 2007, 150, 797–806. [Google Scholar] [CrossRef]
- Pierce, S.K.; Curtis, N.E.; Massey, S.E.; Bass, A.L.; Karl, S.A.; Finney, C.M. A morphological and molecular comparison between Elysia crispata and a new species of kleptoplastic sacoglossan sea slug (Gastropoda: Opisthobranchia) from the Florida Keys, USA. Molluscan Res. 2006, 26, 23–38. [Google Scholar]
- Thompson, T.E.; Jarman, G.M. Nutrition of Tridachia crispata (Mörch) (Sacoglossa). J. Molluscan Stud. 1989, 55, 239–244. [Google Scholar] [CrossRef]
- Trench, R.K.; Trench, M.E.; Muscatine, L. Symbiotic chloroplasts; their photosynthetic products and contribution to mucus synthesis in two marine slugs. Biol. Bull. 1972, 142, 335–349. [Google Scholar] [CrossRef]
- Jensen, K.; Clark, K.B. Annotated checklist of Florida Ascoglossan Opisthobranchia. Nautilus (Philadelphia) 1983, 97, 1–13. [Google Scholar]
- Trench, R.K. Chloroplasts: Presumptive and de facto organelles. Ann. N. Y. Acad. Sci. 1981, 361, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.K.; Massey, S.E.; Hanten, J.J.; Curtis, N.E. Horizontal transfer of functional nuclear genes between multicellular organisms. Biol. Bull. 2003, 204, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Tufiño-Velázquez, R.C.; Pedroche, F.F. Las especies del género Bryopsis (Chlorophyta) presentes en la costa del Atlántico mexicano. Rev. Mex. Biodivers. 2019, 90, 1–12. [Google Scholar]
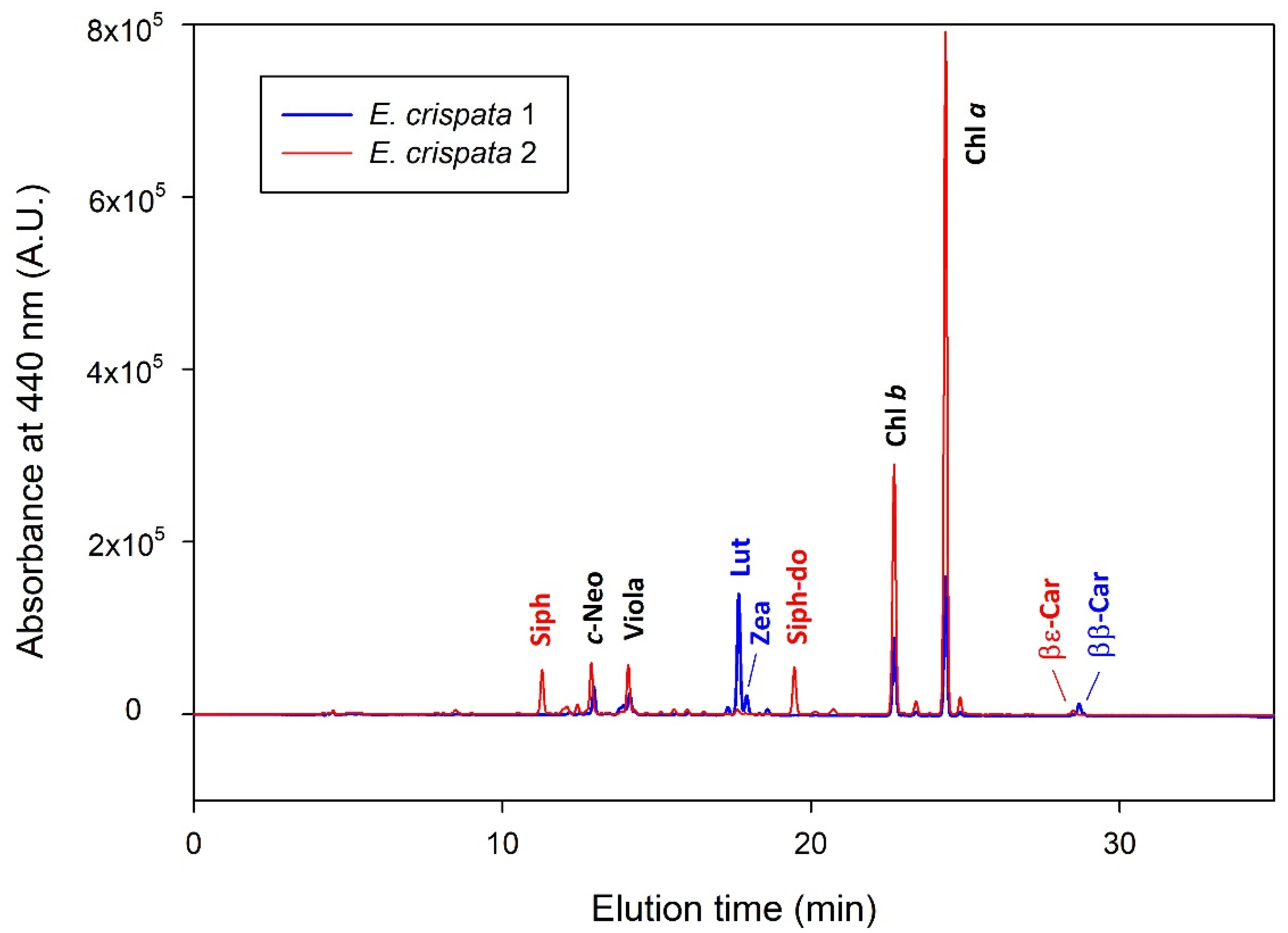
| Pigment | Retention Time (min) | λ max (nm) |
|---|---|---|
| Siphonoxanthin (Siph) | 11.38 | 448 |
| all-trans-neoxanthin (t-Neo) | 12.46 | 440, 471 |
| cis-neoxanthin (c-Neo) | 12.98 | 414, 438, 467 |
| Violaxanthin (Viola) | 14.21 | 418, 442, 471 |
| Antheraxanthin (Anth) | 16.18 | 448, 476 |
| Lutein (Lut) | 17.86 | 448, 476 |
| Zeaxanthin (Zea) | 18.11 | 453, 480 |
| Siphonoxanthin-dodecenoate (Siph-do) | 19.76 | 454 |
| Chlorophyll b (Chl b) | 23.09 | 458, 646 |
| Chlorophyll a (Chl a) | 24.71 | 431, 663 |
| β,ε-Carotene (βε-Car) | 29.01 | 449, 477 |
| β,β-Carotene (ββ-Car) | 29.22 | 454, 478 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital, X.G.; Rey, F.; Cartaxana, P.; Cruz, S.; Domingues, M.R.; Calado, R.; Simões, N. Pigment and Fatty Acid Heterogeneity in the Sea Slug Elysia crispata Is Not Shaped by Habitat Depth. Animals 2021, 11, 3157. https://doi.org/10.3390/ani11113157
Vital XG, Rey F, Cartaxana P, Cruz S, Domingues MR, Calado R, Simões N. Pigment and Fatty Acid Heterogeneity in the Sea Slug Elysia crispata Is Not Shaped by Habitat Depth. Animals. 2021; 11(11):3157. https://doi.org/10.3390/ani11113157
Chicago/Turabian StyleVital, Xochitl Guadalupe, Felisa Rey, Paulo Cartaxana, Sónia Cruz, Maria Rosário Domingues, Ricardo Calado, and Nuno Simões. 2021. "Pigment and Fatty Acid Heterogeneity in the Sea Slug Elysia crispata Is Not Shaped by Habitat Depth" Animals 11, no. 11: 3157. https://doi.org/10.3390/ani11113157
APA StyleVital, X. G., Rey, F., Cartaxana, P., Cruz, S., Domingues, M. R., Calado, R., & Simões, N. (2021). Pigment and Fatty Acid Heterogeneity in the Sea Slug Elysia crispata Is Not Shaped by Habitat Depth. Animals, 11(11), 3157. https://doi.org/10.3390/ani11113157








