Influence of Essential Oils on the Microbiological Quality of Fish Meat during Storage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Composition of Essential Oils
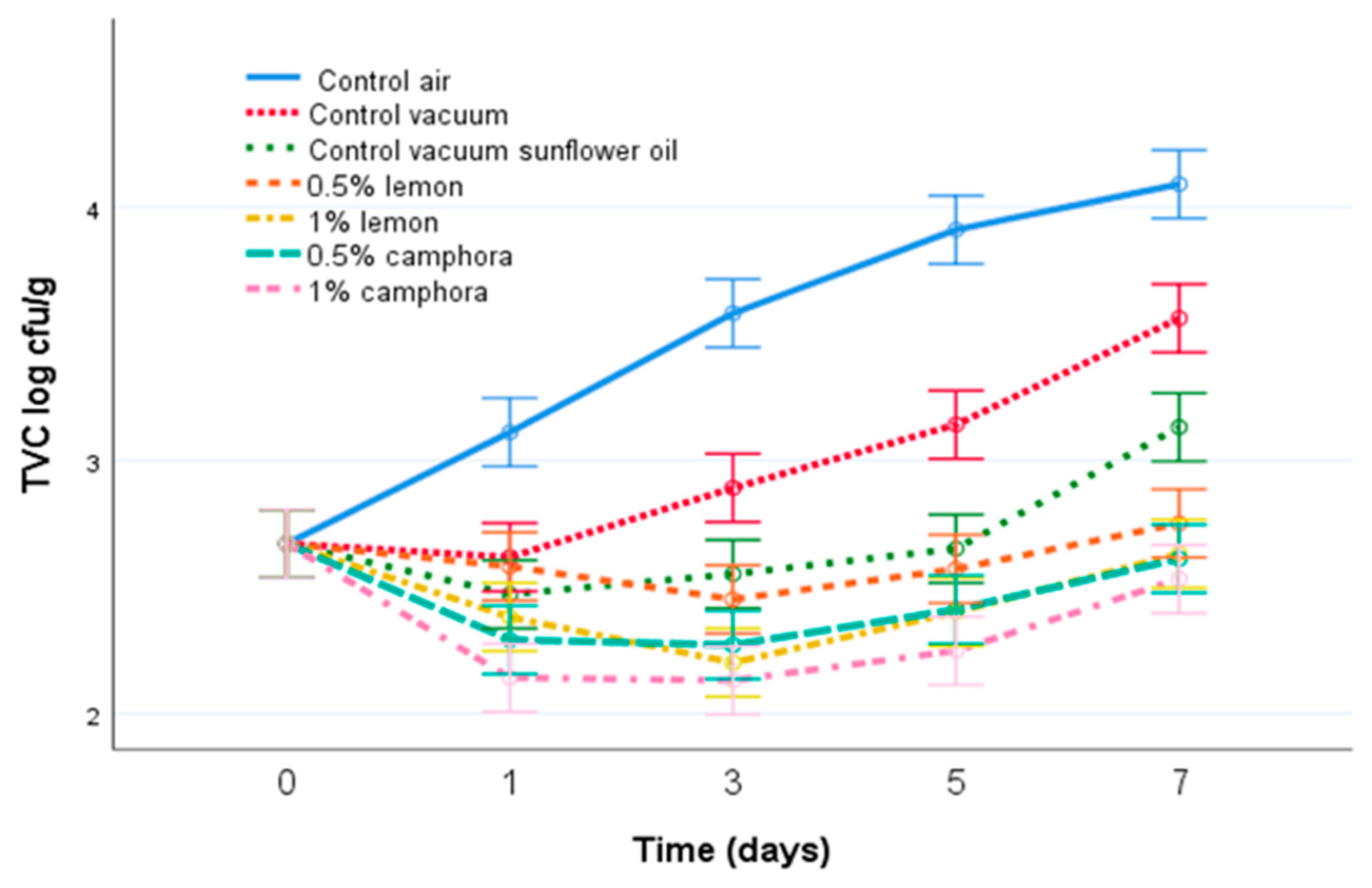
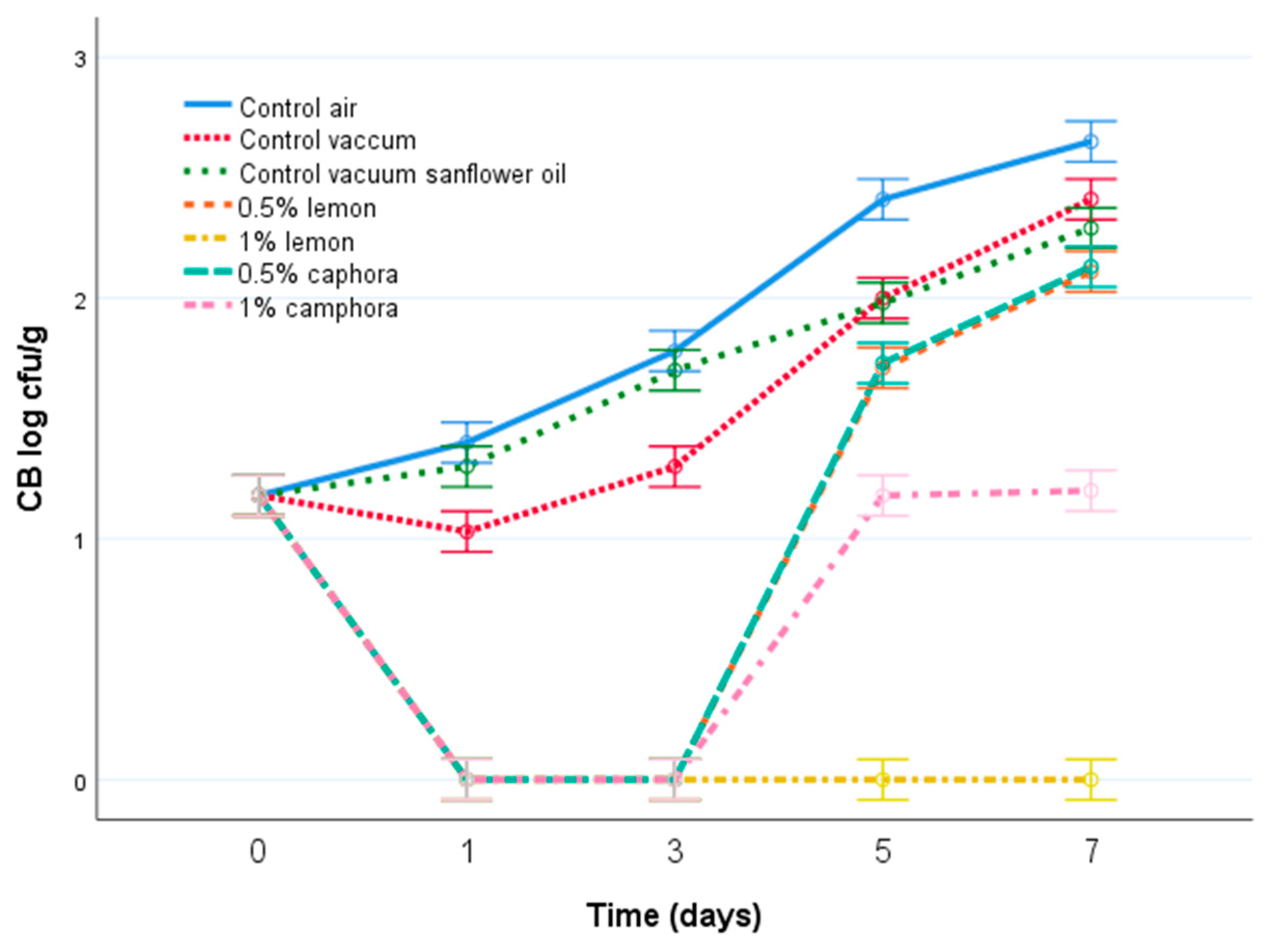
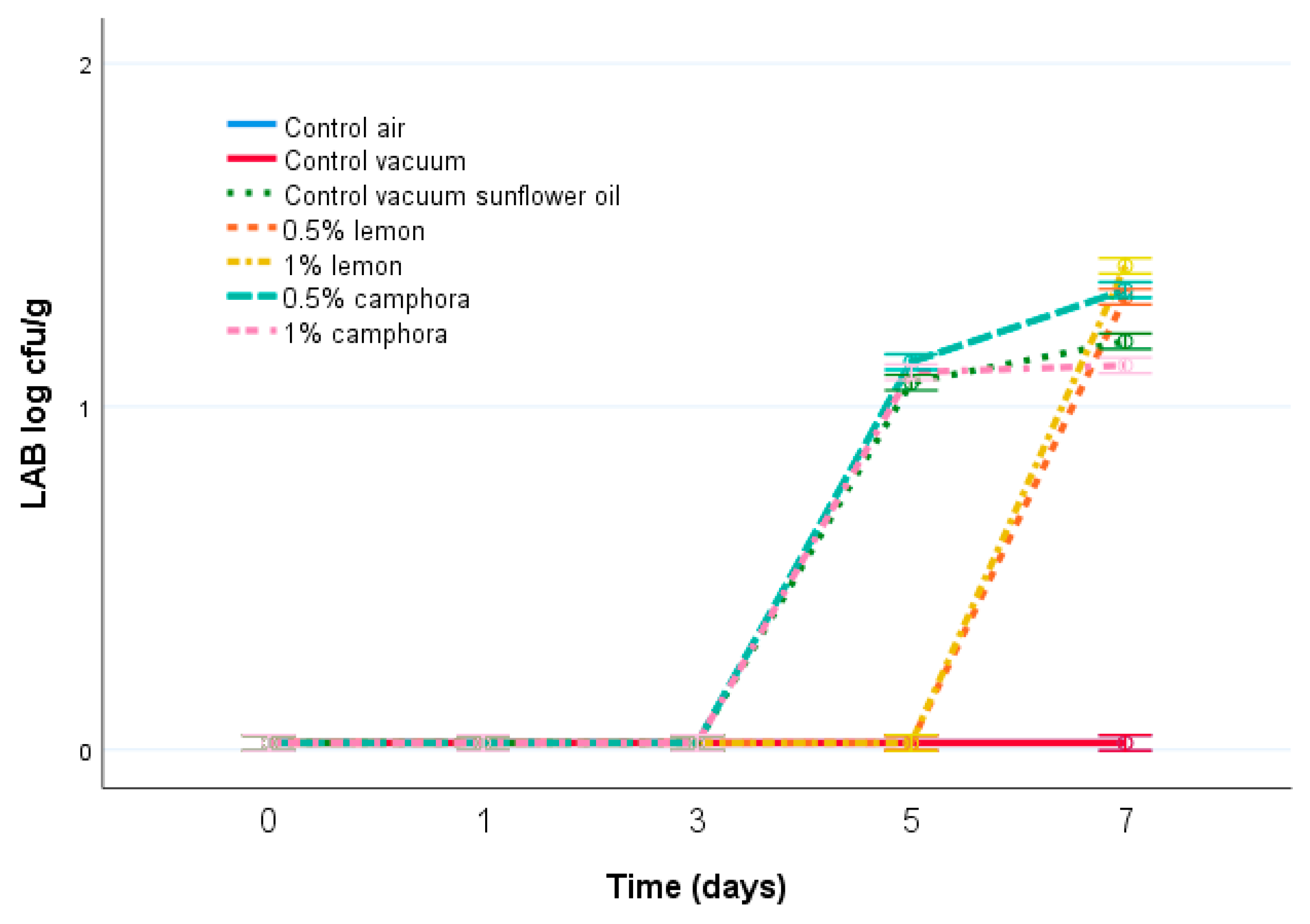
3.2. Microbiological Quality of Fish Meat
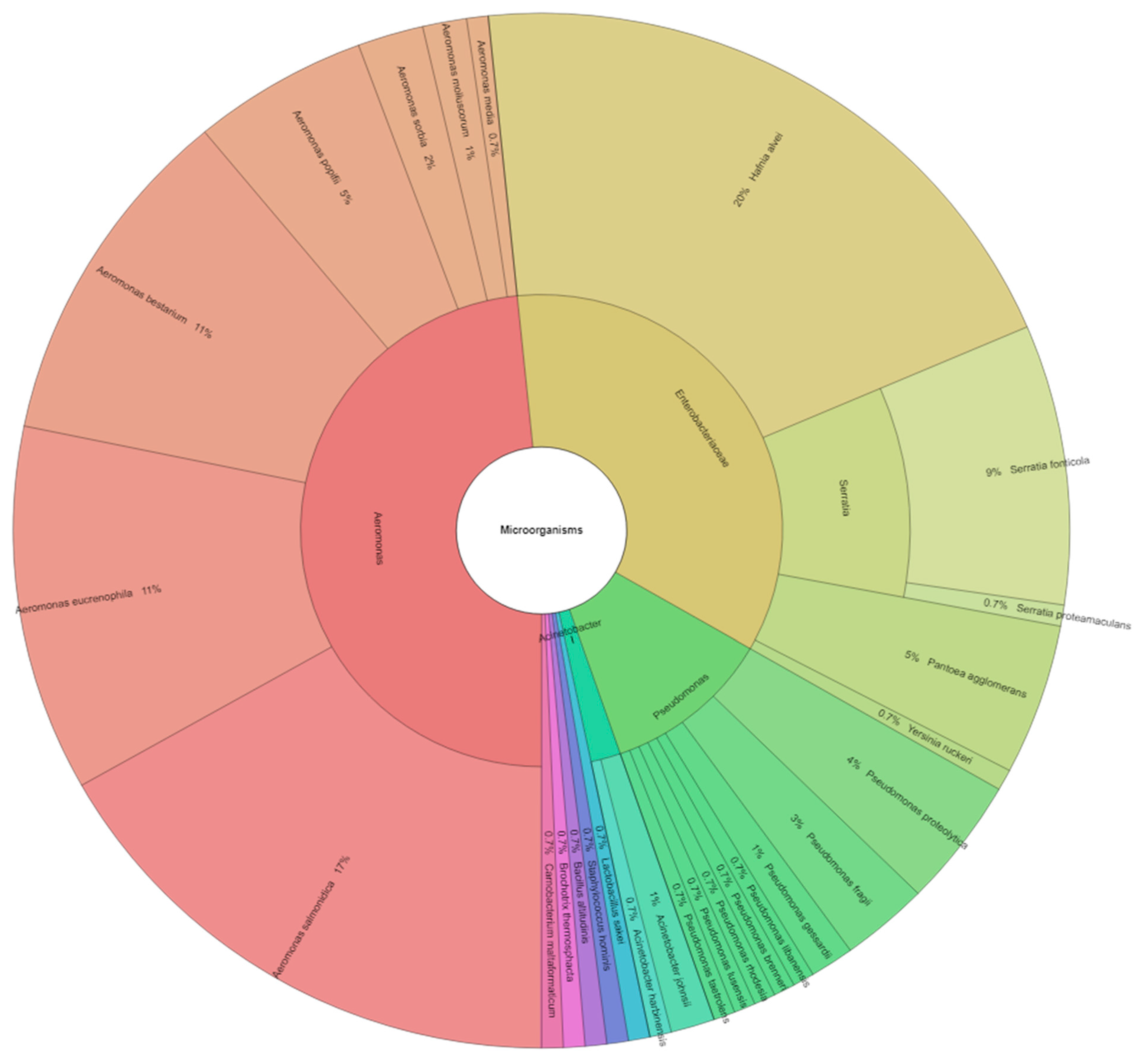
3.3. Identification of Isolated Microorganisms from Samples Using a MALDI-TOF MS Biotyper
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, R.; Roy, K.; Pan, J.; Shah, B.R.; Mraz, J. Critical review on the use of essential oils against spoilage in chilled stored fish: A quantitative meta-analyses. Trends Food Sci. Technol. 2021, 111, 175–190. [Google Scholar] [CrossRef]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, H.-T.; Lee, E.-W.; et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review. Food Control 2021, 129, 108244. [Google Scholar] [CrossRef]
- Saleh, E.A.; Al-Hawary, I.I.; Elnajar, M.M. Antibacterial and anti-oxidant activities of laurel oil against Staphylococcus aureus and Pseudomonas fluorescence in oreochromis niloticus fillets. Slov. Vet. Res. 2019, 55, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Abuibaid, A.A. Essential oils for applications in fish and other seafood products. Eurasian J. Food Sci. Technol. 2019, 3, 58–64. [Google Scholar]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassoun, A.; Coban, O.E. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Klūga, A.; Terentjeva, M.; Vukovic, N.L.; Kačániová, M. Antimicrobial activity and chemical composition of essential oils against pathogenic microorganisms of freshwater fish. Plants 2021, 10, 1265. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; da Cruz Silva, G.; de Aguiar, A.C.; Cipriano, L.; de Azeredo, H.M.C.; Bogusz, S., Jr.; Ferreira, M.D. Chemical composition and antifungal activity of essential oils and their combinations against Botrytis cinerea in strawberries. J. Food Meas. Charact. 2021, 15, 1815–1825. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological activity and antibiofilm molecular profile of Citrus aurantium essential oil and its application in a food model. Molecules 2020, 25, 3956. [Google Scholar] [CrossRef]
- Amiri Resketi, M.; Yeganeh, S.; Jani Khalili, K. Dietary sour lemon (Citrus limon) peel essential oil supplementation for reduction of deltamethrin-induced stress in rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2021, 52, 105–123. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Yousef, Y.M.; El-Tras, W.F.; Khalafallaa, M.M. Dietary essential oil extract from sweet orange (Citrus sinensis) and bitter lemon (Citrus limon) peels improved nile tilapia performance and health status. Aquac. Res. 2021, 52, 1463–1479. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Ziino, G.; Giuffrida, A.; Panebianco, A. Activity of r(+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. Int. J. Food Microbiol. 2016, 237, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Martorana, A.; Guarrasi, V.; Barbera, M.; Gaglio, R.; Santulli, A.; Settanni, L.; Galati, A.; Moschetti, G.; Francesca, N. Effect of the lemon essential oils on the safety and sensory quality of salted sardines (Sardina pilchardus walbaum 1792). Food Control 2017, 73, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Wanyang, S.; Wei, H.; Guangyu, W. Study on chemical constituents of the essential oil and classification of types from Cinnamomum camphora. J. Integr. Plant Biol. 1989, 31, 209–214. [Google Scholar]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. Linaloofera fujita against escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Tang, C.; Zhang, R.; Ye, S.; Zhao, Z.; Huang, Y.; Xu, X.; Lan, W.; Yang, D. Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (linn.) presl. J. Ethnopharmacol. 2020, 253, 112652. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicenço, C.B.; Silvestre, W.P.; Lima, T.S.; Pauletti, G.F. Insecticidal activity of Cinnamomum camphora ness and eberm var. Linaloolifera fujita leaf essential oil and linalool against Anticarsia gemmatalis. J. Essent. Oil Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Mancuso, M.; Catalfamo, M.; Laganà, P.; Rappazzo, A.C.; Raymo, V.; Zampino, D.; Zaccone, R. Screening of antimicrobial activity of citrus essential oils against pathogenic bacteria and candida strains. Flavour Fragr. J. 2019, 34, 187–200. [Google Scholar] [CrossRef]
- Navarro-Segura, L.; Ros-Chumillas, M.; Martinez-Hernandez, G.B.; Lopez-Gomez, A. A new advanced packaging system for extending the shelf life of refrigerated farmed fish fillets. J. Sci. Food Agric. 2020, 100, 4601–4611. [Google Scholar] [CrossRef]
- Kačániová, M.; Kunová, S.; Haščík, P.; Pietrzyk, K.; Kluz, M.; Terentjeva, M.; Savistkaya, T.; Grinshpan, D. The antimicrobial effect of thyme and rosemary essential oils against listeria monocytogenes in sous vide turkey meat during storage. Potravin. Slovak J. Food Sci. 2021, 15, 575–584. [Google Scholar]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klüga, A.K.M.; Kantor, A.; Kovalenko, K.; Terentjeva, M. Identification of microflora of freshwater fish caught in the driksna river and pond in Latvia. FOODBALT Jelgava Latv. Univ. Agric. 2017, 164–168. [Google Scholar]
- Lyu, F.; Hong, Y.-L.; Cai, J.-H.; Wei, Q.-Q.; Zhou, X.; Ding, Y.-T.; Liu, Z.-F.; Liu, L. Antimicrobial effect and mechanism of cinnamon oil and gamma radiation on Shewanella putrefaciens. J. Food Sci. Technol. 2018, 55, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Abdeldaiem, M.H.M.; Ali, H.G.M.; Ramadan, M.F. Impact of different essential oils on the characteristics of refrigerated carp (cyprinus carpio) fish fingers. J. Food Meas. Charact. 2017, 11, 1412–1420. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Lv, J.; Li, Q.; Kong, C.; Luo, Y. Effect of cinnamon essential oil on bacterial diversity and shelf-life in vacuum-packaged common carp (Cyprinus carpio) during refrigerated storage. Int. J. Food Microbiol. 2017, 249, 1–8. [Google Scholar] [CrossRef]
- Haute, S.; Raes, K.; Van der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68. [Google Scholar]
- Andevari, G.T.; Rezaei, M. Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. Int. J. Food Sci. Technol. 2011, 46, 2305–2311. [Google Scholar] [CrossRef]
- Durmus, M. The effects of nanoemulsions based on citrus essential oils (orange, mandarin, grapefruit, and lemon) on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets at 4 +/−2 degrees c. J. Food Saf. 2020, 40. [Google Scholar] [CrossRef]
- Corbo, M.R.; Speranza, B.; Filippone, A.; Granatiero, S.; Conte, A.; Sinigaglia, M.; Del Nobile, M.A. Study on the synergic effect of natural compounds on the microbial quality decay of packed fish hamburger. Int. J. Food Microbiol. 2008, 127, 261–267. [Google Scholar] [CrossRef]
- Sarmast, E.; Fallah, A.A.; Dehkordi, S.H.; Rafieian-Kopaei, M. Impact of glazing based on chitosan-gelatin incorporated with persian lime (Citrus latifolia) peel essential oil on quality of rainbow trout fillets stored at superchilled condition. Int. J. Biol. Macromol. 2019, 136, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Motavaf, F.; Mirvaghefi, A.; Farahmand, H.; Hosseini, S.V. Effect of Zataria multiflora essential oil and potassium sorbate on inoculated Listeria monocytogenes, microbial and chemical quality of raw trout fillet during refrigerator storage. Food Sci. Nutr. 2021, 9, 3015–3025. [Google Scholar] [CrossRef]
- Sayyari, Z.; Rabbani, M.; Farahmandfar, R.; Esmaeilzadeh Kenari, R.; Mousavi Nadoushan, R. Investigation of the effect of essential oil along with nanocoatings containing gums in the development of fish fillet storage time. J. Food Meas. Charact. 2021, 15, 3539–3552. [Google Scholar] [CrossRef]
- Sayyari, Z.; Rabani, M.; Farahmandfar, R.; Esmaeilzadeh Kenari, R.; Mousavi Nadoshan, R. The effect of nanocomposite edible coating enriched with Foeniculum vulgare essential oil on the shelf life of Oncorhynchus mykiss fish fillets during the storage. J. Aquat. Food Prod. Technol. 2021, 30, 579–595. [Google Scholar] [CrossRef]
- Karaton Kuzgun, N.; Erecevit Sönmez, P.; Tanyildizi, M.Ş. Preservative effect of coriander essential oil applied in different proportions on the storage of rainbow trout (Oncorhynchus mykiss) fillets. Prog. Nutr. 2021, 22, e2020081. [Google Scholar]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of rainbow trout fillets stored at 4 degrees c. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mehdizadeh, T.; Mojaddar Langroodi, A.; Rezaei, F. Effect of chitosan edible coating enriched with lemon verbena extract and essential oil on the shelf life of vacuum rainbow trout (Oncorhynchus mykiss). J. Food Saf. 2020, 40, e12781. [Google Scholar] [CrossRef]
- Kuzgun, N.K. Effect of garlic (Allium sativum l.) essential oils on Oncorhynchus mykiss fillets during storage. Prog. Nutr. 2019, 21, 709–714. [Google Scholar]
- Aksoy, A.S.Ç. Combined use of laurel essential oil and vacuum packing to extend the shelf-life of rainbow trout (Oncorhynchus mykiss) fillets. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 779–786. [Google Scholar]
- Çoban, O.E.; Patir, B.; Özpolat, E.; Kuzgun, N.K. Improving the quality of fresh rainbow trout by sage essential oil and packaging treatments. J. Food Saf. 2016, 36, 299–307. [Google Scholar] [CrossRef]
- Angiş, S.O.P. Effect of thyme essential oil and packaging treatments on chemical and microbiological properties of fresh rainbow trout (Oncorhynchus mykiss) fillets during storage at refrigerator temperatures. Afr. J. Microbiol. Res. 2013, 7, 1136–1143. [Google Scholar]
- Yildiz, P.O. Combine effects of essential oils and packaging on smoked rainbow trout stored 4 degrees c. Packag. Technol. Sci. 2015, 28, 987–997. [Google Scholar] [CrossRef]
- Pour, A.R.; Mirzargar, S.S.; Soltani, M.; Ebrahimzadeh Mousavi, H.A.; Mostafavi, S.A. The antibacterial effects of Cuminum cyminum l. And Rosmarinus officinalis extracts and essential oil against Lactococcus garvieae in laboratory conditions on rainbow trout. Eur. J. Exp. Biol. 2014, 4, 456–463. [Google Scholar]
- Ansari, M.S.M.; Hosseini, S.E.; Kamali, A. Study of antibacterial effect of mentha longifolia essential oil on Lactococcus garvieae in rainbow trout fillet at 4 °C. Anim. Vet. Sci. 2014, 4, 556–559. [Google Scholar]
- Pedros-Garrido, S.; Clemente, I.; Calanche, J.B.; Condon-Abanto, S.; Beltran, J.A.; Lyng, J.G.; Brunton, N.; Bolton, D.; Whyte, P. Antimicrobial activity of natural compounds against listeria spp. And their effects on sensory attributes in salmon (salmo salar) and cod (gadus morhua). Food Control 2020, 107. [Google Scholar] [CrossRef]
- Pedrosa, G.; Pimentel, T.; Gavahian, M.; Medeiros, L.; Pagán, R.; Magnani, M. The combined effect of essential oils and emerging technologies on food safety and quality. LWT Food Sci. Technol. 2021, 147, 111593. [Google Scholar] [CrossRef]
- d’Acampora Zellner, B.; Casilli, A.; Dugo, P.; Dugo, G.; Mondello, L. Odour fingerprint acquisition by means of comprehensive two-dimensional gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromatogr. A 2007, 1141, 279–286. [Google Scholar] [CrossRef]
- Jalal, K.C.; Akbar, J.; Nurul, L.; Faizul, H.N.; Isma, Y.; Irwandi, J.; Mahbuba, B. Comparative study on spoilage and pathogenic bacteria in selected commercial marine and freshwater fishes. Int. Food Res. J. 2017, 6, 298–304. [Google Scholar]
- Sørensen, J.S.; Bøknæs, N.; Mejlholm, O.; Dalgaard, P. Superchilling in combination with modified atmosphere packaging resulted in long shelf-life and limited microbial growth in atlantic cod (gadus morhua L.) from capture-based-aquaculture in greenland. Food Microbiol. 2020, 88, 103405. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, S.O. Isolation of enterobacteriaceae and pseudomonas spp. From raw fish sold in fish market in khartoum state. J. Bacteriol. Res. 2009, 1, 85–88. [Google Scholar]
| No | RI a | Compound b | % c |
|---|---|---|---|
| 1 | 992 | β-myrcene | tr |
| 2 | 1004 | α-phellandrene | tr |
| 3 | 1016 | α-terpinene | tr |
| 4 | 1023 | p-cimene | tr |
| 5 | 1028 | α-limonene | tr |
| 6 | 1029 | β-phellandrene | tr |
| 7 | 1033 | 1,8-cineole | tr |
| 8 | 1034 | Benzyl alcohol | tr |
| 9 | 1038 | (Z)-β-ocimene | tr |
| 10 | 1047 | (E)-β-ocimene | tr |
| 11 | 1060 | γ-terpinene | tr |
| 12 | 1074 | Cis-linalool oxide | 0.6 |
| 13 | 1089 | Trans-linalool oxide | 0.9 |
| 14 | 1098 | linalool | 98.1 |
| 15 | 1148 | camphor | tr |
| 16 | 1422 | (E)-caryophyllene | tr |
| Total | 99.5 |
| No | RI a | Compound b | % c |
|---|---|---|---|
| 1 | 926 | α-thujene | 0.6 |
| 2 | 938 | α-pinene | 2.6 |
| 3 | 948 | Camphene | tr |
| 4 | 977 | Sabinene | 2.8 |
| 5 | 980 | β-pinene | 13.3 |
| 6 | 992 | β-myrcene | 2.0 |
| 7 | 998 | n-octanal | tr |
| 8 | 1004 | α-phellandrene | tr |
| 9 | 1016 | α-terpinene | 0.2 |
| 10 | 1023 | p-cimene | 1.0 |
| 11 | 1028 | α-limonene | 58.9 |
| 12 | 1047 | (E)-β-ocimene | tr |
| 13 | 1060 | γ-terpinene | 11.2 |
| 14 | 1088 | α-terpinolene | 0.5 |
| 15 | 1103 | n-nonanal | tr |
| 16 | 1136 | Cis-limonene oxide | tr |
| 17 | 1138 | Trans-limonene oxide | tr |
| 18 | 1152 | Citronellal | tr |
| 19 | 1178 | 4-terpinenol | tr |
| 20 | 1189 | α-terpineol | 0.2 |
| 21 | 1202 | n-decanal | tr |
| 22 | 1238 | Neral | 1.1 |
| 26 | 1266 | Geranial | 1.8 |
| 27 | 1355 | Citronellyl acetate | tr |
| 28 | 1364 | Neryl acetate | 0.6 |
| 29 | 1380 | Geranyl acetate | 0.3 |
| 30 | 1422 | (E)-caryophyllene | 0.3 |
| 31 | 1437 | α-trans-bergamotene | 0.6 |
| 32 | 1497 | Valencene | tr |
| 33 | 1507 | β-bisabolene | 0.9 |
| total | 98.8 |
| Sample | CB | LAB | Other |
|---|---|---|---|
| Control—air | Hafnia alvei, Serratia fonticola, Serratia proteamaculans, Yersinia ruckeri, and Pantoea agglomerans | Bacillus altitudinis, Aeromonas salmonicida, Pseudomonas libanensis, Pseudomonas proteolytica, Pseudomonas fragi, Acinetobacter johnsonii, and Acinetobacter harbinensis | |
| Control—vacuum | Hafnia alvei | Aeromonas bestiarum, Aeromonas salmonicida, Aeromonas eucrenophila, Pseudomonas fragi, and Pseudomonas proteolytica | |
| Control—sunflower oil | Hafnia alvei, Serratia fonticola, and Pantoea agglomerans | Lactobacillus sakei Carnobacterium maltaromaticum | Aeromonas eucrenophila, Aeromonas bestiarum, Aeromonas molluscorum, Aeromonas salmonicida, Aeromonas popoffii, and Aeromonas sobria |
| Lemon EO 0.5 | Hafnia alvei and Serratia fonticola | Aeromonas eucrenophila, Aeromonas popoffii, Aeromonas salmonicida, Aeromonas bestiarum, Aeromonas media, Aeromonas sobria, Pseudomonas lundensis, Pseudomonas proteolytica, and Staphylococcus hominis | |
| Lemon EO 1 | Hafnia alvei and Serratia fonticola | Aeromonas bestiarum, Aeromonas eucrenophila, Aeromonas salmonicida, Aeromonas sobria, Pseudomonas rhodesiae, Pseudomonas proteolytica, and Brochothrix thermosphacta | |
| C. camphora EO 0.5 | Hafnia alvei and Serratia fonticola | Lactobacillus sakei | Aeromonas bestiarum, Aeromonas salmonicida, Aeromonas eucrenophila, Aeromonas molluscorum, Pseudomonas brenneri, and Pseudomonas taetrolens |
| C. camphora EO 1 | Hafnia alvei | Lactobacillus sakei | Aeromonas eucrenophila, Aeromonas salmonicida, Aeromonas bestiarum, Pseudomonas fragi, Pseudomonas proteolytica, and Pseudomonas gessardii |
| Microorganisms | Family |
|---|---|
| Lactobacillus sakei | Lactobacillaceace |
| Staphylococcus hominis | Staphylococcaceae |
| Pseudomonas gessardii, Pseudomonas libanensis, Pseudomonas fragii, Pseudomonas proteolytica, Pseudomonas brenneri, Pseudomonas rhodesia, Pseudomonas lundensis, and Pseudomonas taetrolens | Pseudomonadaceae |
| Hafnia alvei, Serratia fonticola, Serratia proteamaculans, Yersinia ruckeri, and Pantoea agglomerans | Enterobacteriaceae |
| Bacillus altitudinis | Bacilliaceae |
| Aeromonas salmonicida, Aeromonas popoffii, Aeromonas eucrenophila, Aeromonas bestiarum, Aeromonas molluscorum, Aeromonas sobria, and Aeromonas media | Aeromonadaceae |
| Acinetobacter johnsonii Acinetobacter harbinensis | Moraxellaceae |
| Brochothrix thermosphacta | Listeriaceae |
| Carnobacterium maltaromaticum | Carnobacteriaceae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunová, S.; Sendra, E.; Haščík, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Influence of Essential Oils on the Microbiological Quality of Fish Meat during Storage. Animals 2021, 11, 3145. https://doi.org/10.3390/ani11113145
Kunová S, Sendra E, Haščík P, Vukovic NL, Vukic M, Kačániová M. Influence of Essential Oils on the Microbiological Quality of Fish Meat during Storage. Animals. 2021; 11(11):3145. https://doi.org/10.3390/ani11113145
Chicago/Turabian StyleKunová, Simona, Esther Sendra, Peter Haščík, Nenad L. Vukovic, Milena Vukic, and Miroslava Kačániová. 2021. "Influence of Essential Oils on the Microbiological Quality of Fish Meat during Storage" Animals 11, no. 11: 3145. https://doi.org/10.3390/ani11113145
APA StyleKunová, S., Sendra, E., Haščík, P., Vukovic, N. L., Vukic, M., & Kačániová, M. (2021). Influence of Essential Oils on the Microbiological Quality of Fish Meat during Storage. Animals, 11(11), 3145. https://doi.org/10.3390/ani11113145








