Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Mixed Feed
2.2. Chemical Analysis of Fermented Mixed Feed
2.3. Birds, Housing and Dietary Treatments
2.4. Sample Collection
2.5. Morphological Investigations
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) and Real-Time Polymerase Chain Reaction (PCR)
2.7. 16S rRNA Gene Sequencing
2.8. Statistical Analysis
3. Results
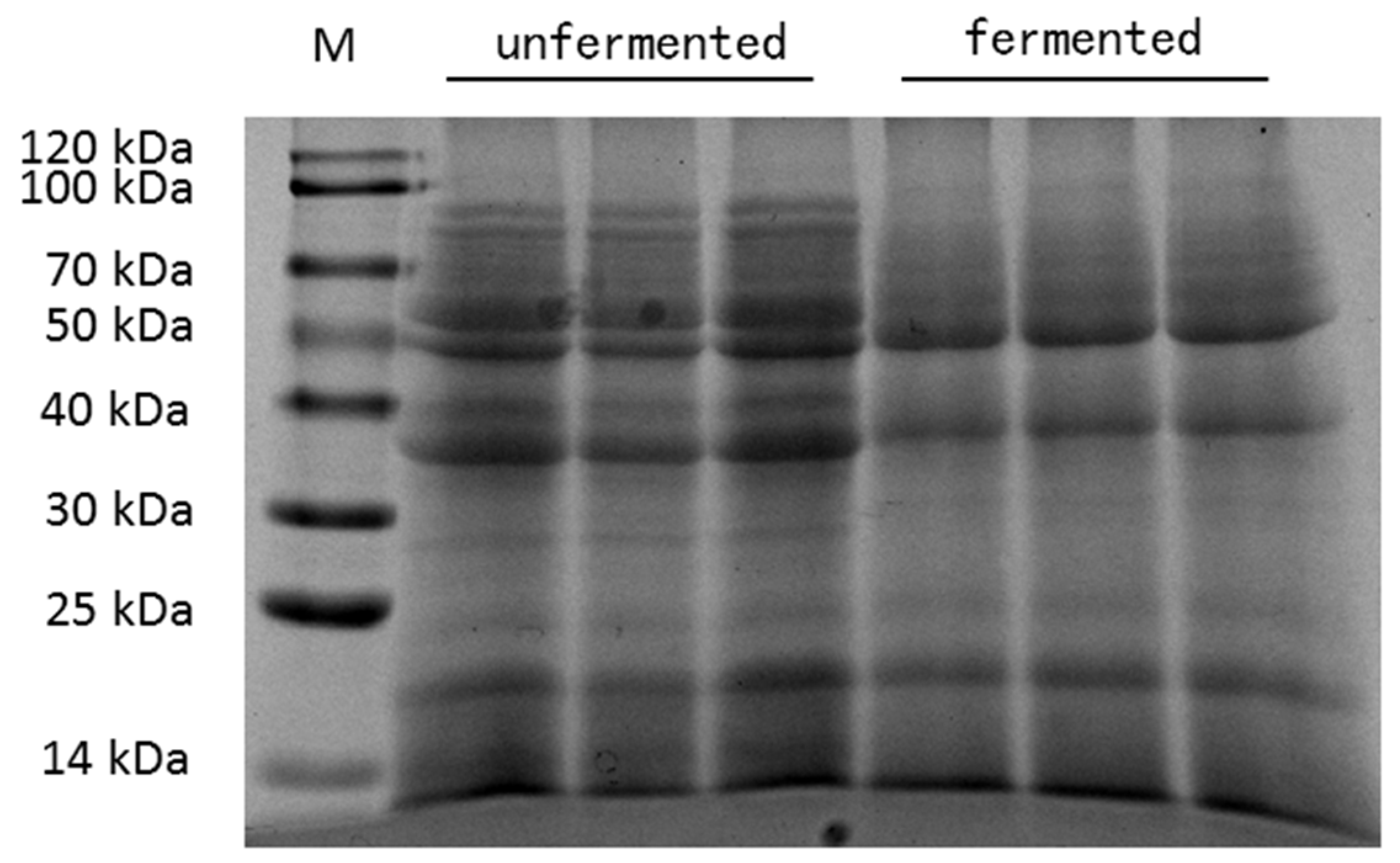
3.1. Chemical Composition of Fermented Mixed Feed
3.2. Intestinal Morphology
3.3. Total sIgA Concentration and Physical Barrier mRNA Abundance in the Intestinal Mucosa
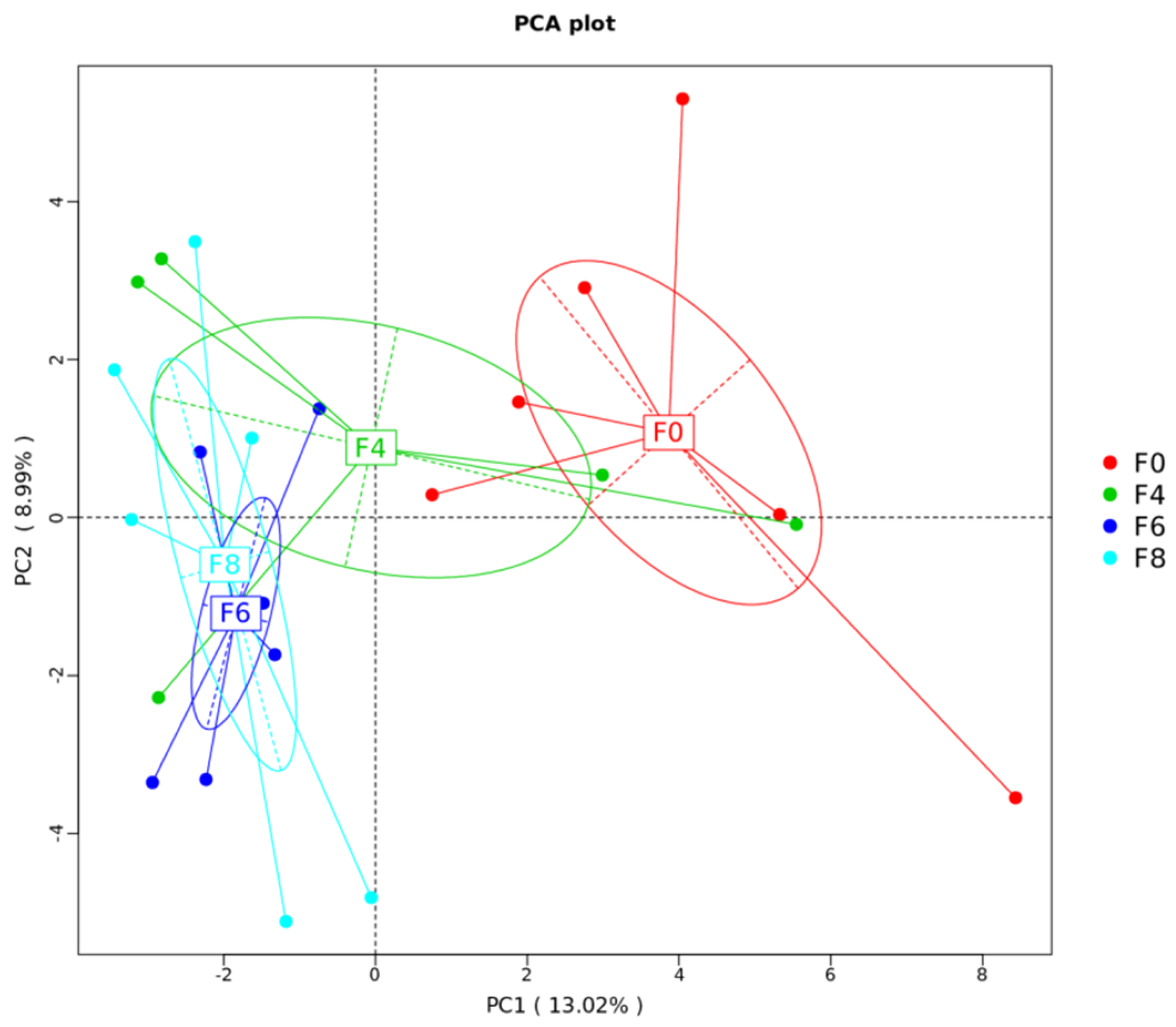
3.4. Cecal Microbial Diversity and Community
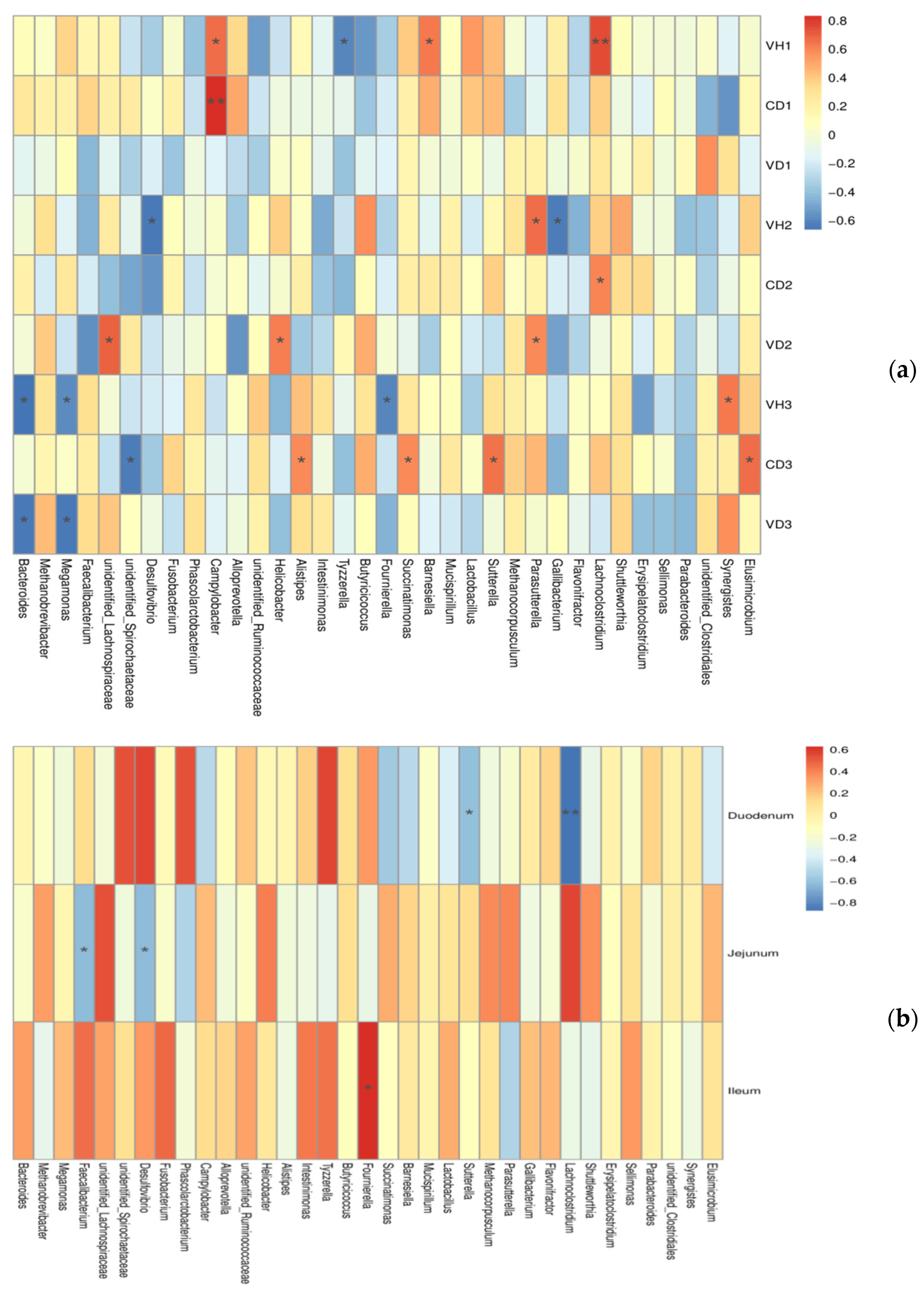
3.5. Correlations between Microbiota and Gut Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khalique, A.; Zeng, D.; Shoaib, M.; Wang, H.; Qing, X.; Rajput, D.S.; Pan, K.; Ni, X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. AMB Express 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Han, Y.; Zhao, J.-z.; Zhou, Z.-j.; Fan, H. Consuming fermented distillers’ dried grains with solubles (DDGS) feed reveals a shift in the faecal microbiota of growing and fattening pigs using 454 pyrosequencing. J. Integr. Agric. 2017, 16, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Plumed-Ferrer, C.; von Wright, A. Fermented pig liquid feed: Nutritional, safety and regulatory aspects. J. Appl. Microbiol. 2009, 106, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, C.; Su, W.; Jin, M.; Xu, B.; Hao, L.; Zhang, Y.; Lu, Z.; Wang, F.; Wang, Y.; et al. Dynamics of the Physicochemical Characteristics, Microbiota, and Metabolic Functions of Soybean Meal and Corn Mixed Substrates during Two-Stage Solid-State Fermentation. mSystems 2020, 5, 501–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.Y.; Wu, Y.H.; Jiang, C.Y.; Liu, Y. Solid state fermented potato pulp can be used as poultry feed. Br. Poult. Sci. 2010, 51, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.R.; Moravej, H.; Tabandeh, F.; Zaghari, M.; Shivazad, M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult. Sci. 2009, 88, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Z.; Zhu, K.; Xue, Y.; Xie, F.; Mao, S. Comprehensive Understanding of the Bacterial Populations and Metabolites Profile of Fermented Feed by 16S rRNA Gene Sequencing and Liquid Chromatography-Mass Spectrometry. Metabolites 2019, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [Green Version]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Canibe, N.; Jensen, B.B. Fermented liquid feed-Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Engberg, R.M.; Hammershoj, M.; Johansen, N.F.; Abousekken, M.S.; Steenfeldt, S.; Jensen, B.B. Fermented feed for laying hens: Effects on egg production, egg quality, plumage condition and composition and activity of the intestinal microflora. Br. Poult Sci. 2009, 50, 228–239. [Google Scholar] [CrossRef]
- Semjon, B.; Bartkovsky, M.; Marcincakova, D.; Klempova, T.; Bujnak, L.; Hudak, M.; Jaduttova, I.; Certik, M.; Marcincak, S. Effect of Solid-State Fermented Wheat Bran Supplemented with Agrimony Extract on Growth Performance, Fatty Acid Profile, and Meat Quality of Broiler Chickens. Animals 2020, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, J.; Wang, H.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Effects of fermented feeds and ginseng polysaccharides on the intestinal morphology and microbiota composition of Xuefeng black-bone chicken. PLoS ONE 2020, 15, e0237357. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.; Lu, W.Q.; Piao, X.S.; Hu, J.K.; Gong, L.M.; Thacker, P.A. Effects of Feeding Solid-state Fermented Rapeseed Meal on Performance, Nutrient Digestibility, Intestinal Ecology and Intestinal Morphology of Broiler Chickens. Asian Australas. J. Anim. Sci. 2009, 23, 263–271. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Zhang, Y.; Song, D.; Lu, Z.; Wang, Y. Microbiota in fermented feed and swine gut. Appl. Microbiol. Biotechnol. 2018, 102, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, R.; Selvaraj, P.; Nanjappan, K. Feeding of Fermented Soybean Meal on Broiler Performance. Int. J. Poult. Sci. 2006, 5, 868–872. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-J.; Xu, Z.-R.; Zhao, S.-H.; Sun, J.-Y.; Yang, X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed Sci. Technol. 2007, 135, 176–186. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Yin, Y.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Amino acid and phosphorus digestibility of fermented corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium fed to pigs. J. Anim. Sci. 2017, 95, 3996–4004. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2006. [Google Scholar]
- Buddrick, O.; Jones, O.A.H.; Cornell, H.J.; Small, D.M. The influence of fermentation processes and cereal grains in wholegrain bread on reducing phytate content. J. Cereal. Sci. 2014, 59, 3–8. [Google Scholar] [CrossRef]
- Hong, K.J.; Lee, C.H.; Kim, S.W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M.; Fanning, A.S. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 2004, 16, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Pan, H.; Sun, Z.; Qin, G. Effect of soybean variety on anti-nutritional factors content, and growth performance and nutrients metabolism in rat. Int. J. Mol. Sci. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-H.; Cho, S.-J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Varsha, K.K.; Priya, S.; Devendra, L.; Nampoothiri, K.M. Control of spoilage fungi by protective lactic acid bacteria displaying probiotic properties. Appl. Biochem. Biotechnol. 2014, 172, 3402–3413. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Lu, Z.; Wang, Y. Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Sokrab, A.M.; Mohamed Ahmed, I.A.; Babiker, E.E. Effect of fermentation on antinutrients, and total and extractable minerals of high and low phytate corn genotypes. J. Food Sci. Technol. 2014, 51, 2608–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, W.; Wang, C.; Zhu, R.; Qiao, J. Characteristics of Solid-state Fermented Feed and its Effects on Performance and Nutrient Digestibility in Growing-finishing Pigs. Asian Australs. J. Anim. Sci. 2008, 21, 1635–1641. [Google Scholar] [CrossRef]
- Silk, D.B.; Chung, Y.C.; Berger, K.L.; Conley, K.; Beigler, M.; Sleisenger, M.H.; Spiller, G.A.; Kim, Y.S. Comparison of oral feeding of peptide and amino acid meals to normal human subjects. Gut 1979, 20, 291–299. [Google Scholar] [CrossRef]
- Missotten, J.A.; Michiels, J.; Dierick, N.; Ovyn, A.; Akbarian, A.; De Smet, S. Effect of fermented moist feed on performance, gut bacteria and gut histo-morphology in broilers. Br. Poult. Sci. 2013, 54, 627–634. [Google Scholar] [CrossRef]
- Li, L.; Li, W.F.; Liu, S.Z.; Wang, H.H. Probiotic fermented feed improved the production, health and nutrient utilisation of yellow-feathered broilers reared in high altitude in Tibet. Br. Poult. Sci. 2020, 61, 746–753. [Google Scholar] [CrossRef]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef]
- Jazi, V.; Foroozandeh, A.D.; Toghyani, M.; Dastar, B.; Rezaie Koochaksaraie, R.; Toghyani, M. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella Typhimurium. Poult. Sci. 2018, 97, 2034–2043. [Google Scholar] [CrossRef]
- Zarkadas, L.N.; Wiseman, J. Influence of processing of full fat soya beans included in diets for piglets. Anim. Feed. Sci. Technol. 2005, 118, 121–137. [Google Scholar] [CrossRef]
- Kumar, N.; Arthur, C.P.; Ciferri, C.; Matsumoto, M.L. Structure of the secretory immunoglobulin A core. Science 2020, 367, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Mitjans, M.; Barniol, G.; Ferrer, R. Mucosal surface area in chicken small intestine during development. Cell Tissue Res. 1997, 290, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4659–4665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib-Naseri, K.; de Paula Dorigam, J.C.; Doranalli, K.; Kheravii, S.; Swick, R.A.; Choct, M.; Wu, S.B. Modulations of genes related to gut integrity, apoptosis, and immunity underlie the beneficial effects of Bacillus amyloliquefaciens CECT 5940 in broilers fed diets with different protein levels in a necrotic enteritis challenge model. J. Anim. Sci. Biotechnol. 2020, 11, 104. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Wang, J.; Van Parys, A.; Haesebrouck, F.; Joossens, M.; Falony, G.; Raes, J.; Ducatelle, R.; Van Immerseel, F. The Probiotic Butyricicoccus pullicaecorum Reduces Feed Conversion and Protects from Potentially Harmful Intestinal Microorganisms and Necrotic Enteritis in Broilers. Front. Microbiol. 2016, 7, 1416. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.; Denman, S.E.; Hughes, R.J.; Geier, M.S.; Crowley, T.M.; Chen, H.; Haring, V.R.; Moore, R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012, 96, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, C.; De Souza-Pilz, M.; Bojesen, A.M.; Bisgaard, M.; Hess, M. Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathol. 2009, 38, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Wiersema, M.L.; Koester, L.R.; Schmitz-Esser, S.; Koltes, D.A. Comparison of intestinal permeability, morphology, and ileal microbial communities of commercial hens housed in conventional cages and cage-free housing systems. Poult. Sci. 2021, 100, 1178–1191. [Google Scholar] [CrossRef] [PubMed]


| Items | Group | |||
|---|---|---|---|---|
| 0 | 4% | 6% | 8% | |
| Ingredients | ||||
| Corn | 56.00 | 56.00 | 56.00 | 56.00 |
| Soybean meal | 20.60 | 20.60 | 20.60 | 20.60 |
| Wheat bran | 2.40 | 2.40 | 2.40 | 2.40 |
| Fermented feed | 0.00 | 4.00 | 6.00 | 8.00 |
| Unfermented feed | 8.00 | 4.00 | 2.00 | 0.00 |
| Stone powder | 7.50 | 7.50 | 7.50 | 7.50 |
| Soybean oil | 0.50 | 0.50 | 0.50 | 0.50 |
| Premix 1 | 5.00 | 5.00 | 5.00 | 5.00 |
| Nutrient levels 2 | ||||
| ME(MJ/kg) | 12.09 | 12.09 | 12.09 | 12.09 |
| Crude protein | 15.99 | 16.00 | 16.01 | 16.01 |
| Calcium | 3.60 | 3.60 | 3.60 | 3.60 |
| Total phosphorus | 0.42 | 0.42 | 0.42 | 0.42 |
| Lys | 0.94 | 0.94 | 0.94 | 0.94 |
| Met | 0.44 | 0.44 | 0.44 | 0.44 |
| Thr | 0.70 | 0.70 | 0.70 | 0.70 |
| Gene | Primers Sequence (5′–3′) |
|---|---|
| β-actin | F: ACACCCACACCCCTGTGATGAA |
| R: TGCTGCTGACACCTTCACCATTC | |
| ZO-1 | F: TATAGAAGATCGTGCCGCCTCC |
| R: GAGGTCTGCCATCGTAGCTC | |
| Occludin | F: ACAGCCCTCAATACCAGGATGTG |
| R: ACCATGCGCTTGATGTGGAA | |
| MUC2 | F: TTCATGATGCCTGCTCTTGTG |
| R: CCTGAGCCTTGGTACATTCTTGT |
| Unfermented Mixed Feed | Fermented Mixed Feed | SEM | p-Value | |
|---|---|---|---|---|
| pH | 6.23 a | 4.49 b | 0.40 | <0.01 |
| Crude protein, % | 15.90 b | 16.18 a | 0.07 | 0.016 |
| Crude fiber, % | 3.33 | 3.20 | 2.80 | 0.680 |
| Ether extract, % | 0.57 | 1.09 | 0.15 | 0.060 |
| Phytic acid, % | 0.65 a | 0.37 b | 0.07 | 0.025 |
| Trypsin inhibitor, μg/g | 359.29 a | 216.39 b | 32.04 | <0.01 |
| β-glucan, μg/g | 1588.89 a | 1204.32 b | 86.35 | <0.01 |
| Fermented Mixed Feed | SEM | p-Value | ||||
|---|---|---|---|---|---|---|
| 0% | 4% | 6% | 8% | |||
| Duodenum | ||||||
| VH 1, μm | 1767.04 | 1731.68 | 1774.26 | 1753.69 | 21.83 | 0.889 |
| CD 2, μm | 294.56 a | 256.96 b | 254.26 b | 221.31 c | 4.24 | <0.01 |
| VH/CD 3, μm/μm | 6.18 bc | 6.86 b | 7.16 b | 8.02 a | 0.13 | <0.01 |
| Jejunum | ||||||
| VH, μm | 922.89 c | 1315.79 a | 1079.89 b | 1121.96 b | 19.41 | <0.01 |
| CD, μm | 177.47 b | 222.91 a | 180.14 b | 169.85 b | 3.94 | <0.01 |
| VH/CD, μm/μm | 5.28 b | 6.23 a | 6.28 a | 6.88 a | 0.12 | <0.01 |
| Ileum | ||||||
| VH, μm | 882.98 | 936.10 | 968.38 | 961.42 | 15.84 | 0.208 |
| CD, μm | 136.40 | 145.14 | 145.96 | 138.04 | 1.97 | 0.201 |
| VH/CD, μm/μm | 6.57 | 6.64 | 6.63 | 7.13 | 0.11 | 0.241 |
| Fermented Mixed Feed | SEM | p-Value | ||||
|---|---|---|---|---|---|---|
| 0% | 4% | 6% | 8% | |||
| Chao1 | 693.32 | 682.26 | 712.44 | 731.14 | 10.61 | 0.398 |
| Ace | 702.49 | 694.70 | 723.97 | 740.77 | 10.52 | 0.419 |
| Shannon | 6.42 | 5.90 | 6.41 | 6.68 | 0.12 | 0.137 |
| Simpson | 0.97 | 0.94 | 0.96 | 0.97 | 0.01 | 0.287 |
| Treatment | R-Value | p-Value |
|---|---|---|
| F0–F4 | 0.07407 | 0.4 |
| F0–F8 | 0.6 | 0.016 |
| F4–F8 | 0.4769 | 0.025 |
| F0–F6 | 0.3457 | 0.045 |
| F4–F6 | 0.2099 | 0.214 |
| F6–F8 | −0.02667 | 0.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Feng, J.; Wang, Y.; Lv, J.; Li, J.; Guo, L.; Min, Y. Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals 2021, 11, 3059. https://doi.org/10.3390/ani11113059
Liu Y, Feng J, Wang Y, Lv J, Li J, Guo L, Min Y. Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals. 2021; 11(11):3059. https://doi.org/10.3390/ani11113059
Chicago/Turabian StyleLiu, Yinglu, Jia Feng, Yamin Wang, Jing Lv, Jinghe Li, Lijuan Guo, and Yuna Min. 2021. "Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens" Animals 11, no. 11: 3059. https://doi.org/10.3390/ani11113059
APA StyleLiu, Y., Feng, J., Wang, Y., Lv, J., Li, J., Guo, L., & Min, Y. (2021). Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals, 11(11), 3059. https://doi.org/10.3390/ani11113059





