Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Corticosterone Administration
2.4. Bird Husbandry
2.5. Sample Collection
2.6. Sample Preparation
2.7. Nuclear Magnetic Resonance Data Acquisition and Processing
2.8. Statistical Analysis
3. Results
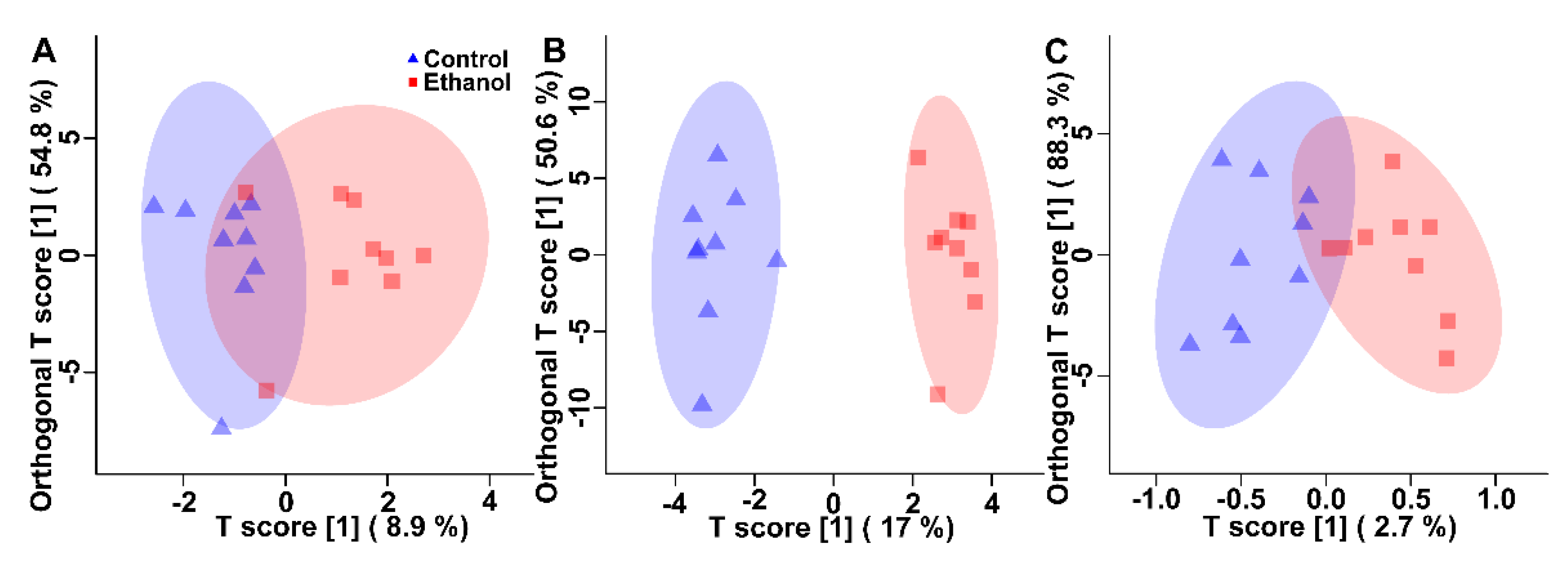
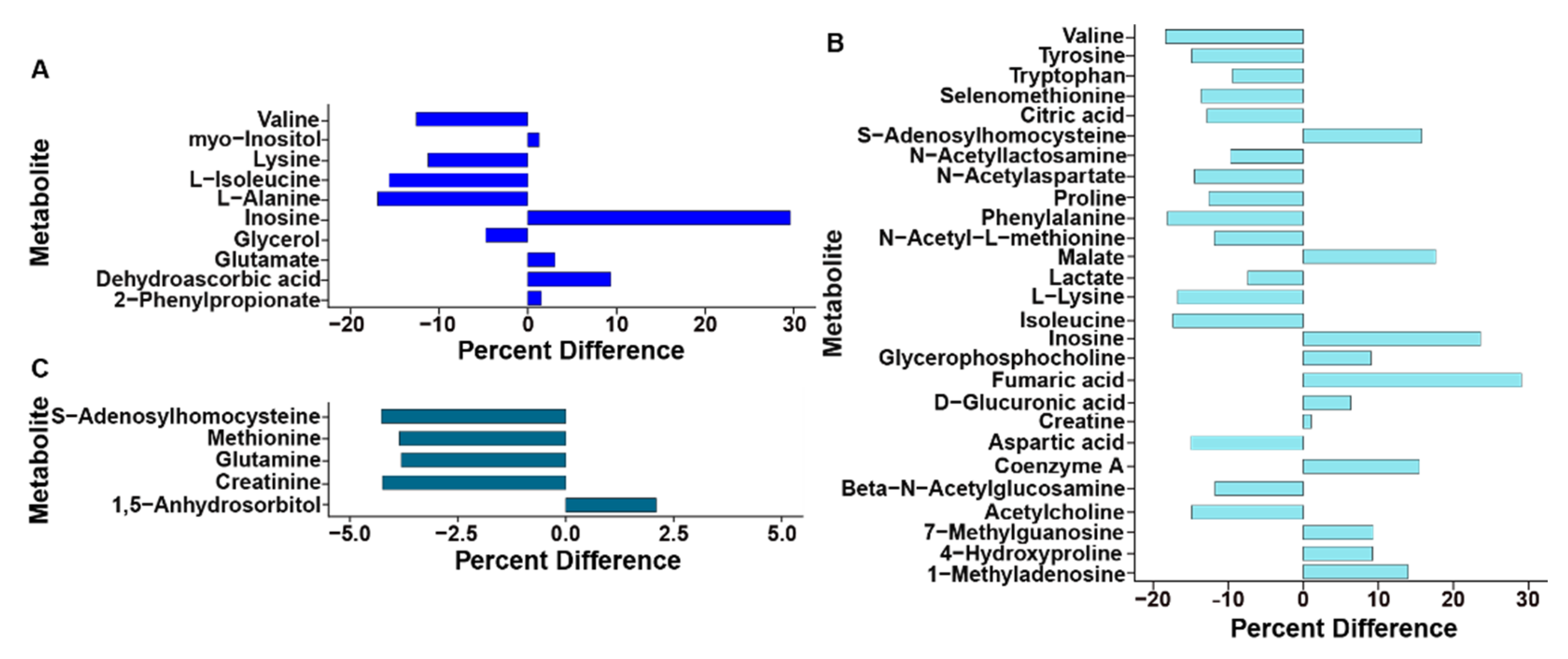
3.1. Ethanol Alters the Metabolome of Kidneys, Liver, and Breast Muscle
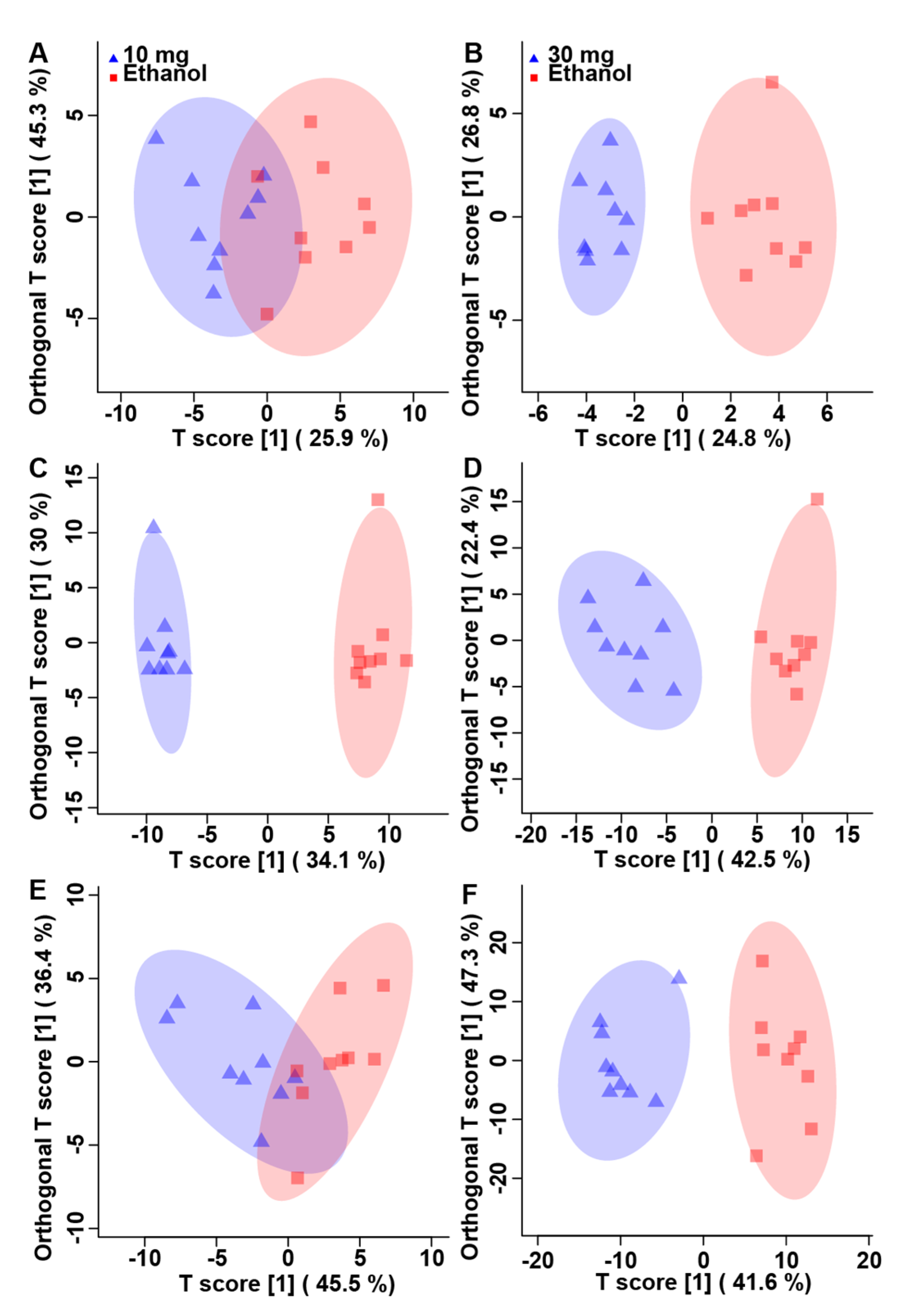
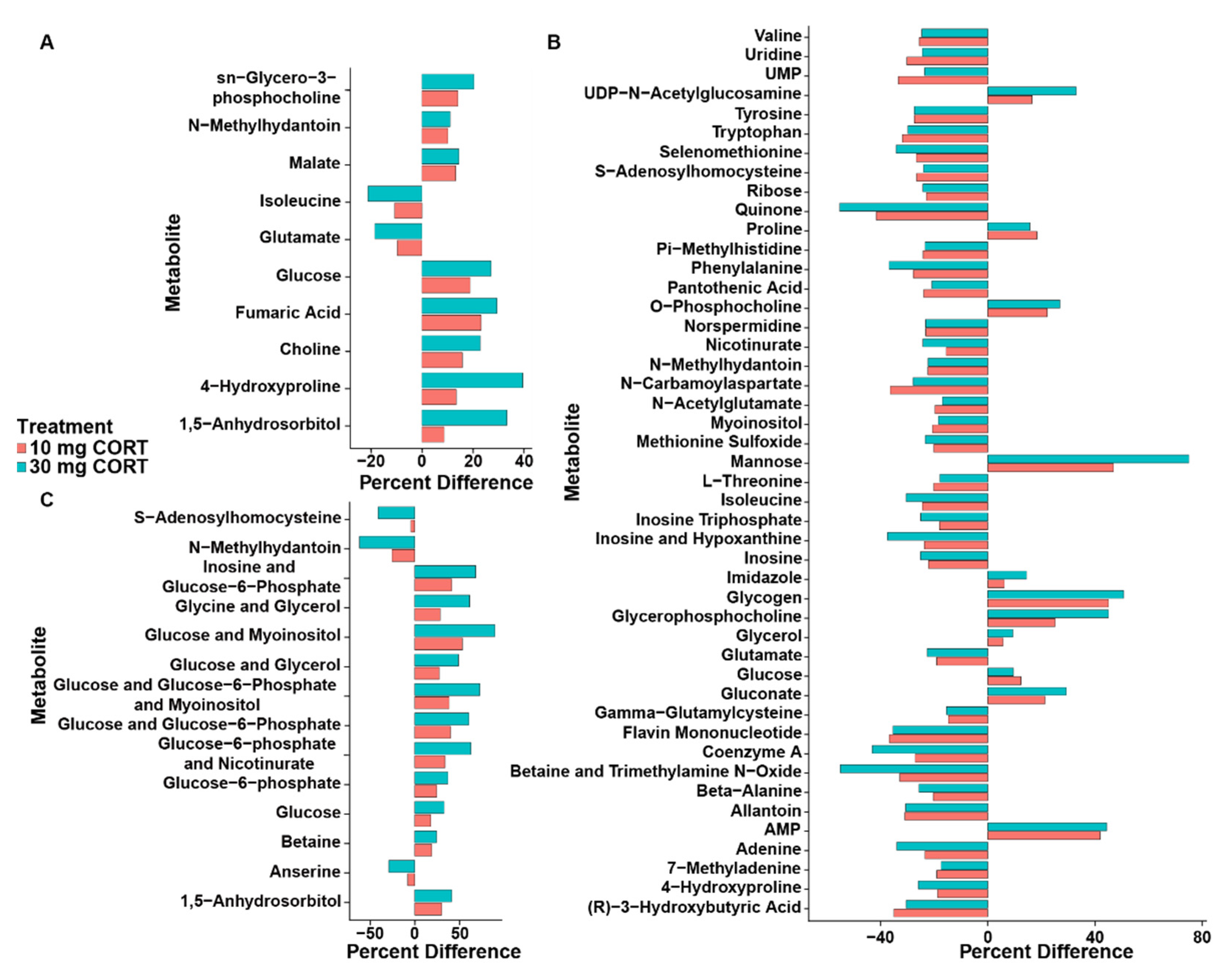
3.2. Corticosterone Substantively Alters the Metabolome of Kidneys, Liver, and Breast Muscle
4. Discussion
4.1. Ethanol Impacts on Kidneys
4.2. Ethanol Impacts on Liver
4.3. Ethanol Impacts on Breast Muscle
4.4. Corticosterone Impacts on the Kidney Metabolome
4.5. Corticosterone Impacts on the Liver Metabolome
4.6. Corticosterone Impacts on the Breast Muscle Metabolome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockrem, J.F. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Lattin, C.R.; Reed, J.M.; DesRochers, D.W.; Romero, L.M. Elevated corticosterone in feathers correlates with corticosterone-induced decreased feather quality: A validation study. J. Avian Biol. 2011, 42, 247–252. [Google Scholar] [CrossRef]
- Shini, S.; Kaiser, P. Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress 2009, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Hangalapura, B.N.; Nieuwland, M.G.B.; Buyse, J.; Kemp, B.; Parmentier, H.K. Effect of duration of cold stress on plasma adrenal and thyroid hormone levels and immune responses in chicken lines divergently selected for antibody responses. Poult. Sci. Assoc. 2004, 83, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Zhang, Z.W.; Qu, J.P.; Yao, H.D.; Li, M.; Li, S.; Xu, S.W. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperon 2014, 19, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulkifli, I.; Najafi, P.; Nurfarahin, A.J.; Soleimani, A.F.; Kumari, S.; Aryani, A.A.; O’Reilly, E.L.; Eckersall, P.D. Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poult. Sci. 2014, 93, 3112–3118. [Google Scholar] [CrossRef]
- Mu, P.; Xu, N.; Chai, T.; Jia, Q.; Yin, Z.; Yang, S.; Qian, Y.; Qiu, J. Simultaneous determination of 14 antiviral drugs and relevant metabolites in chicken muscle by UPLC–MS/MS after QuEChERS preparation. J. Chromatogr. B 2016, 1023-1024, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Beier, R.C.; Cao, X.; Shen, J.; Zhang, S. Simultaneous determination of mequindox, quinocetone, and their major metabolites in chicken and pork by UPLC–MS/MS. Food Chem. 2014, 160, 171–179. [Google Scholar] [CrossRef]
- Monajjemzadeh, F.; Farajzadeh, M.A.; Mogaddam, M.R.A. Combination of pressurised liquid extraction with dispersive liquid–liquid microextraction method for the extraction of some pesticides and their related metabolites from chicken liver. Int. J. Environ. Anal. Chem. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Yong, Y.; Liu, Y.; He, L.; Xu, L.; Zhang, Y.; Fang, B. Simultaneous determination of quinocetone and its major metabolites in chicken tissues by high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2013, 919-920, 30–37. [Google Scholar] [CrossRef]
- Cajka, T.; Danhelova, H.; Zachariasova, M.; Riddellova, K.; Hajslova, J. Application of direct analysis in real time ionization–mass spectrometry (DART–MS) in chicken meat metabolomics aiming at the retrospective control of feed fraud. Metabolomics 2013, 9, 545–557. [Google Scholar] [CrossRef]
- Alexandrakis, D.; Brunton, N.P.; Downey, G.; Scannell, A.G.M. Identification of spoilage marker metabolites in Irish chicken breast muscle using HPLC, GC–MS coupled with SPME and traditional chemical techniques. Food Bioprocess Technol. 2012, 5, 1917–1923. [Google Scholar] [CrossRef]
- Lytou, A.E.; Nychas, G.-J.; Panagou, E.Z. Effect of pomegranate based marinades on the microbiological, chemical and sensory quality of chicken meat: A metabolomics approach. Int. J. Food Microbiol. 2018, 267, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.D.; Wright, B.D.; Sheppard, S.A.; Abbott, D.W.; Oryschak, M.A.; Montina, T. Expeller-pressed canola (Brassica napus) meal modulates the structure and function of the cecal microbiota, and alters the netabolome of the pancreas, liver, and breast muscle of broiler chickens. Animals 2021, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Aggrey, S.E.; Milfort, M.C.; Fuller, A.L.; Yuan, J.; Rekaya, R. Effect of host genotype and Eimeria acervulina infection on the metabolome of meat-type chickens. PLoS ONE 2019, 14, e0223417. [Google Scholar] [CrossRef]
- Beauclercq, S.; Nadal-Desbarats, L.; Hennequet-Antier, C.; Collin, A.; Tesseraud, S.; Bourin, M.; Le Bihan-Duval, E.; Berri, C. Serum and muscle metabolomics for the prediction of ultimate pH, a key factor for chicken-meat quality. J. Proteome Res. 2016, 15, 1168–1178. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, H.Y.; Zhang, H.J.; Xu, L.; Wu, S.G.; Yan, H.J.; Gong, Y.S.; Qi, G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 2009, 88, 2033–2041. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, Y.; Wang, G.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1 H-NMR-based water-soluble low molecular weight compound characterization and fatty acid composition of boiled Wuding chicken during processing. J. Sci. Food Agric. 2018, 99, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ’Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, C.I.; Mappley, L.J.; La Ragione, R.M.; Woodward, M.J.; Claus, S.P. NMR-based metabolic characterization of chicken tissues and biofluids: A model for avian research. Metabolomics 2016, 12, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauclercq, S.; Nadal-Desbarats, L.; Hennequet-Antier, C.; Gabriel, I.; Tesseraud, S.; Calenge, F.; Le Bihan-Duval, E.; Mignon-Grasteau, S. Relationships between digestive efficiency and metabolomic profiles of serum and intestinal contents in chickens. Sci. Rep. 2018, 8, 6678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, D.; Ambeskovic, M.; Montina, T.; Metz, G.A.S. Stress transgenerationally programs metabolic pathways linked to altered mental health. Cell. Mol. Life Sci. 2016, 73, 4547–4557. [Google Scholar] [CrossRef] [PubMed]
- Paxman, E.J.; Boora, N.S.; Kiss, D.; Laplante, D.P.; King, S.; Montina, T.; Metz, G.A.S. Prenatal maternal stress from a natural disaster alters urinary metabolomic profiles in project ice storm participants. Sci. Rep. 2018, 8, 12932. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, J.; Radmilovic, A.; Montina, T.D.; Metz, G.A.S. Cardiorenal metabolic biomarkers link early life stress to risk of non-communicable diseases and adverse mental health outcomes. Sci. Rep. 2020, 10, 13295. [Google Scholar] [CrossRef] [PubMed]
- Zaytsoff, S.J.M.; Brown, C.L.J.; Montina, T.; Metz, G.A.S.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 19225. [Google Scholar] [CrossRef] [PubMed]
- Post, J.; Rebel, J.; ter Huurne, A. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 2003, 82, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.E.; Mahle, D.A.; Doom, T.E.; Reo, N.V.; DelRaso, N.J.; Raymer, M.L. Dynamic adaptive binning: An improved quantification technique for NMR spectroscopic data. Metabolomics 2011, 7, 179–190. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Liang, F.; Deng, B.-C.; Lai, G.-B.; Gonçalves, C.M.V.; Lu, H.-M.; Yan, J.; Huang, X.; Yi, L.-Z.; Liang, Y.-Z. Informative metabolites identification by variable importance analysis based on random variable combination. Metabolomics 2015, 11, 1539–1551. [Google Scholar] [CrossRef]
- Goodpaster, A.M.; Romick-Rosendale, L.; Kennedy, M.A. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal. Biochem. 2010, 401, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-based metabolomics in human disease diagnosis: Applications, limitations, and recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- Hazard, D.; Fernandez, X.; Pinguet, J.; Chambon, C.; Letisse, F.; Portais, J.-C.; Wadih-Moussa, Z.; Rémignon, H.; Molette, C. Functional genomics of the muscle response to restraint and transport in chickens. J. Anim. Sci. 2011, 89, 2717–2730. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, S.; Okuyama, H.; Tachibana, T.; Makino, R. Effects of high ambient temperature on plasma metabolomic profiles in chicks. Anim. Sci. J. 2018, 89, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Huff, G.R.; Shini, A.; Kaiser, P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010, 89, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.K.; Li, Q.Y.; Piao, X.S.; Liu, J.D.; Zhao, P.F.; Xu, X.; Zhang, S.; Niu, S. Forsythia suspensa extract attenuates corticosterone-induced growth inhibition, oxidative injury, and immune depression in broilers. Poult. Sci. 2014, 93, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lin, H.; Jiao, H.C.; Song, Z.G.; Zhao, J.P.; Jiang, K.J. Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp. Biochem. Physiol. 2007, 147, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J. Sci. Food Agric. 2019, 99, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Bartov, I. Effects of dietary protein concentration and corticosterone injections on energy and nitrogen balances and fat deposition in broiler chicks. Br. Poult. Sci. 1985, 26, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nagai, Y.; Ohtsuka, A.; Tomita, Y. Effects of dietary corticosterone and trilostane on growth and skeletal muscle protein turnover in broiler cockerels. Br. Poult. Sci. 1994, 35, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G.; Braun, E.J. Avian metabolism: Its control and evolution. Front. Biol. 2013, 8, 134–159. [Google Scholar] [CrossRef]
- Elkomy, N.M.I.M.; Ibrahim, I.A.A.E.-H.; Elshazly, S.M.; El-Fayoumi, H.M. Ameliorative effects of clonidine on ethanol induced kidney injury in rats: Potential role for imidazoline-1 receptor. Eur. J. Pharmacol. 2018, 824, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Van Thiel, D.H.; Gavaler, J.S.; Little, J.M.; Lester, R. Alcohol: Its effect on the kidney. Metabolism 1977, 26, 857–866. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Nagy, L.E.; McIntyre, T.M. Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation. Free. Radic. Biol. Med. 2014, 69, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Hughes, C.; Ayers, C.R.; Malloy, C.R.; Jin, E.S. Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism 2017, 67, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.-C.; Lian, L.-Y.; Zhang, Y.; He, Q.-D.; Chen, J.-L.; Zhang, L.-B.; Huang, M.-S.; Liu, M.; Qian, L.-C.; Liu, C.-C.; et al. Dynamic analysis of metabolic response in gastric ulcer (GU) rats with electroacupuncture treatment using 1H NMR-based metabolomics. Evid.-Based Complement. Altern. Med. 2019, 2019, 1291427-12. [Google Scholar] [CrossRef]
- Watford, M. Gluconeogenesis in the chicken: Regulation of phosphoenolpyruvate carboxykinase gene expression. Fed. Proc. 1985, 44, 2469–2474. [Google Scholar] [PubMed]
- Karmen, A.; Wróblewski, F.; LaDue, J.S. Transaminase activity in human blood. J. Clin. Investig. 1955, 34, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, R.J.; Wang, Y.; Cline, G.W.; Rabin-Court, A.; Song, J.; Dufour, S.; Zhang, X.M.; Petersen, K.F.; Shulman, G.I. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell 2018, 172, 234–248.e17. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Nagy, L.E.; McIntyre, T.M. Myeloperoxidase formation of PAF receptor ligands induces PAF receptor-dependent kidney injury during ethanol consumption. Free. Radic. Biol. Med. 2015, 86, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afkhami, M.; Kermanshahi, H.; Heravi, R.M. Evaluation of whey protein sources on performance, liver antioxidants and immune responses of broiler chickens challenged with ethanol. J. Anim. Physiol. Anim. Nutr. 2020, 104, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.F.; Wang, L.K.; Wen, A.Y.; Wang, L.X.; Jin, G.M. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Askanazi, J.; Carpentier, Y.A.; Michelsen, C.B.; Elwyn, D.H.; Furst, P.; Kantrowitz, L.R.; Gump, F.E.; Kinney, J.M. Muscle and plasma amino acids following injury influence of intercurrent infection. Ann. Surg. 1980, 192, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Bai, X.; Shah, A.A.; Dai, S.; Wang, L.; Hua, J.; Che, C.; He, S.; Wen, A.; Jiang, J. Interactive effects of glutamine and gamma-aminobutyric acid on growth performance and skeletal muscle amino acid metabolism of 22–42-day-old broilers exposed to hot environment. Int. J. Biometeorol. 2016, 60, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Dryer, S.E.; Alfieri, C.; Kavvadas, P.; Simonini, P.; Ikehata, M.; Dussaule, J.C.; Chadjichristos, C.E.; Rastaldi, M.P.; Messa, P.; Chatziantoniou, C. Glutamate receptors in the kidney. Nephrol. Dial. Transplant. 2015, 30, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phang, J.M.; Donald, S.P.; Pandhare, J.; Liu, Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 2008, 35, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Phang, J.M.; Liu, W.E.I.; Zabirnyk, O. Proline metabolism and microenvironmental stress. Annu. Rev. Nutr. 2010, 30, 441–463. [Google Scholar] [CrossRef] [Green Version]
- Roginski, A.C.; Cecatto, C.; Wajner, S.M.; Camera, F.D.M.; Castilho, R.F.; Wajner, M.; Amaral, A.U. Experimental evidence that maleic acid markedly compromises glutamate oxidation through inhibition of glutamate dehydrogenase and α-ketoglutarate dehydrogenase activities in kidney of developing rats. Mol. Cell. Biochem. 2019, 458, 99–112. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, L.; Li, J.; Xing, T.; Jiang, Y.; Gao, F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021, 100, 215–223. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrebski, S.F.; Lamont, S.J.; Schmidt, C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 2017, 12, e0181900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, A.H.; Zhang, X.; Jastrebski, S.; Singh, A.; Schmidt, C. Understanding the liver under heat stress with statistical learning: An integrated metabolomics and transcriptomics computational approach. BMC Genom. 2019, 20, 502. [Google Scholar] [CrossRef] [PubMed]
- Henle, K.J.; Kaushal, G.P.; Nagle, W.A.; Nolen, G.T. Prompt protein glycosylation during acute heat stress. Exp. Cell Res. 1993, 207, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, Q.; Liu, J.; Jia, Y.; Cai, D.; Idriss, A.A.; Omer, N.A.; Zhao, R. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci. Rep. 2017, 7, 40251. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.; Hu, Y.; Hou, Z.; Zong, Y.; Omer, N.A.; Abobaker, H.; Zhao, R. Corticosterone-induced lipogenesis activation and lipophagy inhibition in chicken liver are alleviated by maternal betaine supplementation. J. Nutr. 2018, 148, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef] [Green Version]
- Bequette, B.J. Amino acid metabolism in animals: An overview. In Amino Acids in Animal Nutrition, 2nd ed.; D’Mello, J.P.F., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 87–102. [Google Scholar]
- Urdaneta-Rincon, M.; Leeson, S. Muscle (pectoralis major) protein turnover in young broiler chickens fed graded levels of lysine and crude protein. Poult. Sci. 2004, 83, 1897–1903. [Google Scholar] [CrossRef]
- Chowdhury, V.S. Heat stress biomarker amino acids and neuropeptide afford thermotolerance in chicks. J. Poult. Sci. 2019, 56, 0180024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girón, M.D.; Vilchez, J.D.; Shreeram, S.; Salto, R.; Manzano, M.; Cabrera, E.; Campos, N.; Edens, N.K.; Rueda, R.; López-Pedrosa, J.M. β-Hydroxy-β-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. PLoS ONE 2015, 10, e0117520. [Google Scholar] [CrossRef]
- Jahromi, M.F.; Altaher, Y.W.; Shokryazdan, P.; Ebrahimi, R.; Ebrahimi, M.; Idrus, Z.; Tufarelli, V.; Liang, J.B. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int. J. Biometeorol. 2016, 60, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Savenije, B.; Lambooij, E.; Gerritzen, M.; Venema, K.; Korf, J. Effects of feed deprivation and transport on preslaughter blood metabolites, early postmortem muscle metabolites, and meat quality. Poult. Sci. 2002, 81, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Hao, Y.; Feng, J.; Lin, H.; Feng, Y.; Wu, X.; Yang, X.; Gu, X. The expression of carnosine and its effect on the antioxidant capacity of Longissimus dorsi muscle in finishing pigs exposed to constant heat stress. Asian-Australas. J. Anim. Sci. 2014, 27, 1763–1772. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, C.L.J.; Zaytsoff, S.J.M.; Montina, T.; Inglis, G.D. Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens. Animals 2021, 11, 3056. https://doi.org/10.3390/ani11113056
Brown CLJ, Zaytsoff SJM, Montina T, Inglis GD. Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens. Animals. 2021; 11(11):3056. https://doi.org/10.3390/ani11113056
Chicago/Turabian StyleBrown, Catherine L. J., Sarah J. M. Zaytsoff, Tony Montina, and G. Douglas Inglis. 2021. "Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens" Animals 11, no. 11: 3056. https://doi.org/10.3390/ani11113056
APA StyleBrown, C. L. J., Zaytsoff, S. J. M., Montina, T., & Inglis, G. D. (2021). Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens. Animals, 11(11), 3056. https://doi.org/10.3390/ani11113056





