Ecological Analysis of the Helminth Community of Microtus lusitanicus (Gerbe, 1879) (Rodentia) in Asturias (NW Spain)
Abstract
:Simple Summary
Abstract
1. Introduction
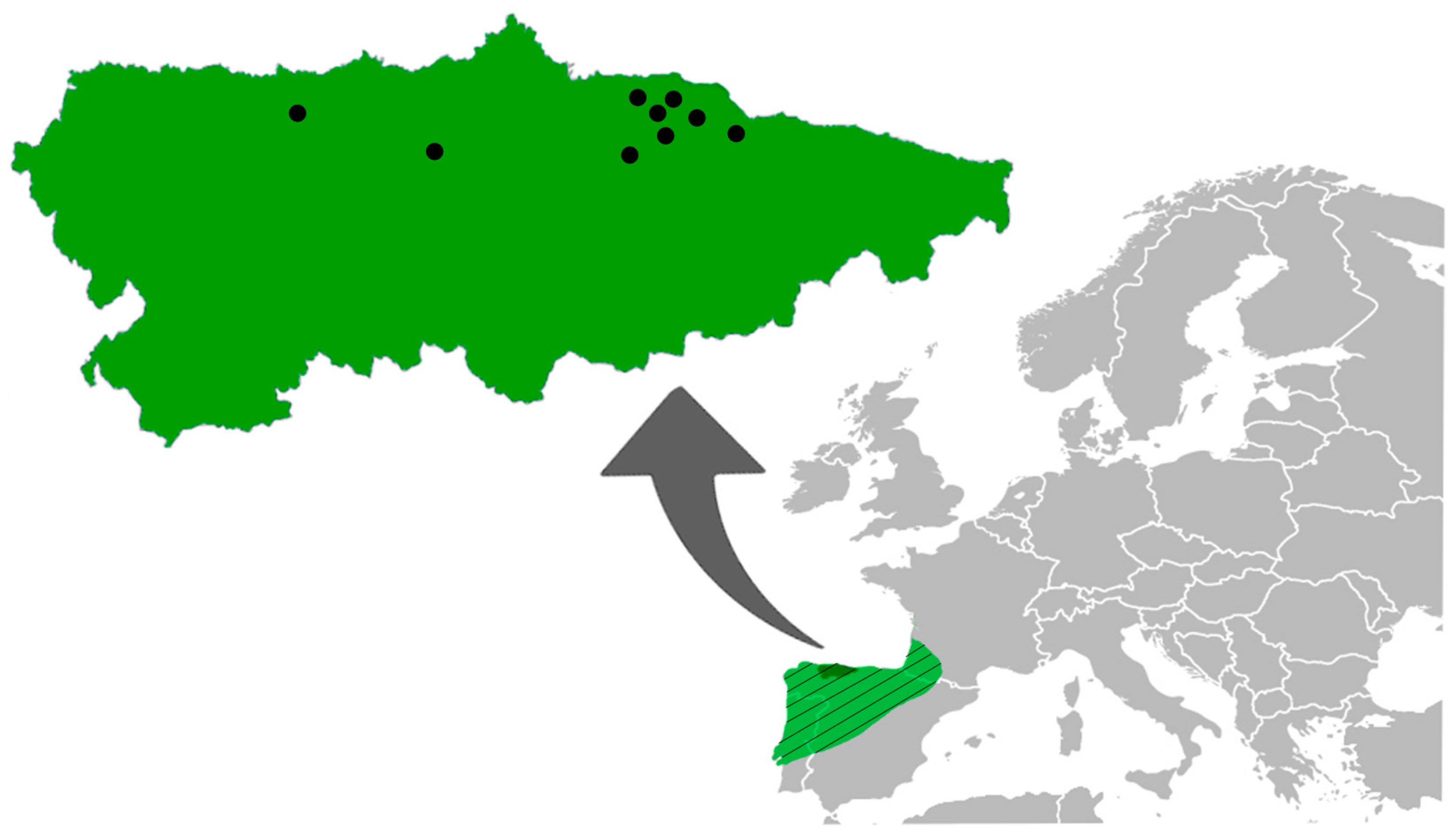
2. Materials and Methods
2.1. Zoological and Helminthological Procedures
2.2. Helminth Community Analysis
3. Results
3.1. Helminth Species
3.1.1. Hydatigera taeniaeformis (Batsch, 1786) Larvae
3.1.2. Paranoplocephala omphalodes (Hermann, 1783)
3.1.3. Rodentolepis asymmetrica (Janicki, 1904)
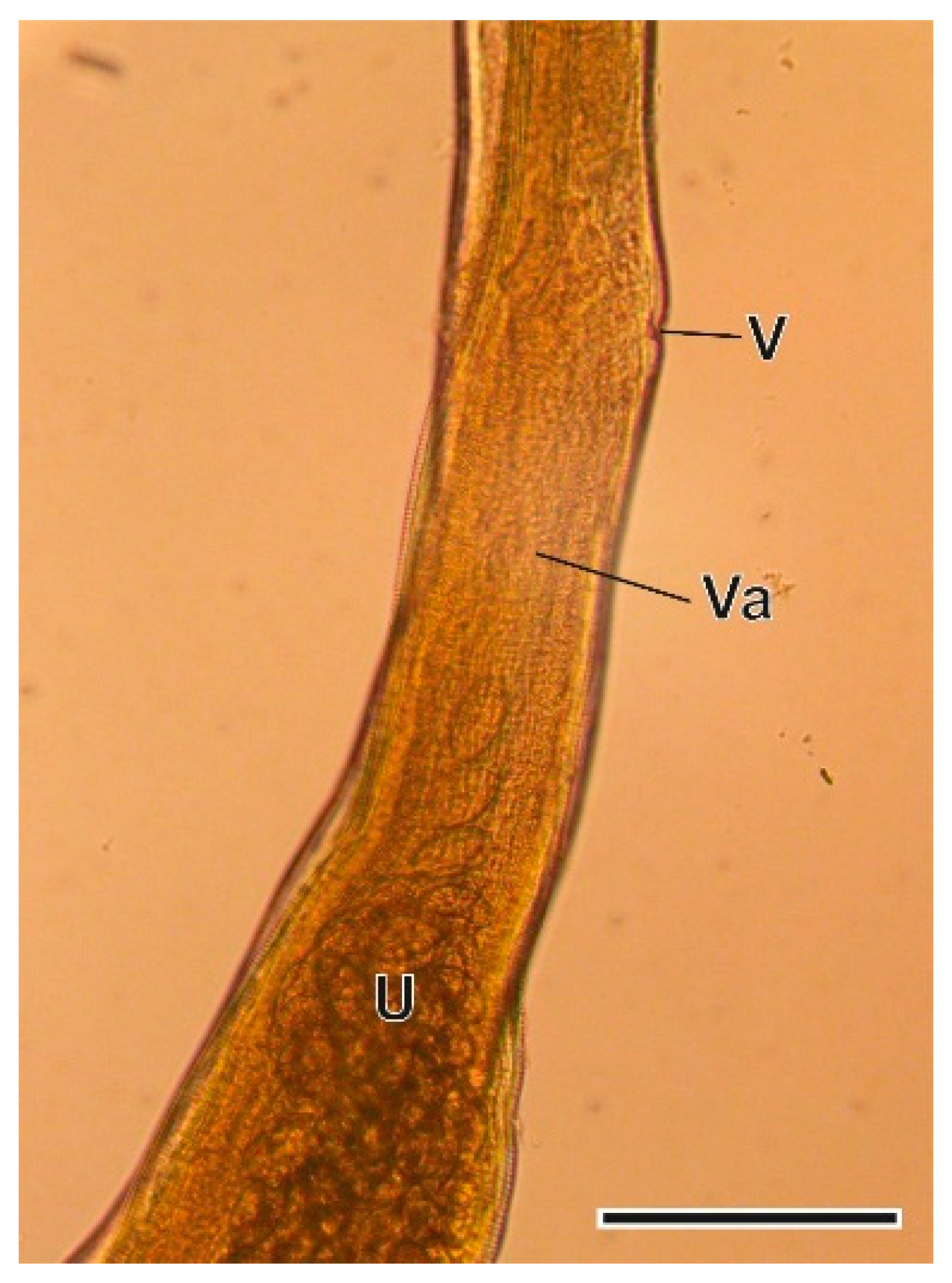
3.1.4. Trichuris arvicolae Feliu et al., 2000
3.1.5. Carolinensis minutus (Dujardin, 1845)
3.1.6. Heligmosomum costellatum (Dujardin, 1845)
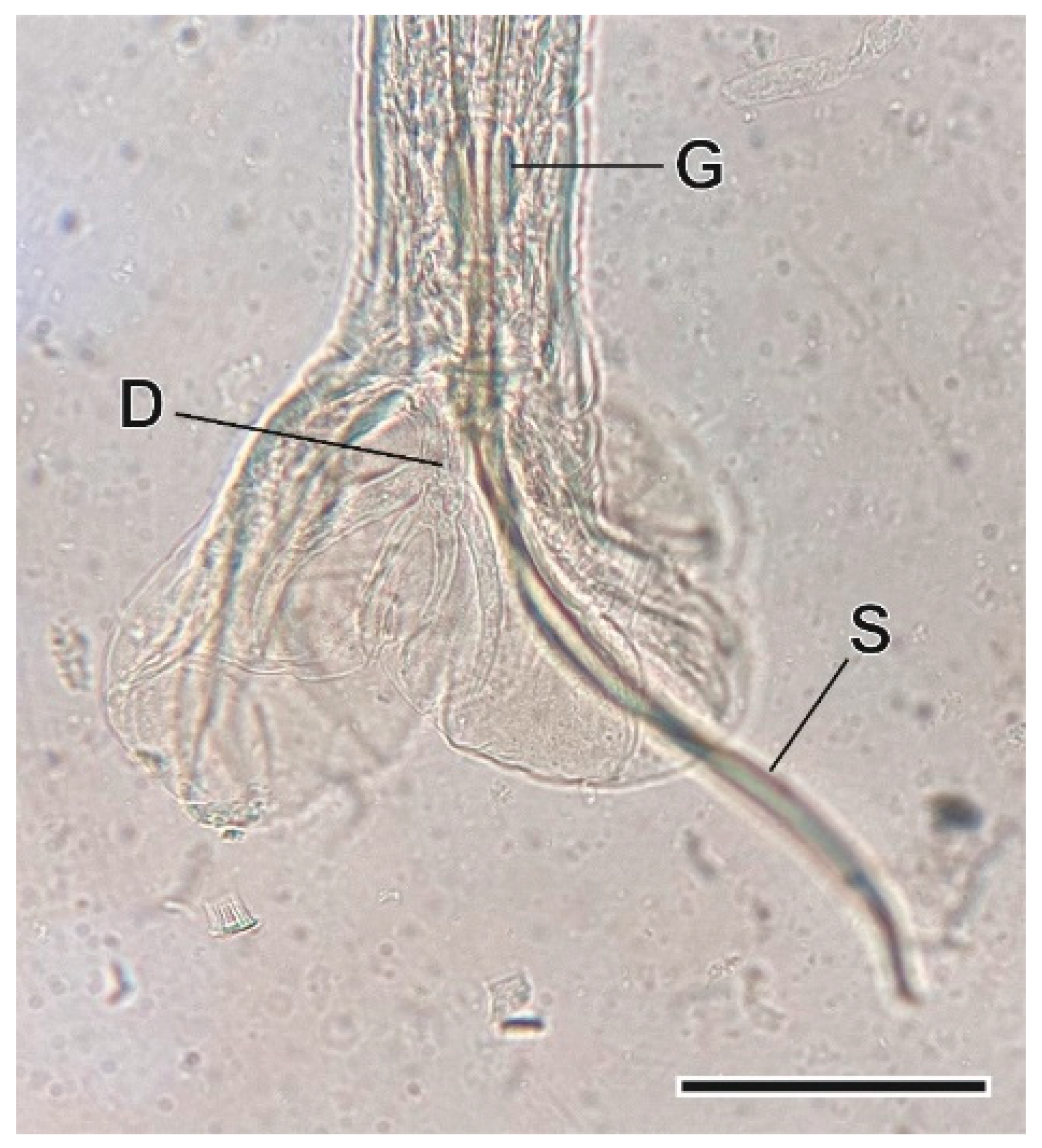
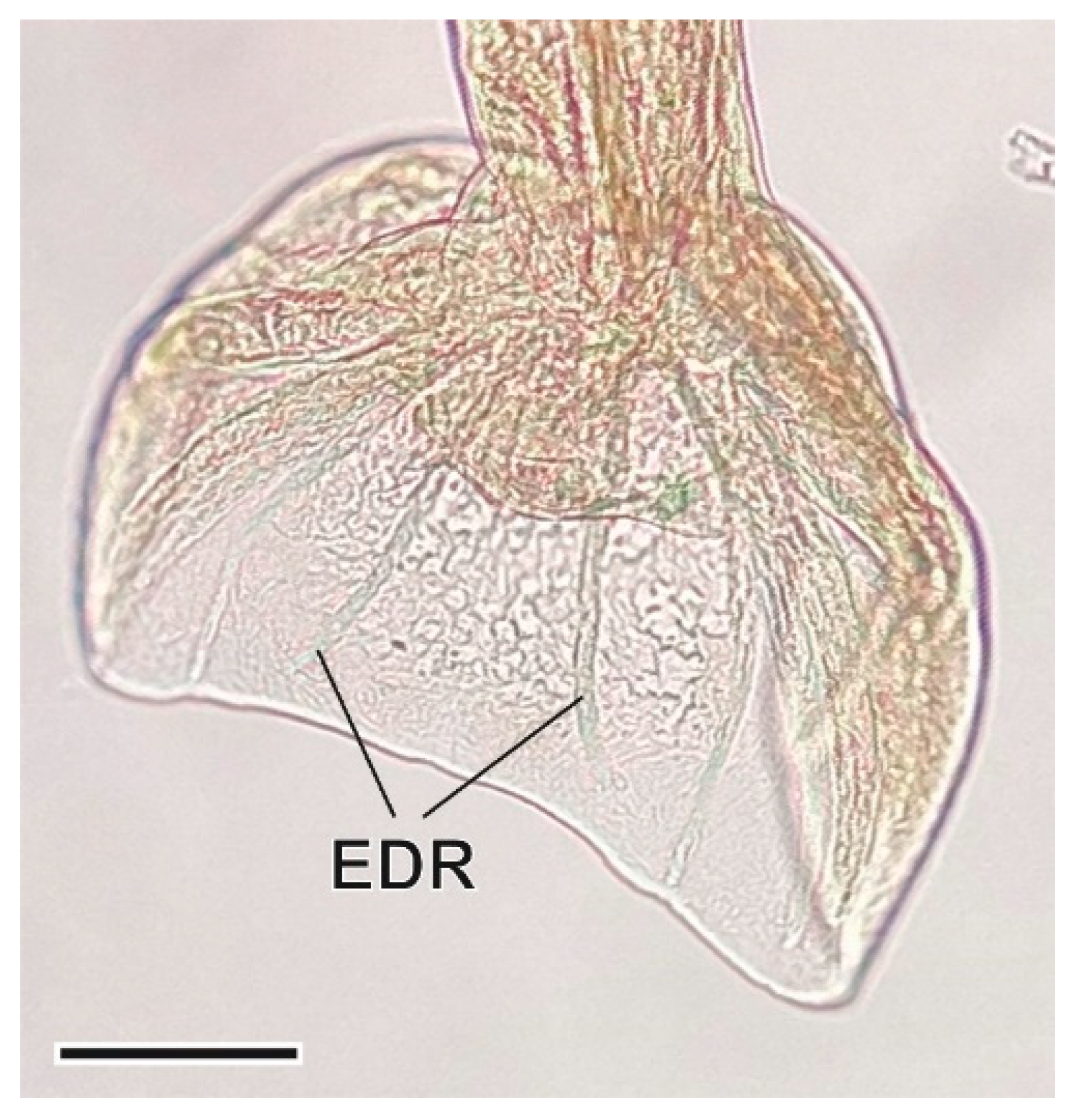
3.1.7. Syphacia nigeriana Baylis, 1928
3.2. Helminth Community Analysis
4. Discussion
4.1. Remarks on the Helminth Species
4.1.1. Hydatigera taeniaeformis Larvae
4.1.2. Paranoplocephala omphalodes
4.1.3. Rodentolepis asymmetrica
4.1.4. Trichuris arvicolae
4.1.5. Carolinensis minutus
4.1.6. Heligmosomum costellatum
4.1.7. Syphacia nigeriana
4.2. Helminth Community Analysis
4.3. The Use of the Helminths of Microtus lusitanicus in Pest Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mira, A.; Mathias, M. Microtus lusitanicus (Gerbe, 1879). In Atlas y Libro Rojo de los Mamíferos Terrestres de España; Gisbert, J., Palomo, J.L., Eds.; Dirección General para la Biodiversidad-SECEM-SECEMU: Madrid, Spain, 2007; pp. 418–442. [Google Scholar]
- Santos, S.M.; Lourenço, R.F.; Mathias, M.L.; Mira, A.P. Spatial and temporal ecology of the Lusitanian pine vole (Microtus lusitanicus) in a Mediterranean polyculture. Anim. Biol. 2010, 60, 209–227. [Google Scholar] [CrossRef]
- Miñarro, M.; Somoano, A.; Ventura, J. Intra-annual continuous reproduction of the apple pest Microtus lusitanicus: Implications for management. Crop Prot. 2017, 96, 164–172. [Google Scholar] [CrossRef]
- Ventura, J.; Jiménez, L.; Gisbert, J. Breeding characteristics of the Lusitanian pine vole, Microtus lusitanicus. Anim. Biol. 2010, 60, 1–14. [Google Scholar] [CrossRef]
- Duarte, M.A.; Mathias, M.D.L.; Bastos-Silveira, C. Pair-bonding behaviour of the sister species Microtus lusitanicus and M. duodecimcostatus. J. Ethol. 2015, 33, 213–223. [Google Scholar] [CrossRef]
- Somoano, A.; Ventura, J.; Miñarro, M. Continuous breeding of fossorial water voles in northwestern Spain: Potential impact on apple orchards. Folia Zool. 2017, 66, 37–49. [Google Scholar] [CrossRef]
- Ribeiro, J.N. Colony Social Structure and Burrow Architecture of the Lusitanian Pine Vole, Microtus lusitanicus (Gerbe, 1879). Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2019. [Google Scholar]
- Miñarro, M.; Montiel, C.; Dapena, E. Vole pests in apple orchards: Use of presences signs to estimate the abundance of Arvicola terrestris cantabriae and Microtus lusitanicus. J. Pest. Sci. 2012, 85, 477–488. [Google Scholar] [CrossRef]
- BOE. Royal decree 409/2008, of 28 March, that established national program for controlling pests of field voles. Boletín Oficial del Estado 2018, 86, 19217–19219. (In Spanish) [Google Scholar]
- EU. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products Text with EEA relevance. OJEU 2012, 167, 1–123. [Google Scholar]
- Jacob, J. Vertebrate pest management: Science and application. Pest. Manag. Sci. 2013, 69, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Ranchelli, E.; Barfknecht, R.; Capizzi, D.; Riga, F.; Mazza, V.; Dell’Agnello, F.; Zaccaroni, M. From biology to management of Savi’s pine vole (Microtus savii). Pest. Manag. Sci. 2016, 72, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Bernot, R.J. Parasite-host elemental content and the effects of a parasite on host-consumer-driven nutrient recycling. Freshw. Sci. 2013, 32, 299–308. [Google Scholar] [CrossRef]
- Anderson, R.M. The regulation of host population growth by parasitic species. Parasitology 1978, 76, 119–157. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Regulation and stability of host-parasite population interactions. I. Regulatory processes. J. Anim. Ecol. 1978, 47, 219–247. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Blackwell Sci.: Oxford, UK, 1996. [Google Scholar]
- Leirs, H.; Singleton, G.R. Parasites and pest population management. In Micromammals and Macroparasites. From Evolutionary Ecology to Management; Morand, S., Krasnov, B.R., Poulin, R., Eds.; Springer: Tokyo, Japan, 2006; pp. 565–591. [Google Scholar]
- Feliu, C.; Renaud, F.; Catzeflis, F.; Hugot, J.P.; Durand, P.; Morand, S. A comparative analysis of parasite species richness of Iberian rodents. Parasitology 1997, 115, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Feliu, C.; Ventura, J.; Segovia, J.M.; Jiménez, L.; Ribas, A.; Gisbert, J. Helminths of Microtus lusitanicus (Gerbe, 1879) (Rodentia: Cricetidae) in the Iberian Peninsula: Faunistic and ecological considerations. Rev. Ibero-Latinoam. Parasitol. 2009, 68, 159–166. [Google Scholar]
- Díaz-González, T.E.; Fernández-Prieto, J.A. Prados y pastos cantábricos: Origen y diversidad. In Producciones Agroganaderas: Gestión Eficiente y Conservación del Medio Natural. XLV Reunión Científica de la SEEP; De la Roza, B., Martínez, A., Carballal, A., Eds.; SERIDA: Gijón, Spain, 2005; Volume 2, pp. 699–730. [Google Scholar]
- Miñarro, M. Weed communities in apple orchards under organic and conventional fertilization and tree-row management. Crop. Prot. 2012, 39, 89–96. [Google Scholar] [CrossRef]
- EU Directive 2010/63/EU horizontal legislation on the protection of animal used for scientific Purposes. OJEU 2010, 276, 33–79.
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Bush, A.O. An Ecological Analysis of the Helminth Parasites of the White Ibis in Florida. Master’s Thesis, University of Florida, Gainesville, FL, USA, 1973. [Google Scholar]
- Pence, D.B.; Eason, S. Comparison of the helminth faunas of two sympatric top carnivores from the Rolling Plains of Texas. J. Parasitol. 1980, 66, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lefkovitch, L.P. An index of spatial distribution. Res. Popul. Ecol. 1966, 8, 89–92. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Croom Helm: London, UK, 1998. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Berger, W.H.; Parker, F.L. Diversity of planktonic Foraminifera in deep sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- May, R.M. Patterns of species abundance and diversity. In Ecology and Evolution Communities; Cody, M.L., Diamond, J.M., Eds.; Harvard University Press: Cambridge, MA, USA, 1975; pp. 81–120. [Google Scholar]
- Pielou, E.C. An Introduction to Mathematical Ecology; John Wiley & Sons: New York, NY, USA, 1969. [Google Scholar]
- Murai, É. Taeniid species in Hungary (Cestoda: Taeniidae). II. Larval stages of taeniids parasitizing rodents and lagomorphs. Misc. Zool. Hung. 1981, 1, 27–44. [Google Scholar]
- Murai, É. Review of Tapeworms in Microtinae from Hungary. Parasitol. Hung. 1974, 7, 111–141. [Google Scholar]
- Feliu, C.; Spakulová, M.; Casanova, J.C.; Renaud, F.; Morand, S.; Hugot, J.P.; Santalla, F.; Durand, P. Genetic and morphological heterogeneity in small rodent whipworms in southwestern Europe: Characterization of Trichuris muris and description of Trichuris arvicolae n. sp. (Nematoda: Trichuridae). J. Parasitol. 2000, 86, 442–449. [Google Scholar] [CrossRef]
- Mészáros, F. Parasitic Nematodes of Microtus arvalis (Rodentia) in Hungary. Parasitol. Hung. 1977, 10, 67–83. [Google Scholar]
- Rossin, A.; Malizia, A.I.; Denegri, G.M. The role of the subterranean rodent Ctenomys talarum (Rodentia: Octodontidae) in the life cycle of Taenia taeniaeformis (Cestoda: Taeniidae) in urban environments. Vet. Parasitol. 2004, 122, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Haukisalmi, V.; Wickström, L.M.; Henttonen, H.; Hantula, J.; Gubányi, A. Molecular and morphological evidence for multiple species within Paranoplocephala omphalodes (Cestoda, Anoplocephalidae) in Microtus voles (Arvicolinae). Zool. Scr. 2004, 33, 277–290. [Google Scholar] [CrossRef]
- Deter, J.; Chaval, Y.; Galán, M.; Berthier, K.; Salvador, A.R.; Casanova Garcia, J.C.; Morand, S.; Cosson, J.F.; Charbonnel, N. Linking demography and host dispersal to Trichuris arvicolae distribution in a cyclic vole species. Int. J. Parasitol. 2007, 37, 813–824. [Google Scholar] [CrossRef]
- Callejón, R.; De Rojas, M.; Feliu, C.; Balao, F.; Marrugal, Á.; Henttonen, H.; Guevara, D.; Cutillas, C. Phylogeography of Trichuris populations isolated from different Cricetidae rodents. Parasitology 2012, 139, 1795–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, J. Sobre las Helmintofaunas de las Especies de Insectívoros y Roedores del Delta del Ebro (NE de la Península Ibérica). Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 1988. [Google Scholar]
- Janova, E.; Skoric, M.; Heroldova, M.; Tenora, F.; Fictum, P.; Pavlik, I. Determinants of the prevalence of Heligmosomum costellatum (Heligmosomidae: Trichostrongyloidea) in a common vole population in southern Moravia, Czech Republic. J. Helminthol. 2010, 84, 410–414. [Google Scholar] [CrossRef]
- Feliu, C.; Torres, J.; Miquel, J.; Gisbert, J.; Garcia-Perea, R. Helmintofauna of Microtus (Microtus) cabrerae (Thomas, 1906) (Rodentia: Arvicolidae) in the Iberian Peninsula: Faunistic and ecological considerations. Ann. Parasit. Hum. Comp. 1991, 66, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Feliu, C.; Torres, J.; Casanova, J.C.; Miquel, J. Consideraciones sobre el binomio helmintofauna-modo de vida de los roedores ibéricos. In In Memoriam al Profesor Doctor D.F. de Martínez Gómez; Hernández Rodríguez, S., Ed.; Universidad de Córdoba: Córdoba, Spain, 1992; pp. 351–366. [Google Scholar]
- Diamond, J.M. The islands dilemma: Lessons of modern biogeographic studies for the design of natural reserves. Biol. Conserv. 1975, 7, 129–146. [Google Scholar] [CrossRef]
- Somoano, A.; Bastos-Silveira, C.; Ventura, J.; Miñarro, M.; Heckel, G. Bocage landscape restricts the gene flow of pest vole populations. Manuscript in preparation.
- Dobson, A.P. The population biology of parasite induced changes in host behaviour. Q. Rev. Biol. 1988, 63, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.P.; Hudson, P.J. Regulation and stability of a free-living host-parasite system: Trychostrongylus tenuis in red grouse. II. Population models. J. Anim. Ecol. 1992, 61, 487–498. [Google Scholar] [CrossRef]
- Shaw, D.J.; Dobson, A.P. Patterns of microparasite abundance and aggregation in wildlife populations: A quantitative review. Parasitology 1995, 111, S111–S133. [Google Scholar] [CrossRef]
- Wilson, K.; Bjørnstad, O.N.; Dobson, A.P.; Merler, S.; Poglayen, G.; Randolph, S.E.; Read, A.F.; Skorping, A. Heterogeneities in macroparasite infections: Patterns and processes. In The Ecology of Wildlife Diseases; Hudson, P.J., Rizzoli, A., Grenfell, B.T., Heesterbeek, H., Dobson, A.P., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 6–44. [Google Scholar]
- Morand, S.; Krasnov, B. Why apply ecological laws to epidemiology? Trends Parasitol. 2008, 24, 304–309. [Google Scholar] [CrossRef]
- Theis, J.H.; Schwab, R.G. Seasonal prevalence of Taenia taeniaeformis: Relationship to age, sex, reproduction and abundance of an intermediate host (Peromyscus maniculatus). J. Wildl. Dis. 1992, 28, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, P.J.; Cattadori, I.M.; Boag, B.; Dobson, A.B. Climate disruption and parasite-host dynamics: Patterns and processes associated with warming and the frequency of extreme climatic events. J. Helminthol. 2006, 80, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elton, C.; Ford, E.B.; Baker, J.R.; Gardiner, A.D. The health and parasites of a wild mouse population. Proc. Zool. Soc. Lond. 1931, 1931, 453–466. [Google Scholar] [CrossRef]
- Kisielewska, K. Intestinal helminths as indicatory of the age structure of Microtus arvalis Pallas, 1778 population. Bull. Acad. Polon. Sci. Ser. Sci. Biol. 1971, 19, 275–282. [Google Scholar]
- Montgomery, S.S.J.; Montgomery, W.I. Spatial and temporal variation in the infracommunity structure of helminths of Apodemus sylvaticus (Rodentia: Muridae). Parasitology 1989, 98, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.D. On the interpretation of host-parasite ecology: Heligmosomoides polygyrus (Nematoda) in the wood mouse (Apodemus sylvaticus) populations. J. Zool. 1992, 226, 109–121. [Google Scholar] [CrossRef]
- Gregory, R.D.; Montgomery, S.S.J.; Montgomery, W.I. Population biology of Heligmosomoides polygyrus (Nematoda) in the wood mouse. J. Anim. Ecol. 1992, 61, 749–757. [Google Scholar] [CrossRef]
- Behnke, J.M.; Lewis, J.W.; Mohd Zain, S.N.; Gibert, F.S. Helminth infections in Apodemus sylvaticus in Southern England: Interactive effects of host age, sex and year on the prevalence and abundance of infections. J. Helminthol. 1999, 22, 861–907. [Google Scholar] [CrossRef] [Green Version]
- Eira, C.; Torres, J.; Vingada, J.; Miquel, J. Ecological aspects influencing the helminth community of the wood mouse Apodemus sylvaticus in Dunas de Mira, Portugal. Acta Parasitol. 2006, 51, 300–308. [Google Scholar] [CrossRef]
- Fuentes, M.V.; Sáez, S.; Trelis, M.; Galán-Puchades, M.T.; Esteban, J.G. The helminth community of the wood mouse, Apodemus sylvaticus, in the Sierra Espuña, Murcia, Spain. J. Helminthol. 2004, 78, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.V.; Sainz-Elipe, S.; Galán-Puchades, M.T. Ecological study of the wood mouse helminth community in a burned Mediterranean ecosystem in regeneration five years after a wildfire. Acta Parasitol. 2007, 52, 403–413. [Google Scholar] [CrossRef]
- Fuentes, M.V.; Sainz-Elipe, S.; Sáez-Durán, S.; Galán-Puchades, M.T. The helminth community of the wood mouse Apodemus sylvaticus in a Mediterranean ecosystem in regeneration ten years after a wildfire. J. Helminthol. 2010, 84, 39–48. [Google Scholar] [CrossRef]
- Jackson, J.A.; Friberg, I.M.; Bolch, I.; Lowe, A.; Ralli, C.; Harris, P.D.; Behnke, J.M.; Bradley, J.E. Immunomodulatory parasites and toll-like receptor-mediated tumour necrosis factor alpha responsiveness in wild mammals. BMC Biol. 2009, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.A.; Hall, A.J.; Friberg, I.M.; Ralli, C.; Lowe, A.; Zawadzka, M.; Turner, A.K.; Stewart, A.; Birtles, R.J.; Paterson, S.; et al. An immunological marker of tolerance to infection in wild rodents. PLoS Biol. 2014, 12, e1001901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friberg, I.M.; Little, S.; Ralli, C.; Lowe, A.; Hall, A.; Jackson, J.A.; Bradley, P.J. Macroparasites at peripheral sites of infection are major and dynamic modifiers of systematic antimicrobial patterns recognition responses. Mol. Ecol. 2013, 22, 2810–2826. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Durán, S.; Debenedetti, A.L.; Sainz-Elipe, S.; Galán-Puchade, M.T.; Fuentes, M.V. The helminth community component species of the wood mouse as biological indicator tags of a ten post-fire-year regeneration process in a Mediterranean ecosystem. Parasitol. Res. 2018, 117, 2217–2231. [Google Scholar] [CrossRef] [PubMed]
- Behnke, J.M.; Rogan, M.T.; Craig, P.S.; Jackson, J.A.; Hide, G. Long-term trends in helminth infections of wood mice (Apodemus sylvaticus) from the vicinity of Malhalm Tarn in North Yorkshire, England. Parasitology 2021, 148, 451–463. [Google Scholar] [CrossRef]
- Sanchez, A.; Devevey, G.; Bize, P. Female-biased infection and transmission of the gastrointestinal nematode Trichuris arvalis infecting the common vole, Microtus arvalis. Int. J. Parasitol. 2011, 41, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Grzybek, M.; Bajer, A.; Bednarska, M.; Al-Sarraf, M.; Behnke-Borowczyk, J.; Harris, P.D.; Price, S.J.; Brown, G.S.; Osborne, S.J.; Siński, E.; et al. Long-term spatiotemporal stability and dynamic changes in helminth infracommunities of bank voles (Myodes glareolus) in NE Poland. Parasitology 2015, 142, 1722–1743. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, C.; Di Bella, C.; Casanova, J.C.; Ribas, A.; Cagnin, M. Helminth communities of wood mouse (Apodemus sylvaticus) on the river Avena (Calabria, southern Italy). Hystrix 2010, 21, 171–176. [Google Scholar] [CrossRef]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Morand, S.; Hawlena, H.; Khokhlova, I.S.; Shenbrot, G.I. Sex biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 2005, 146, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, B.R.; Bordes, F.; Khokhlova, I.S.; Morand, S. Gender-biased parasitism in small mammals: Patterns, mechanisms, consequences. Mammalia 2012, 76, 1–13. [Google Scholar] [CrossRef]
- Bordes, F.; Ponlet, N.; Göuy de Bellocq, J.; Ribas, A.; Krasnov, B.R.; Morand, S. Is there sex-biased resistance and tolerance in Mediterranean wood mouse (Apodemus sylvaticus) populations facing multiple helminth infections? Oecologia 2012, 170, 123–135. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode Parasites of Vertebrates. Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Bajer, A.; Behnke, J.M.; Pawelczyk, A.; Kuliś, K.; Sereda, M.J.; Siński, E. Medium-term temporal stability of the helminth component community in bank voles (Clethrionomys glareolus) from the Mazury Lake District region of Poland. Parasitology 2005, 130, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Arneberg, P.; Shorping, A.; Grenfell, B.; Read, A.F. Host-densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond. B 1998, 265, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Abu-Madi, M.A.; Behnke, J.M.; Lewis, J.W.; Gilbert, F.S. Seasonal and site variation community structure of intestinal helminths in Apodemus sylvaticus from three contrasting habitats in south-east England. J. Helminthol. 2000, 74, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnke, J.M.; Bajer, A.; Harris, P.D.; Newington, L.; Pidgeon, E.; Rowlands, G.; Sheriff, C.; Kuliś-Malkowska, K.; Siński, E.; Gilbert, F.S.; et al. Temporal and between-side variation in helminth communities of bank voles (Myodes glareolus) from N.E. Poland. 1. Regional fauna and component community levels. Parasitology 2008, 135, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Gulland, F.M.D. The impact of infectious diseases on wild animal populations: A review. In Ecology of Infectious Diseases in Natural Populations; Grenfell, B.T., Dobson, A.P., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 20–51. [Google Scholar]
- Albon, S.D.; Stein, A.; Irvine, R.J.; Langvatn, R.; Ropstad, E.; Halvorsen, O. The role of parasites in the dynamics of a reindeer population. Proc. R. Soc. Lond. B 2002, 269, 1625–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, P.J.; Rizzoli, A.; Grenfell, B.T.; Heesterbeek, H.; Dobson, A.P. The Ecology of Wildlife Diseases; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Stien, A.; Irvine, R.J.; Langvatn, R.; Ropstad, E.; Halvorsen, O.; Albon, S.D. The impact of gastrointestinal nematodes on wild reindeer: Experimental and cross-sectional studies. J. Anim. Ecol. 2002, 71, 937–945. [Google Scholar] [CrossRef]
- Newey, S.; Thirgood, S.J. Parasite-mediated reduction in fecundity of mountain hares. Proc. R. Soc. Lond. B 2004, 271, S413–S415. [Google Scholar] [CrossRef] [Green Version]
- Grenfell, B.; Wilson, K.; Finkenstädt, B.F.; Coulson, T.N.; Murray, S.; Albon, S.D.; Pemberton, J.M.; Clutton-Brock, T.H.; Crawley, M.J. Noise and determinism in synchronised sheep dynamics. Nature 1998, 394, 674–677. [Google Scholar] [CrossRef]
- Redpath, S.M.; Mougeot, F.; Leckie, F.L.; Elston, D.A.; Hudson, P.J. Testing the role of parasites in driving the cyclic population dynamics of a gamebird. Ecol. Lett. 2006, 9, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Deter, J.; Cosson, J.F.; Chaval, Y.; Charbonnel, N.; Morand, S. The intestinal nematode Trichuris arvicolae affects the fecundity of its host, the common vole Microtus arvalis. Parasitol. Res. 2007, 101, 1161–1164. [Google Scholar] [CrossRef]
- Deter, J.; Charbonnel, N.; Cosson, J.F.; Morand, S. Regulation of vole populations by the nematode Trichuris arvicolae: Insights from modelling. Eur. J. Wildl. Res. 2008, 54, 60–70. [Google Scholar] [CrossRef]
- Mair, I.; Else, K.J.; Forman, R. Trichuris muris as a tool for holistic discovery research: From translational research to environmental bio-tagging. Parasitology 2021, 1–13. [Google Scholar] [CrossRef]
- Lõhmus, M.; Moalem, S.; Björklund, M. Leptin, a tool of parasites? Biol. Lett. 2012, 8, 849–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, J.R.; Kacelnik, A. Decision-making. In Behavioural Ecology and Evolutionary Approach; Krebs, J.R., Davies, N.B., Eds.; Blackwell Scientific: Oxford, UK, 1991; pp. 105–136. [Google Scholar]
- Lhmus, M.; Sundström, L.F. Leptin and social environment influence the risk-taking and feeding behaviour of Asian blue quail. Anim. Behav. 2004, 68, 607–612. [Google Scholar] [CrossRef]





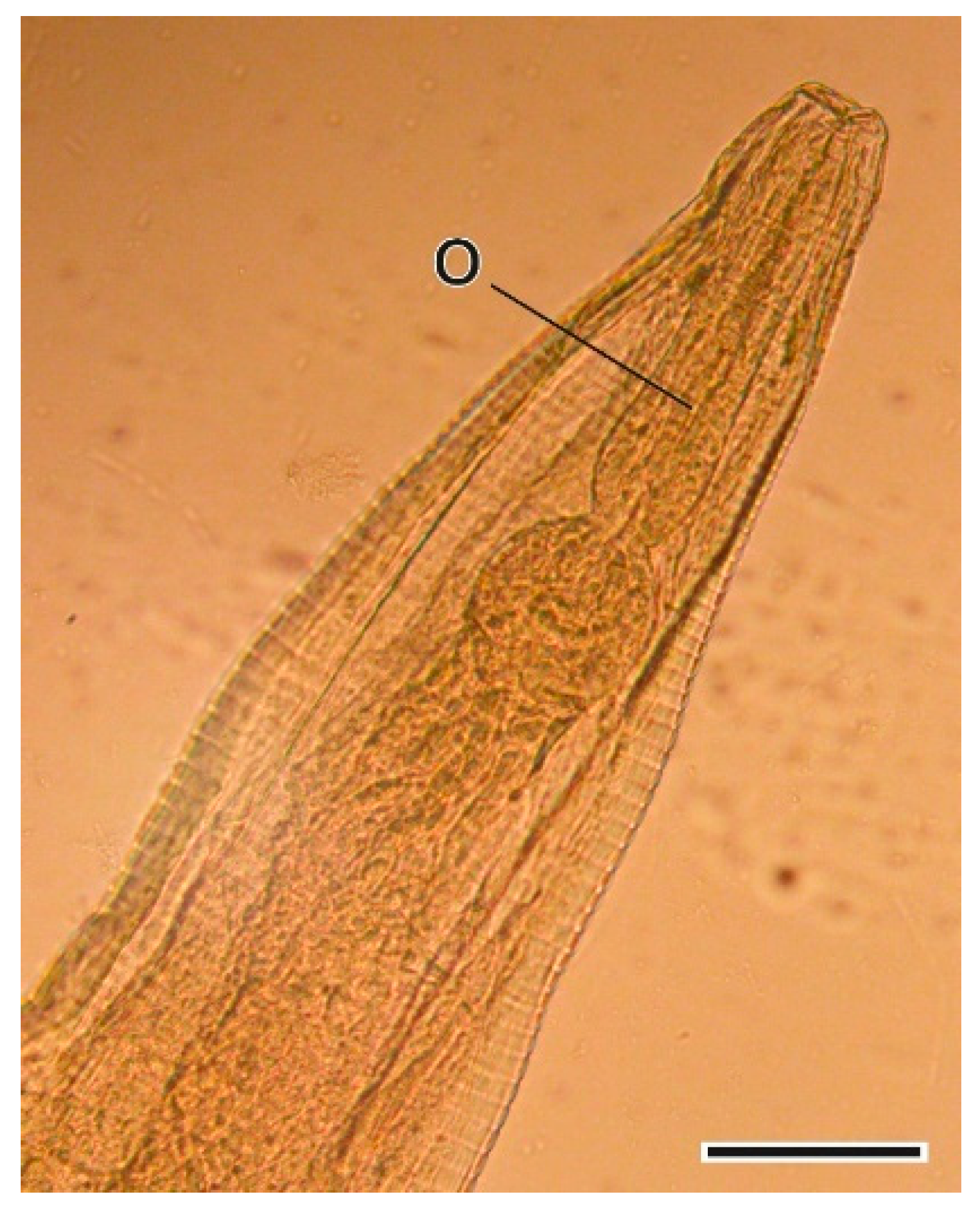
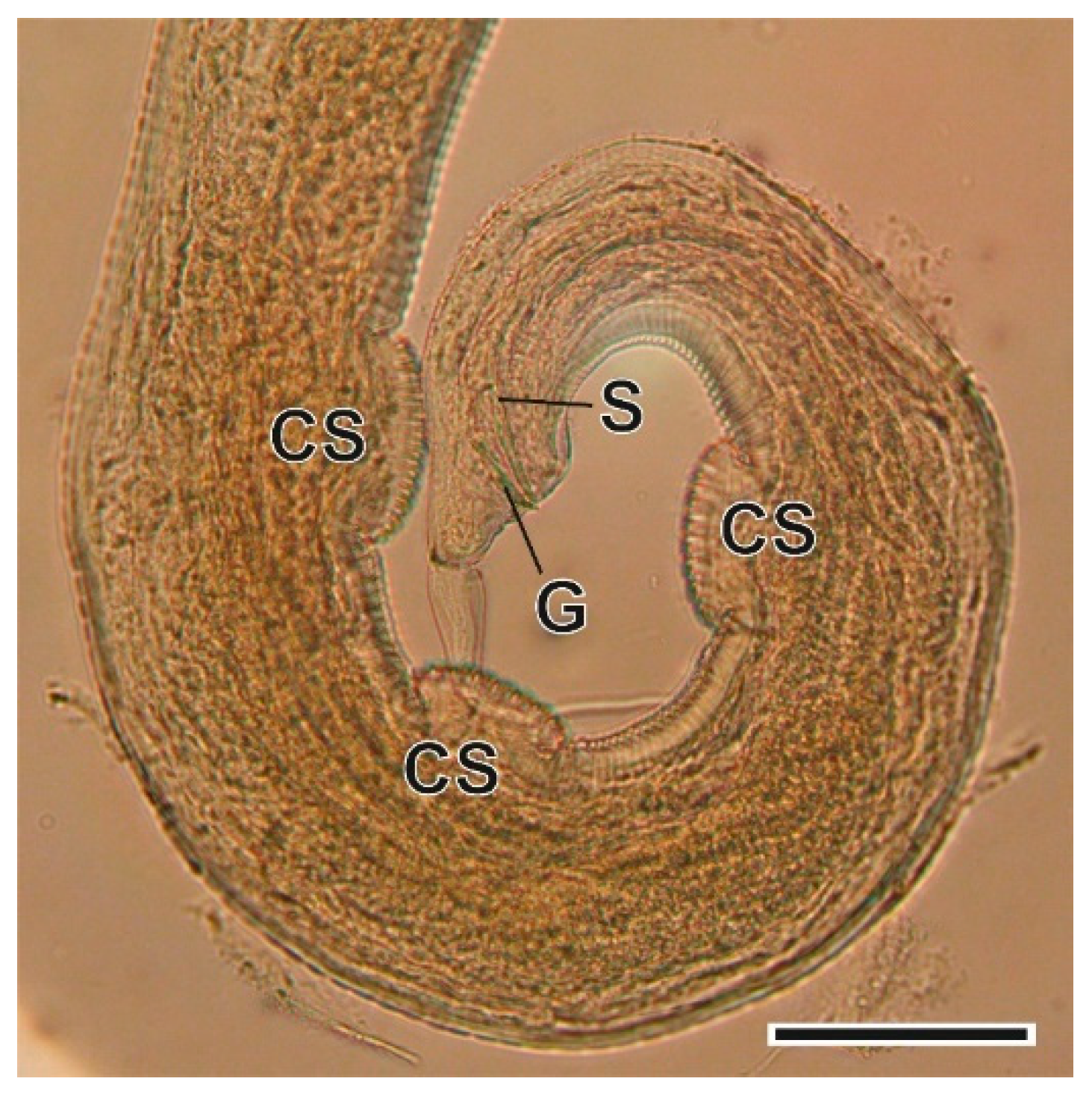
| Host Sex | Season | Host Age | |||
|---|---|---|---|---|---|
| Juveniles | Sub-Adults | Adults | Total | ||
| Males | Winter | 3 | 26 | 39 | 68 |
| Spring | 10 | 35 | 51 | 96 | |
| Summer | 6 | 63 | 36 | 105 | |
| Autumn | 5 | 63 | 26 | 94 | |
| Total | 24 | 187 | 152 | 363 | |
| Females | Winter | 3 | 33 | 35 | 71 |
| Spring | 8 | 38 | 45 | 91 | |
| Summer | 18 | 36 | 37 | 91 | |
| Autumn | 10 | 40 | 44 | 94 | |
| Total | 39 | 147 | 161 | 347 | |
| Year | Season | ||||
|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | Total | |
| 2011 | 25 | 92 | 110 | 116 | 343 |
| 2012 | 101 | 95 | 86 | 72 | 354 |
| 2013 | 13 | 0 | 0 | 0 | 13 |
| Total | 139 | 187 | 196 | 188 | 710 |
| n | Mean Temperature (°C) | Mean Rainfall (mm) | Cumulative Rainfall (mm) | |
|---|---|---|---|---|
| Winter 2010 | 25 | 10.1 | 3.0 | 269.0 |
| Spring 2010 | 92 | 15.0 | 4.0 | 338.4 |
| Summer 2010 | 110 | 19.9 | 0.7 | 65.9 |
| Autumn 2010 | 116 | 12.6 | 5.5 | 503.4 |
| Winter 2011 | 101 | 11.1 | 2.9 | 246.0 |
| Spring 2011 | 95 | 16.2 | 1.0 | 88.5 |
| Summer 2011 | 86 | 19.7 | 1.3 | 116.2 |
| Autumn 2011 | 72 | 14.6 | 2.2 | 199.0 |
| Winter 2012 | 13 | 10.2 | 1.4 | 125.1 |
| Helminth Species | Site | LC | n | Prevalence (95% CI) | Mean Abundance (SE) | Median Intensity (Range) |
|---|---|---|---|---|---|---|
| CESTODA | ||||||
| Cysticercoid sp. larvae | I | F | 1 | 0.15 (0.1–0.5) | <0.00 (<0.00) | 1 (1) |
| Hydatigera taeniaeformis larvae | L | F | 59 | 8 (6–10) | 0.10 (0.01) | 1 (1–5) |
| Paranoplocephala omphalodes | I | NF | 2 | 0.3 (0.1–0.7) | <0.00 (<0.00) | 1 (1) |
| Rodentolpeis asymmetrica | I | NF | 5 | 0.7 (0.4–2) | 0.01 (<0.00) | 1 (1–2) |
| NEMATODA | ||||||
| Trichuris arvicolae | C | F | 36 | 5 (4–7) | 0.06 (0.01) | 1 (1–4) |
| Carolinensis minutus | I | F | 308 | 44 (39–47) | 5.80 (0.91) | 3 (1–417) |
| Heligmosomum costellatum | I | F | 25 | 4 (3–6) | 0.16 (0.05) | 3 (1–22) |
| Syphacia nigeriana | C | F | 158 | 22 (19–25) | 4.54 (0.81) | 5 (1–262) |
| Species | |||||||
|---|---|---|---|---|---|---|---|
| H. taeniaeformis | T. arvicolae | C. minutus | H. costellatum | S. nigeriana | |||
| Sex | Males | Prevalence (CI 95%) | 9 (6–12) | 3 (2–5) | 21 (17–26) | 2 (1–3) | 13 (10–17) |
| Mean Abundance (SE) | 0.10 (0.02) | 0.07 (0.02) | 4.60 (0.93) | 0.18 (0.07) | 5.28 (1.22) | ||
| Median Intensity (range) | 1 (1–2) | 1 (1–2) | 3 (1–239) | 3 (1–21) | 6 (1–262) | ||
| Females | Prevalence (CI 95%) | 7 (5–10) | 4 (2–6) | 47 (42–52) | 3 (2–5) | 19 (15–23) | |
| Mean Abundance (SE) | 0.10 (0.02) | 0.06 (0.02) | 7.07 (1.58) | 0.13 (0.07) | 3.76 (1.04) | ||
| Median Intensity (range) | 1 (1–5) | 1 (1–4) | 3 (1–417) | 2 (1–22) | 4 (1–191) | ||
| Age | Juveniles | Prevalence (CI 95%) | - | - | 62 (50–74) | 3 (0.4–11) | 14 (7–25) |
| Mean Abundance (SE) | - | - | 20.19 (7.21) | 0.11 (0.09) | 2.51 (1.08) | ||
| Median Intensity (range) | - | - | 9 (1–417) | 3.5 (2–5) | 13 (1–41) | ||
| Sub-adults | Prevalence (CI 95%) | 5 (3–8) | 3 (2–5) | 45 (40–50) | 3 (2–5) | 23 (19–28) | |
| Mean Abundance (SE) | 0.05 (0.01) | 0.04 (0.01) | 5.56 (1.06) | 0.22 (0.10) | 5.71 (1.42) | ||
| Median Intensity (range) | 1 (1–2) | 1 (1–2) | 3 (1–239) | 4 (1–22) | 4 (1–262) | ||
| Adults | Prevalence (CI 95%) | 14 (10–18) | 8 (5–12) | 38 (33–43) | 4 (2–7) | 23 (19–28) | |
| Mean Abundance (SE) | 0.17 (0.03) | 0.10 (0.02) | 3.16 (0.87) | 0.10 (0.04) | 3.70 (1.00) | ||
| Median Intensity (range) | 1 (1–5) | 1 (1–4) | 2 (1–238) | 1 (1–8) | 5 (1–191) | ||
| Species | |||||||
|---|---|---|---|---|---|---|---|
| H. taeniaeformis | T. arvicolae | C. minutus | H. costellatum | S. nigeriana | |||
| Season | Winter | Prevalence (CI 95%) | 10 (6–16) | 2 (0.4–6) | 28 (21-36) | 6 (3–11) | 30 (23–38) |
| Mean Abundance (SE) | 0.12 (0.03) | 0.02 (0.01) | 0.78 (0.21) | 030 (0.17) | 4.15 (1.40) | ||
| Median Intensity (range) | 1 (1–2) | 1 (1) | 1 (1–22) | 2.5 (1–22) | 3 (1–128) | ||
| Spring | Prevalence (CI 95%) | 10 (6–15) | 7 (4–12) | 51 (44–59) | 5 (2–9) | 24 (18–31) | |
| Mean Abundance (SE) | 0.10 (0.02) | 0.08 (0.02) | 6.14 (0.99) | 0.22 (0.12) | 9.65 (2.71) | ||
| Median Intensity (range) | 1 (1) | 1 (1–2) | 4.5 (1–80) | 2 (1–21) | 11 (1–262) | ||
| Summer | Prevalence (CI 95%) | 6 (3–10) | 7 (4–12) | 59 (52–66) | 3 (1–6) | 17 (12–23) | |
| Mean Abundance (SE) | 0.07 (0.02) | 0.09 (0.03) | 13.30 (3.07) | 0.12 (0.06) | 1.60 (0.43) | ||
| Median Intensity (range) | 1 (1–3) | 1 (1–2) | 5 (1–417) | 4 (1–7) | 4.5 (1–54) | ||
| Autumn | Prevalence (CI 95%) | 8 (5–13) | 3 (1–7) | 31 (25–38) | 0.5 (0.1–4) | 20 (15–26) | |
| Mean Abundance (SE) | 0.11 (0.04) | 0.05 (0.02) | 1.36 (0.29) | 0.02 (0.02) | 2.80 (0.73) | ||
| Median Intensity (range) | 1 (1–5) | 1 (1–4) | 2 (1–31) | 3 (3) | 6 (1–75) | ||
| Year | 2011 | Prevalence (CI 95%) | 8 (5–11) | 5 (3–8) | 51 (46–57) | 3 (2–5) | 16 (12–20) |
| Mean Abundance (SE) | 0.10 (0.02) | 0.06 (0.01) | 8.11 (1.64) | 0.17 (0.07) | 2.37 (0.61) | ||
| Median Intensity (range) | 1 (1–5) | 1 (1–2) | 3.5 (1–417) | 4.5 (1–21) | 4 (1–130) | ||
| 2012 | Prevalence (CI 95%) | 9 (6–12) | 5 (3–8) | 36 (31–41) | 4 (2–7) | 29 (24–34) | |
| Mean Abundance (SE) | 0.10 (0.02) | 0.07 (0.20) | 3.76 (0.88) | 0.15 (0.07) | 6.81 (1.50) | ||
| Median Intensity (range) | 1 (1–3) | 1 (1–4) | 2 (1–238) | 2 (1–22) | 5 (1–262) | ||
| 2013 | Prevalence (CI 95%) | 8 (0.2–34) | - | 31 (9–57) | - | - | |
| Mean Abundance (SE) | 0.08 (0.08) | - | 0.54 (0.27) | - | - | ||
| Median Intensity (range) | 1 (1) | - | 1.5 (1–3) | - | - | ||
| No. of Helminth Species | n | % |
|---|---|---|
| 0 | 260 | 36.6 |
| 1 | 320 | 45.1 |
| 2 | 117 | 16.5 |
| 3 | 12 | 1.7 |
| 4 | 1 | 0.1 |
| Helminth Species | AI | L |
|---|---|---|
| Hydatigera taeniaeformis larvae | - | 0.23 |
| Trichuris arvicolae | 0.06 | 0.24 |
| Carolinensis minutus | 5.80 | 0.99 |
| Heligmosomum costellatum | 0.16 | 0.88 |
| Syphacia nigeriana | 4.54 | 0.99 |
| Sex | Age | |||||
|---|---|---|---|---|---|---|
| Males | Females | Juveniles | Sub-Adults | Adults | Total | |
| Shannon index | 0.86 | 0.78 | 0.38 | 0.82 | 0.93 | 0.84 |
| Simpson index | 0.53 | 0.48 | 0.20 | 0.53 | 0.55 | 0.52 |
| Berger–Parker index | 0.48 | 0.36 | 0.11 | 0.51 | 0.49 | 0.46 |
| Shannon evenness index | 0.44 | 0.40 | 0.34 | 0.46 | 0.45 | 0.40 |
| Season | Year | ||||||
|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | 2011 | 2012 | 2013 | |
| Shannon index | 0.76 | 0.81 | 0.45 | 0.81 | 0.68 | 0.81 | 0.38 |
| Simpson index | 0.38 | 0.50 | 0.22 | 0.48 | 0.39 | 0.49 | 0.22 |
| Berger–Parker index | 0.23 | 0.41 | 0.12 | 0.35 | 0.25 | 0.38 | 0.13 |
| Shannon evenness index | 0.39 | 0.41 | 0.25 | 0.50 | 0.38 | 0.39 | 0.54 |
| Sex | Age | ||||||
|---|---|---|---|---|---|---|---|
| Male | Female | Juveniles | Sub-Adults | Adults | Total | ||
| Mean species richness | X | 0.86 | 0.81 | 0.79 | 0.80 | 0.88 | 0.84 |
| SE | 0.04 | 0.04 | 0.08 | 0.04 | 0.05 | 0.03 | |
| Brillouin index | 0.06 | 0.06 | 0.05 | 0.06 | 0.07 | 0.06 | |
| SE | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |
| Max | 0.60 | 0.75 | 0.62 | 0.65 | 0.75 | 0.75 | |
| BI infected M.l. only | X | 0.07 | 0.06 | 0.04 | 0.06 | 0.06 | 0.06 |
| SE | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |
| % of M.l. infected | 62.5 | 64.3 | 68.3 | 61.1 | 64.9 | 63.38 | |
| Season | Year | |||||||
|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | 2011 | 2012 | 2013 | ||
| Mean species richness | X | 0.78 | 1.01 | 0.92 | 0.62 | 0.83 | 0.86 | 0.38 |
| SE | 0.07 | 0.06 | 0.05 | 0.05 | 0.04 | 0.04 | 0.14 | |
| Brillouin index | X | 0.06 | 0.07 | 0.05 | 0.05 | 0.06 | 0.06 | 0.02 |
| SE | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | |
| Max | 0.65 | 0.62 | 0.55 | 0.75 | 0.75 | 0.65 | 0.28 | |
| BI infected M.l. only | X | 0.07 | 0.08 | 0.06 | 0.04 | 0.06 | 0.06 | 0.06 |
| SE | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| % of M.l. infected | 56.8 | 73.8 | 71.4 | 49.5 | 65.0 | 62.7 | 38.5 | |
| Helminth Species/Independent Variables Included in the Model | df | χ2 | p |
|---|---|---|---|
| Global M. lusitanicus parasitation | |||
| Season of capture | 3 | 32.471 | <0.0001 |
| Hydatigera taeniaeformis larvae | |||
| Host age | 2 | 27.540 | <0.0001 |
| Trichuris arvicolae | |||
| Host age | 2 | 13.789 | 0.001 |
| Carolinensis minutus | |||
| Host age | 2 | 13.333 | 0.001 |
| Host age and sex | 2 | 7.189 | 0.027 |
| Season of capture | 3 | 49.574 | <0.0001 |
| Year of capture | 2 | 17.216 | <0.0001 |
| Heligmosomum costellatum | |||
| Season of capture | 3 | 11.074 | 0.011 |
| Syphacia nigeriana | |||
| Season of capture | 3 | 8.711 | 0.033 |
| Year of capture | 2 | 23.797 | <0.0001 |
| Helminth Species/Independent Variables Included in the Model | df | U/χ2 | p |
|---|---|---|---|
| Hydatigera taeniaeformis larvae | |||
| Host age | 2 | 23.327 | <0.0001 |
| Trichuris arvicolae | |||
| Host age | 2 | 10.992 | 0.004 |
| Carolinensis minutus | |||
| Host age | 2 | 23.558 | <0.0001 |
| Season of capture | 3 | 68.329 | <0.0001 |
| Year of capture | 2 | 21.101 | <0.0001 |
| Heligmosomum costellatum | |||
| Season of capture | 3 | 8.868 | 0.031 |
| Syphacia nigeriana | |||
| Season of capture | 3 | 8.386 | 0.039 |
| Year of capture | 2 | 20.706 | <0.0001 |
| Correlations | rs | p |
|---|---|---|
| Trichuris arvicolae prevalence and mean temperature | 0.691 | 0.009 |
| Trichuris arvicolae abundance and mean temperature | 0.663 | 0.014 |
| Carolinensis minutus abundance and mean temperature | 0.698 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adalid, R.; Feliu, C.; Somoano, A.; Miñarro, M.; Ventura, J.; Torres, J.; Miquel, J.; Fuentes, M.V. Ecological Analysis of the Helminth Community of Microtus lusitanicus (Gerbe, 1879) (Rodentia) in Asturias (NW Spain). Animals 2021, 11, 3055. https://doi.org/10.3390/ani11113055
Adalid R, Feliu C, Somoano A, Miñarro M, Ventura J, Torres J, Miquel J, Fuentes MV. Ecological Analysis of the Helminth Community of Microtus lusitanicus (Gerbe, 1879) (Rodentia) in Asturias (NW Spain). Animals. 2021; 11(11):3055. https://doi.org/10.3390/ani11113055
Chicago/Turabian StyleAdalid, Roser, Carles Feliu, Aitor Somoano, Marcos Miñarro, Jacint Ventura, Jordi Torres, Jordi Miquel, and Màrius Vicent Fuentes. 2021. "Ecological Analysis of the Helminth Community of Microtus lusitanicus (Gerbe, 1879) (Rodentia) in Asturias (NW Spain)" Animals 11, no. 11: 3055. https://doi.org/10.3390/ani11113055
APA StyleAdalid, R., Feliu, C., Somoano, A., Miñarro, M., Ventura, J., Torres, J., Miquel, J., & Fuentes, M. V. (2021). Ecological Analysis of the Helminth Community of Microtus lusitanicus (Gerbe, 1879) (Rodentia) in Asturias (NW Spain). Animals, 11(11), 3055. https://doi.org/10.3390/ani11113055







