Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Experiment One: Feed Composition and Feeding
2.2.1. Feed Source
2.2.2. Coral Feeding
2.2.3. Analysis of Coral Body Composition and Feed
2.3. Experiment Two: Effects of Different Diets on Body Composition, Digestion Enzyme, Growth and Survival of Coral
2.3.1. Experimental Conditions
2.3.2. Coral Feeding
2.3.3. Determination of Coral Growth and Polyp Count
2.3.4. Analysis of Zooxanthellae Density and Chlorophyll a
2.3.5. Analysis of Coral Body Composition and Digestive Enzymes
2.4. Experiment Three: Diurnal Change Analysis of Coral Body Composition and Digestive Enzymes
2.5. Statistical Analysis
3. Results
3.1. Experiment One: Feed Composition and Ingestion
Effects of Feed on Nutrient Intake
3.2. Experiment Two: Effects of Different Diets on Body Composition, Digestion Enzyme, Growth and Survival of Coral
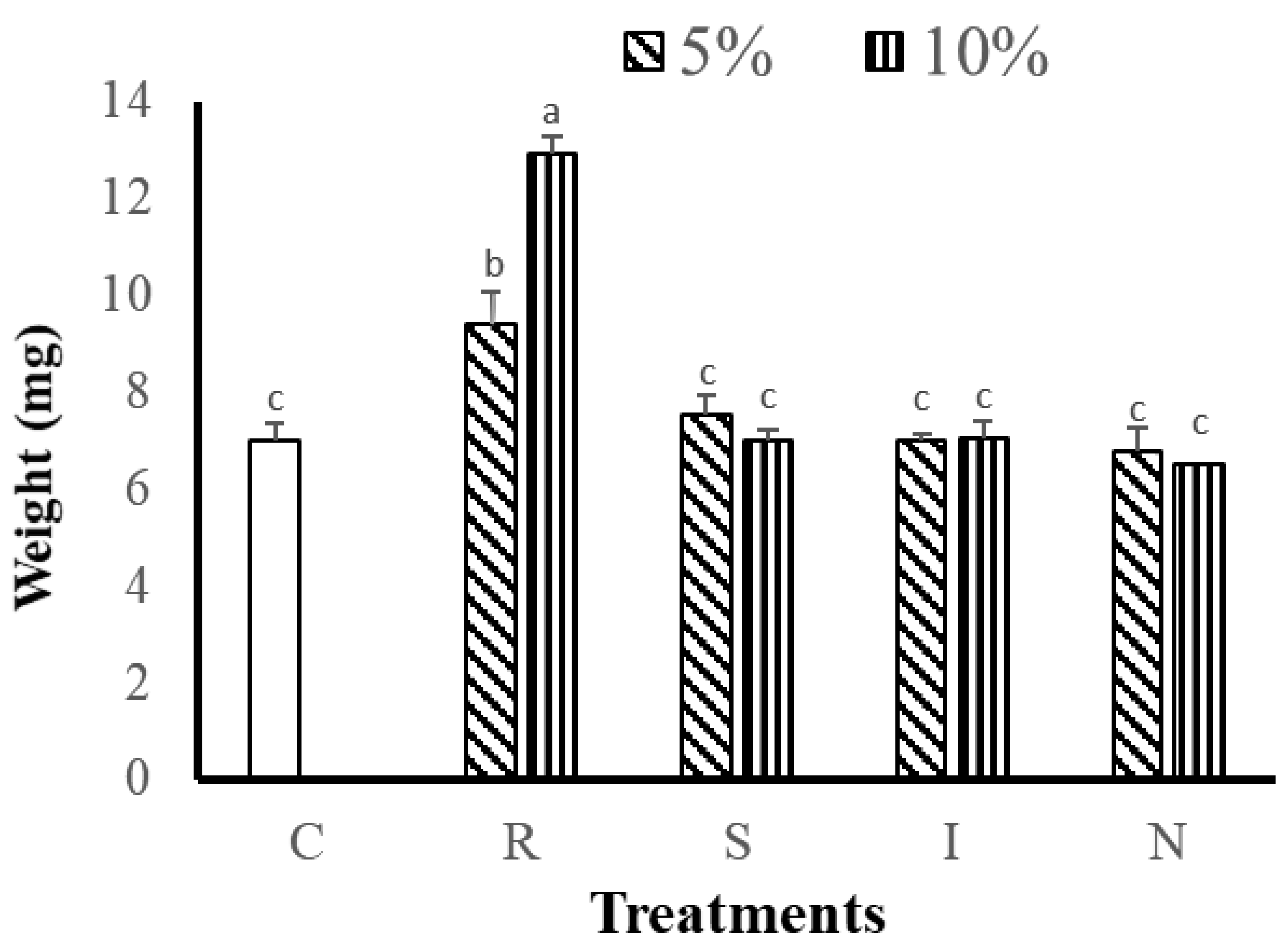
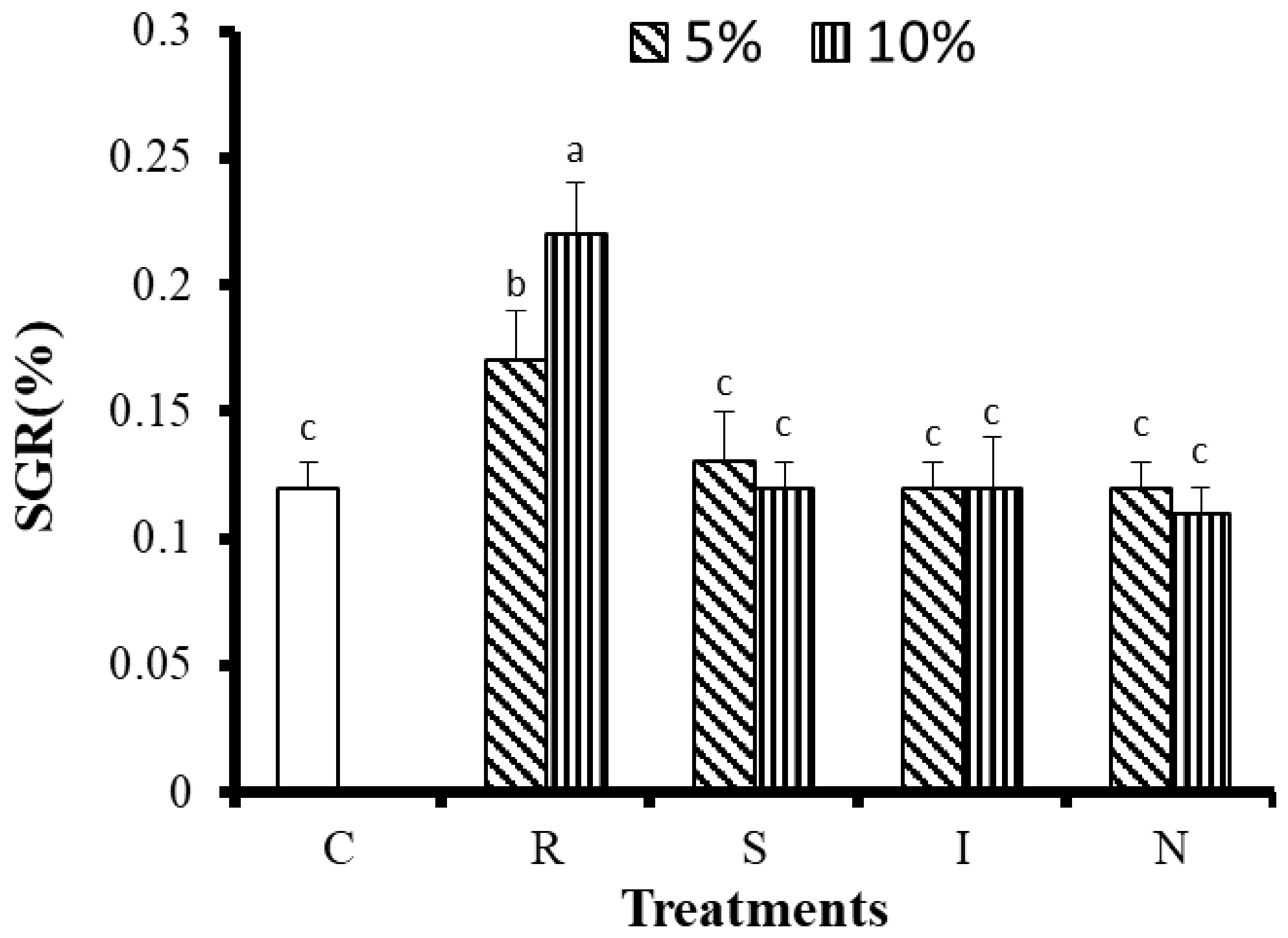
3.2.1. Effects of Feed on Growth and Survival of G. Columna
3.2.2. Influence of Different Feeds on Body Composition
3.2.3. Influence of Different Feeds on Digestive Enzymes
3.2.4. Influence of Different Feeds on Zooxanthellae and Chlorophyll a
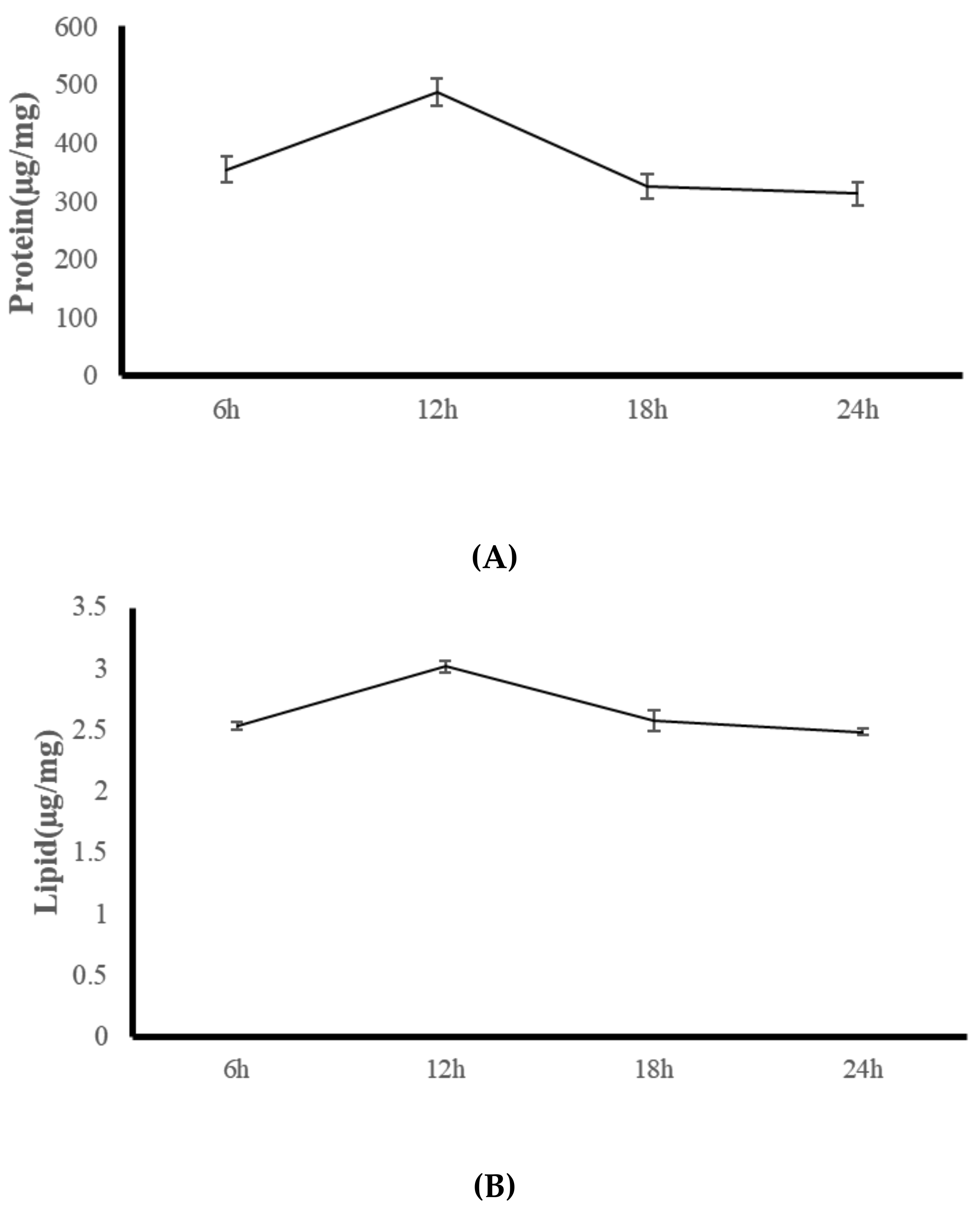
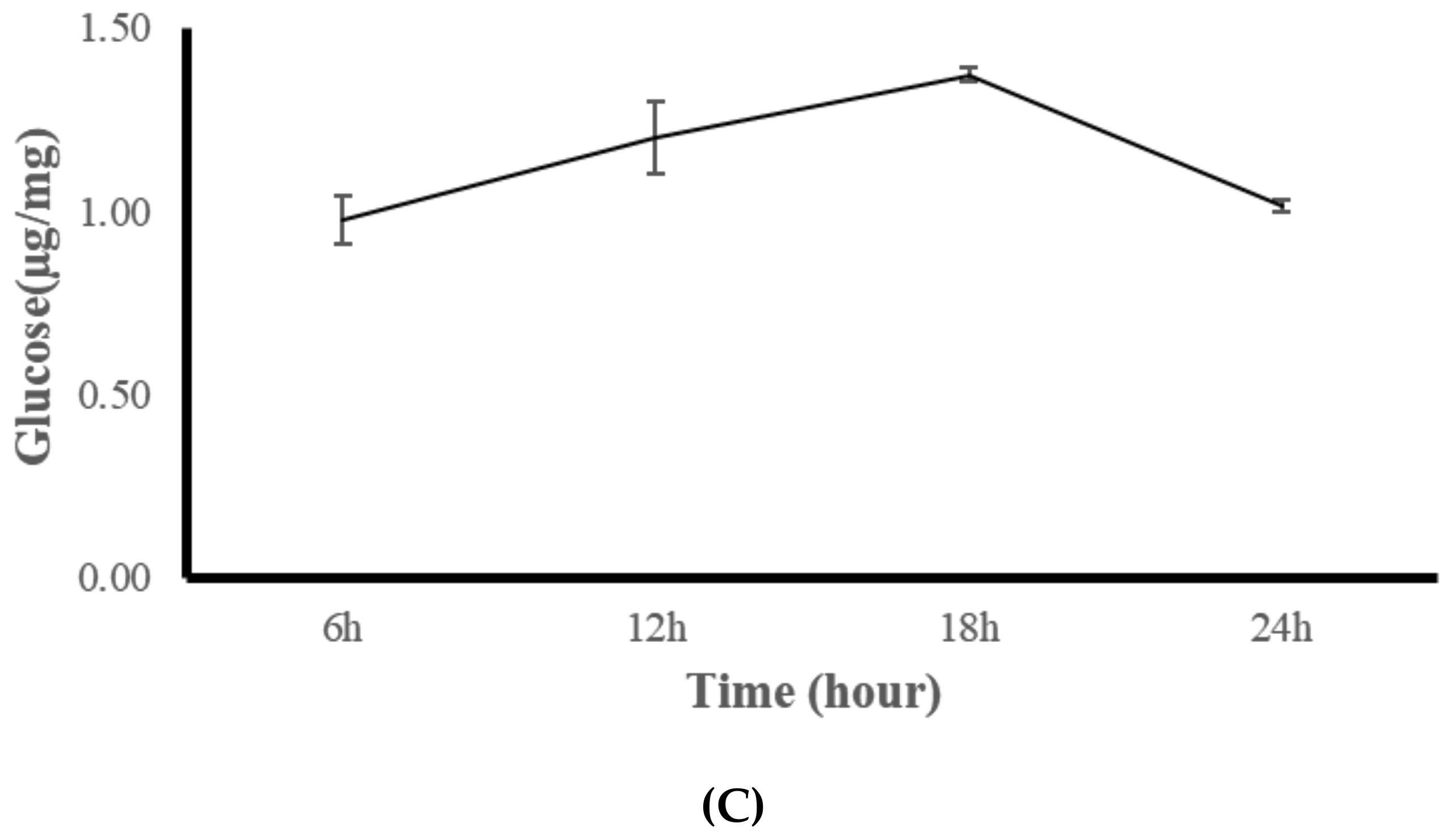
3.3. Experiment Three: Coral Feeding Time Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connell, J.H. Some mechanisms producing structure in natural communities: A model and evidence from field experiments. Ecol. Evol. Communities 1975, 460–490. [Google Scholar]
- Volkov, I.; Banavar, J.R.; Hubbell, S.P.; Maritan, A. Patterns of relative species abundance in rainforests and coral reefs. Nat. Cell Biol. 2007, 450, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wabnitz, C.; Taylor, M.; Green, E.; Razak, T. From Ocean to Aquarium: The Global Trade in Marine Ornamental Species; UNEP World Conservation Monitoring Centre: Cambridge, UK, 2003. [Google Scholar]
- Barton, J.; Willis, B.L.; Hutson, K.S. Coral propagation: A review of techniques for ornamental trade and reef restoration. Rev. Aquac. 2015, 9, 238–256. [Google Scholar] [CrossRef]
- Osinga, R.; Schutter, M.; Griffioen, B.; Wijffels, R.H.; Verreth, J.; Shafir, S.; Henard, S.; Taruffi, M.; Gili, C.; Lavorano, S. The Biology and Economics of Coral Growth. Mar. Biotechnol. 2011, 13, 658–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hii, Y.-S.; Soo, C.L.; Liew, H.-C. Feeding of scleractinian coral, Galaxea fascicularis, on Artemia salina nauplii in captivity. Aquac. Int. 2008, 17, 363–376. [Google Scholar] [CrossRef]
- Hodgson, G. A Global Assessment of Human Effects on Coral Reefs. Mar. Pollut. Bull. 1999, 38, 345–355. [Google Scholar] [CrossRef]
- Crabbe, M.J.C.; Smith, D. Modelling variations in corallite morphology of Galaxea fascicularis coral colonies with depth and light on coastal fringing reefs in the Wakatobi Marine National Park (S.E. Sulawesi, Indonesia). Comput. Biol. Chem. 2006, 30, 155–159. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Stark, J.; Fischer, A.B. Evaluation of artificial light regimes and substrate types for aquaria propagation of the staghorn coral Acropora solitaryensis. Aquaculture 2007, 269, 278–289. [Google Scholar] [CrossRef]
- Lasker, H. Comparison of the Particulate Feeding Abilities of Three Species of Gorgonian Soft Coral. Mar. Ecol. Prog. Ser. 1981, 5, 61–67. [Google Scholar] [CrossRef]
- Ferrier, M.D. Net uptake of dissolved free amino acids by four scleractinian corals. Coral Reefs 1991, 10, 183–187. [Google Scholar] [CrossRef]
- Lewis, J. Heterotrophy in corals: Zooplankton predation by the hydrocoral Millepora complanata. Mar. Ecol. Prog. Ser. 1992, 90, 251–256. [Google Scholar] [CrossRef]
- Al-Moghrabi, S.; Allemand, D.; Couret, J.; Jaubert, J. Fatty acids of the scleractinian coral Galaxea fascicularis: Effect of light and feeding. J. Comp. Physiol. B 1995, 165, 183–192. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Allemand, D.; Gattuso, J.-P.; Jaubert, J.; Rassoulzadegan, F. Microheterotrophy in the zooxanthellate coral Stylophora pistillata: Effects of light and ciliate density. Limnol. Oceanogr. 1998, 43, 1639–1648. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Witting, J.; Tambutté, E.; Sebens, K.P. Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs 2003, 22, 229–240. [Google Scholar] [CrossRef]
- Picciano, M.; Ferrier-Pagès, C. Ingestion of pico- and nanoplankton by the Mediterranean red coral Corallium rubrum. Mar. Biol. 2007, 150, 773–782. [Google Scholar] [CrossRef]
- Anthony, K.R.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Titlyanov, E.; Bil’, K.; Fomina, I.; Titlyanova, T.; Leletkin, V.; Eden, N.; Malkin, A.; Dubinsky, Z. Effects of dissolved ammonium addition and host feeding with Artemia salina on photoacclimation of the hermatypic coral Stylophora pistillata. Mar. Biol. 2000, 137, 463–472. [Google Scholar] [CrossRef]
- Mills, M.M.; Lipschultz, F.; Sebens, K.P. Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs 2004, 23, 311–323. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Meibom, A.; Stolarski, J.; Marrocchi, Y.; Ferrier-Pagès, C.; Domart-Coulon, I.; Dunbar, R.B.; Cuif, J.-P. Strontium-86 labeling experiments show spatially heterogeneous skeletal formation in the scleractinian coral Porites porites. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef] [Green Version]
- James, N.P. Diagenesis of scleractinian corals in the subaerial vadose environment. J. Paleontol. 1974, 785–799. [Google Scholar]
- Goreau, T.F.; Goreau, N.I.; Yonge, C.M. Reef Corals: Autotrophs or Heterotrophs? Biol. Bull. 1971, 141, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Forsman, Z.H.; Rinkevich, B.; Hunter, C.L. Investigating fragment size for culturing reef-building corals (Porites lobata and P. compressa) in ex situ nurseries. Aquaculture 2006, 261, 89–97. [Google Scholar] [CrossRef]
- Miller, M.W. Growth of a temperate coral:effects of temperature, light, depth, and heterotrophy. Mar. Ecol. Prog. Ser. 1995, 122, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Ferrier-Pagès, C.; Gattuso, J.P.; Dallot, S.; Jaubert, J. Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 2000, 19, 103–113. [Google Scholar] [CrossRef]
- Titlyanov, E.; Titlyanova, T.; Yamazato, K.; van Woesik, R. Photo-acclimation of the hermatypic coral Stylophora pistillata while subjected to either starvation or food provisioning. J. Exp. Mar. Biol. Ecol. 2001, 257, 163–181. [Google Scholar] [CrossRef]
- Grottoli, A.G. Effect of light and brine shrimp on skeletal δ13C in the Hawaiian coral Porites compressa: A tank experiment. Geochim. et Cosmochim. Acta 2002, 66, 1955–1967. [Google Scholar] [CrossRef]
- Houlbreèque, F.; Tambutteé, E.; Allemand, D.; Ferrier-Pagès, C. Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Biol. 2004, 207, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Davy, S.K.; Withers, K.J.; Hinde, R. Effects of host nutritional status and seasonality on the nitrogen status of zooxanthellae in the temperate coral Plesiastrea versipora (Lamarck). J. Exp. Mar. Biol. Ecol. 2006, 335, 256–265. [Google Scholar] [CrossRef]
- Anderson, D.; Siwicki, A.; Rumsey, G. Injection or immersion delivery of selected immunostimulants to trout demonstrate enhancement of nonspecific defense mechanisms and protective immunity. In Diseases in Asian Aquaculture, Vol. 11, Fish Health Section Section; Sharff, M., Subasing, R.P., Arthur, J.R., Eds.; Asian Fisheries Society: Manila, Philippines, 1995; pp. 413–426. [Google Scholar]
- Oliva-Teles, A.; Gonçalves, P. Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2001, 202, 269–278. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Hay, M.E. Seaweed-Coral Interactions: Variance in Seaweed Allelopathy, Coral Susceptibility, and Potential Effects on Coral Resilience. PLoS ONE 2014, 9, e85786. [Google Scholar] [CrossRef]
- AOAC. Official Methods for the Analysis, 14th ed.; Association of Official Analytical Chemists, Arlington: Washington, DC, USA, 1984. [Google Scholar]
- Bishop, M. Clinical Chemistry: Principles, Techniques, and Correlations; Jones & Bartlett Publishers: Burlington, MA, USA, 2020. [Google Scholar]
- Price, C.P.; Bossuyt, P.; Bruns, D.E. Tietz fundamentals of clinical chemistry. Medicine 1976, 3. [Google Scholar]
- Conlan, J.A.; Humphrey, C.; Severati, A.; Francis, D. Influence of different feeding regimes on the survival, growth, and biochemical composition of Acropora coral recruits. PLoS ONE 2017, 12, e0188568. [Google Scholar] [CrossRef]
- Conlan, J.A.; Bay, L.K.; Severati, A.; Humphrey, C.; Francis, D. Comparing the capacity of five different dietary treatments to optimise growth and nutritional composition in two scleractinian corals. PLoS ONE 2018, 13, e0207956. [Google Scholar] [CrossRef]
- Conlan, J.; Humphrey, C.; Severati, A.; Parrish, C.; Francis, D. Elucidating an optimal diet for captive Acropora corals. Aquaculture 2019, 513, 734420. [Google Scholar] [CrossRef]
- Rocha, R.J.; Pimentel, T.; Serôdio, J.; Rosa, R.; Calado, R. Comparative performance of light emitting plasma (LEP) and light emitting diode (LED) in ex situ aquaculture of scleractinian corals. Aquaculture 2013, 402–403, 38–45. [Google Scholar] [CrossRef]
- Schutter, M.; van Velthoven, B.; Janse, M.; Osinga, R.; Janssen, M.; Wijffels, R.; Verreth, J. The effect of irradiance on long-term skeletal growth and net photosynthesis in Galaxea fascicularis under four light conditions. J. Exp. Mar. Biol. Ecol. 2008, 367, 75–80. [Google Scholar] [CrossRef]
- Levy, O.; Dubinsky, Z.; Achituv, Y. Photobehavior of stony corals: Responses to light spectra and intensity. J. Exp. Biol. 2003, 206, 4041–4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffrey, S.T.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Du, J.; Qian, L.-C.; Jing, M.-Y.; Weng, X.-Y. Distribution and characteristics of endogenous digestive enzymes in the red-eared slider turtle, Trachemys scripta elegans. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, I. Studies on the digestive lipases of milkfish, Chanos chanos. Aquaculture 1990, 89, 315–325. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, α and β. Methods Enzymol. 1955, 1, 149–158. [Google Scholar]
- Raz-Bahat, M.; Douek, J.; Moiseeva, E.; Peters, E.C.; Rinkevich, B. The digestive system of the stony coral Stylophora pistillata. Cell Tissue Res. 2017, 368, 311–323. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Van Woesik, R.; Yamazato, K. Acclimation of the Hermatypic Coral Stylophora pistillata to Bright Light. Russ. J. Mar. Biol. 2002, 28, S41–S47. [Google Scholar] [CrossRef]
- Mieog, J.C.; Olsen, J.L.; Berkelmans, R.; Bleuler-Martinez, S.A.; Willis, B.L.; van Oppen, M.J. The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS ONE 2009, 4, e6364. [Google Scholar] [CrossRef]
- Nahon, S.; Richoux, N.B.; Kolasinski, J.; Desmalades, M.; Pages, C.F.; Lecellier, G.; Planes, S.; Lecellier, V.B. Spatial and Temporal Variations in Stable Carbon (δ13C) and Nitrogen (δ15N) Isotopic Composition of Symbiotic Scleractinian Corals. PLoS ONE 2013, 8, e81247. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.R.; Barrett, S.M.; Volkman, J.K.; Nearhos, S.P.; Nell, J.A.; Allan, G.L. Biochemical composition of new yeasts and bacteria evaluated as food for bivalve aquaculture. Aquaculture 1996, 143, 341–360. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abdel-Rahman, A.M.; Ismael, N.E. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 2008, 280, 185–189. [Google Scholar] [CrossRef]
- Li, P.; Gatlin, D.M. Nucleotide nutrition in fish: Current knowledge and future applications. Aquaculture 2006, 251, 141–152. [Google Scholar] [CrossRef]
- Gatesoupe, F. Live yeasts in the gut: Natural occurrence, dietary introduction, and their effects on fish health and development. Aquaculture 2007, 267, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Gopalakannan, A.; Arul, V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquac. Res. 2009, 41, 884–892. [Google Scholar] [CrossRef]
- Refstie, S.; Baeverfjord, G.; Seim, R.R.; Elvebø, O. Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture 2010, 305, 109–116. [Google Scholar] [CrossRef]
- Rumsey, G.; Hughes, S.G.; Smith, R.R.; Kinsella, J.E.; Shetty, K.J. Digestibility and energy values of intact, disrupted and extracts from brewer’s dried yeast fed to rainbow trout (Oncorhynchus mykiss). Animal Feed Sci. Technol. 1991, 33, 185–193. [Google Scholar] [CrossRef]
- Nasseri, A.; Rasoul-Ami, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Rodrussamee, N.; Lertwattanasakul, N.; Hirata, K.; Limtong, S.; Kosaka, T.; Yamada, M. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2011, 90, 1573–1586. [Google Scholar] [CrossRef]
- Kim, S.H.; Lin, D.P.; Matsumoto, S.; Kitazono, A.; Matsumoto, T. Fission Yeast Slp1: An Effector of the Mad2-Dependent Spindle Checkpoint. Science 1998, 279, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.I.; Olsen, Y.; Attramadal, Y.; Christie, K.; Birkbeck, T.H.; Skjermo, J.; Vadstein, O. Effects of short term feeding of microalgae on the bacterial flora associated with juvenile Artemia franciscana. Aquaculture 2000, 190, 11–25. [Google Scholar] [CrossRef]
- Lemahieu, C.; Bruneel, C.; Dejonghe, C.; Buyse, J.; Foubert, I. The cell wall of autotrophic microalgae influences the enrichment of long chain omega-3 fatty acids in the egg. Algal Res. 2016, 16, 209–215. [Google Scholar] [CrossRef]
- Dhont, J.; Van Stappen, G. Biology, Tank Production and Nutritional Value of Artemia. Live Feeds Marine Aquac. 2003, 65–121. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Kumar, R.R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2010, 27, 1737–1746. [Google Scholar] [CrossRef]
- Fujii, K.; Nakashima, H.; Hashidzume, Y.; Uchiyama, T.; Mishiro, K.; Kadota, Y. Potential use of the astaxanthin-producing microalga, Monoraphidium sp. GK12, as a functional aquafeed for prawns. Environ. Boil. Fishes 2009, 22, 363–369. [Google Scholar] [CrossRef]
- Roselet, F.F.G.; Escalonamento do Cultivo e da flo Culação da Microalga Marinha Nannochloropsis Oculata. Repositorio Institucional da Universidade Federal do Rio Grande. 2015. Available online: http://repositorio.furg.br/handle/1/8532 (In Spanish) (accessed on 1 September 2021).
- Sebens, K.P.; Koehl, M.A.R. Predation on zooplankton by the benthic anthozoans Alcyonium siderium (Alcyonacea) and Metridium senile (Actiniaria) in the New England subtidal. Mar. Biol. 1984, 81, 255–271. [Google Scholar] [CrossRef]
- Muscatine, L. The role of symbiotic algae in carbon and energy flux in reef corals. Coral Reefs 1990, 25, 75–87. [Google Scholar]
- Osinga, R.; Janssen, M.; Janse, M. The role of light in coral physiology and its implications for coral husbandry. Adv. Coral Husb. Public Aquar. 2008, 2, 173–183. [Google Scholar]
| Nutritional Indicators | R | S | I | N |
|---|---|---|---|---|
| Protein (µg) | 76.67 a −1.56 | 37.00 d −1.33 | 66.00 b −1.33 | 49.00 c −0.67 |
| Lipid (µg) | 6.00 c −0.67 | 5.00 c −1.33 | 32.00 a −2 | 20.33 b −1.56 |
| Glucose (µg) | 22.00 b −0.67 | 29.00 a −0.67 | 0.63 c −0.16 | 0.67 c −0.11 |
| 5% | 10% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water Quality Conditions | C | R | S | I | N | R | S | I | N |
| Temperature (°C) Salinity (PSU) pHAmmonia nitrogen (mg/L) Nitrous acid (mg/L) Nitric acid (PPM) Calcium (PPM) Magnesium (PPM) Phosphate (PPM) | 26.03 (0.24) 35.04 (0.22) | 26.19 (0.14) 35.21(0.39) | 26.20 (0.21) 35.12 (0.17) | 26.32 (0.19) 35.21 (0.12) | 26.31 (0.15) 34.22 (0.82) | 26.21 (0.21) 35.21 (0.19) | 26.03 (0.24) 35.12 (0.11) | 26.47 (0.52) 34.20 (0.21) | 26.93 (0.33) 35.04 (0.19) |
| 8.01 (0.31) 0.04 (0.01) 0.02 (0.01) | 8.03 (0.21) 0.04 (0.02) 0.02 (0.01) | 8.12 (0.42) 0.04 (0.03) 0.02 (0.01) | 8.05 (0.13) 0.05 (0.02) 0.01 (0.01) | 8.21 (0.42) 0.04 (0.03) 0.02 (0.01) | 8.21 (0.93) 0.04 (0.02) 0.02 (0.01) | 8.04 (0.21) 0.04 (0.04) 0.02 (0.01) | 8.21 (0.48) 0.03 (0.01) 0.02 (0.01) | 8.21 (0.24) 0.03 (0.01) 0.02 (0.01) | |
| 0.20 (0.03) 409 (42.32) 1385(69.12) | 0.18 (0.04) 415 (33.92) 1350(63.29) | 0.30 (0.02) 410 (13.03) 1349 (31.03) | 0.13 (0.02) 418 (20.03) 1320 (22.43) | 0.05 (0.02) 424 (32.01) 1381 (53.22) | 0.25 (0.08) 412 (34.21) 1351 (21.04) | 0.20 (0.02) 427 (12.15) 1328 (31.26) | 0.19 (0.04) 413 (30.21) 1365 (23.41) | 0.20 (0.03) 410 (30.22) 1350 (30.34) | |
| 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | |
| Nutritional Indicators | Feeding | ||||
|---|---|---|---|---|---|
| C | R | S | I | N | |
| Protein (µg/mg) | 321.52 b −11.51 | 430.45 a −12.3 | 322.05 b −11.22 | 332.35 b −13.12 | 325.43 b −13.2 |
| Lipid (µg/mg) | 1.10 b −0.15 | 1.20 b −0.11 | 1.40 b −0.12 | 3.11 a −0.21 | 2.85 a 0.14 |
| Glucose (µg/mg) | 1.11 c −0.06 | 3.93 b −0.31 | 4.82 a −0.06 | 1.15 c −0.12 | 1.14 c −0.09 |
| Treatments | Feed | Zooxanthellae (Cells×107 m2) | Chlorophyll a (µg/cm2) | Initial Polyps (Number ± 95%) | End Polyp (Number ± 95%) | Net Increase (Number ± 95%) |
|---|---|---|---|---|---|---|
| 5% | C | 3.71 (0.17) | 53.22 (1.04) | 5.00 (0.00) | 33.00 (1.37) | 28.00 (1.37) |
| R | 4.05 (0.71) | 54.29 (1.23) | 5.00 (0.00) | 40.00 (2.34) | 35.00 (2.34) | |
| S | 3.79 (1.43) | 53.73 (1.17) | 5.00 (0.00) | 32.67 (2.22) | 27.67 (2.22) | |
| I | 3.98 (0.94) | 53.98 (1.39) | 5.00 (0.00) | 34.00 (0.67) | 29.00 (0.67) | |
| N | 4.12 (1.02) | 54.05 (0.28) | 5.00 (0.00) | 32.33 (1.56) | 27.33 (1.56) | |
| 10% | R | 3.93 (1.76) | 54.02 (1.32) | 5.00 (0.00) | 47.33 (0.89) | 42.33 (0.89) |
| S | 4.21(1.53) | 53.69 (1.75) | 5.00 (0.00) | 35.35.00 (2.00) | 30.00 (2.00) | |
| I | 4.27(0.92) | 54.38 (1.43) | 5.00 (0.00) | 36.00 (0.67) | 31.00 (0.67) | |
| N | 4.36(1.47) | 54.64 (0.00) | 5.00 (0.00) | 32.67 (1.78) | 27.67 (1.78) |
| Nutritional Indicators | Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | R | S | I | N | |||||
| 5% (I) | 10% (II) | 5% (I) | 10% (II) | 5% (I) | 10% (II) | 5% (I) | 10% (II) | ||
| Protein (µg/mg) | 374.73 b (11.50) | 474.01 a (23.00) | 486.78 a (36.41) | 327.72 b (21.80) | 383.18 b (12.60) | 386.87 b (12.60) | 385.57 b (19.80) | 339.23 b (12.14) | 321.79 b (8.85) |
| Lipid (µg/mg) | 1.96 b (0.15) | 2.85 a (0.13) | 3.02 a (0.29) | 1.65 b (0.15) | 1.72 b (0.10) | 1.98 b (0.12) | 2.13 b (0.21) | 1.69 b (0.42) | 1.64 b (0.14) |
| Glucose (µg/mg) | 1.11 (0.06) | 1.28 (0.20) | 1.32 (0.22) | 1.13 (0.07) | 1.26 (0.06) | 1.22 (0.09) | 1.15 (0.12) | 1.13 (0.07) | 1.11 (0.09) |
| Test Items | Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | R | S | I | N | |||||
| 5% (I) | 10% (II) | 5% (I) | 10% (II) | 5% (I) | 10% (II) | 5% (I) | 10% (II) | ||
| Protease (U/mg protein) | 214.37 b (11.22) | 324.67 a (26.68) | 325.70 a (34.88) | 206.24 b (7.31) | 196.66 b (12.13) | 182.13 b (42.85) | 168.68 b (30.17) | 191.75 b (35.94) | 190.17 b (33.92) |
| Lipase (U/mg protein) | 10.01 (0.80) | 12.16 (0.52) | 12.84 (0.43) | 10.04 (0.39) | 10.13 (0.37) | 9.42 (0.64) | 10.30 (0.42) | 9.09 (1.30) | 9.42 (0.64) |
| Amylase (U/mg protein) | 1.47 (0.08) | 1.45 (0.15) | 1.61 (0.24) | 1.38 (0.43) | 1.17 (0.54) | 1.27 (0.08) | 1.26 (0.06) | 1.18 (0.52) | 1.26 (0.58) |
| Test Items | Time (Hour) | |||
|---|---|---|---|---|
| 6 | 12 | 18 | 24 | |
| Protease U/mg protein | 153.25 (20.32) c | 385.67 (16.48) a | 285.15 (17.12) b | 167.85 (19.35) c |
| Lipase U/mg protein | 4.01 (0.08) c | 10.16 (0.52) a | 9.06 (0.42) b | 4.39 (0.12) c |
| Amylase U/mg protein | 0.95 (0.21) | 1.29 (0.11) | 1.17 (0.08) | 1.04 (0.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, D.-S.; Sun, W.-T.; Pan, C.-H. Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity. Animals 2021, 11, 3009. https://doi.org/10.3390/ani11113009
Ding D-S, Sun W-T, Pan C-H. Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity. Animals. 2021; 11(11):3009. https://doi.org/10.3390/ani11113009
Chicago/Turabian StyleDing, De-Sing, Wei-Ting Sun, and Chih-Hung Pan. 2021. "Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity" Animals 11, no. 11: 3009. https://doi.org/10.3390/ani11113009
APA StyleDing, D.-S., Sun, W.-T., & Pan, C.-H. (2021). Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity. Animals, 11(11), 3009. https://doi.org/10.3390/ani11113009






