Seroprevalence and Risk Factors of Anaplasma spp. in German Small Ruminant Flocks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
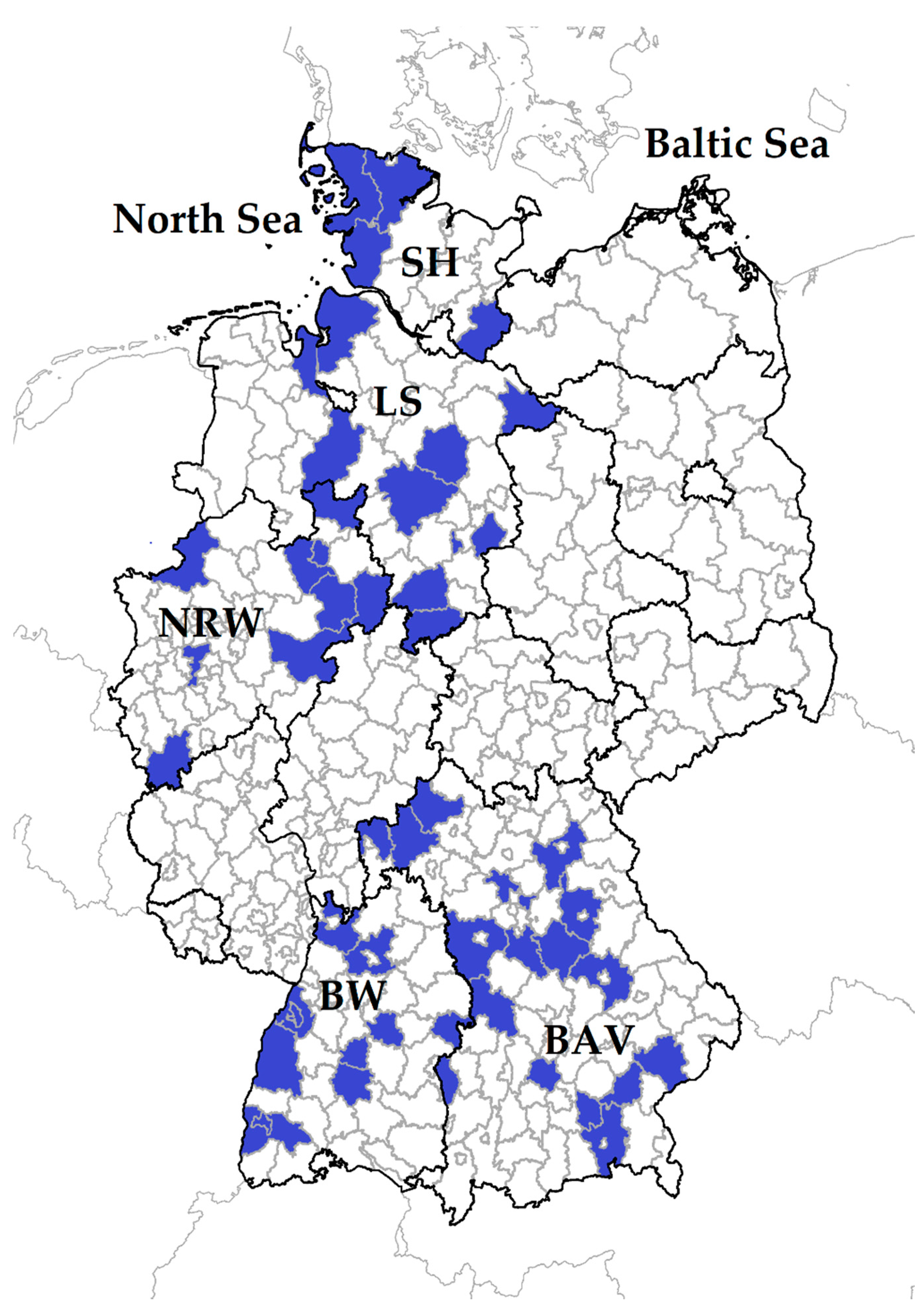
2.1. Animals
2.2. Detection Method
2.3. Risk Analysis
2.4. Statistical Analysis
2.4.1. Seroprevalence
2.4.2. Risk Analysis
Correlation Analysis
Risk Factor Analysis
3. Results
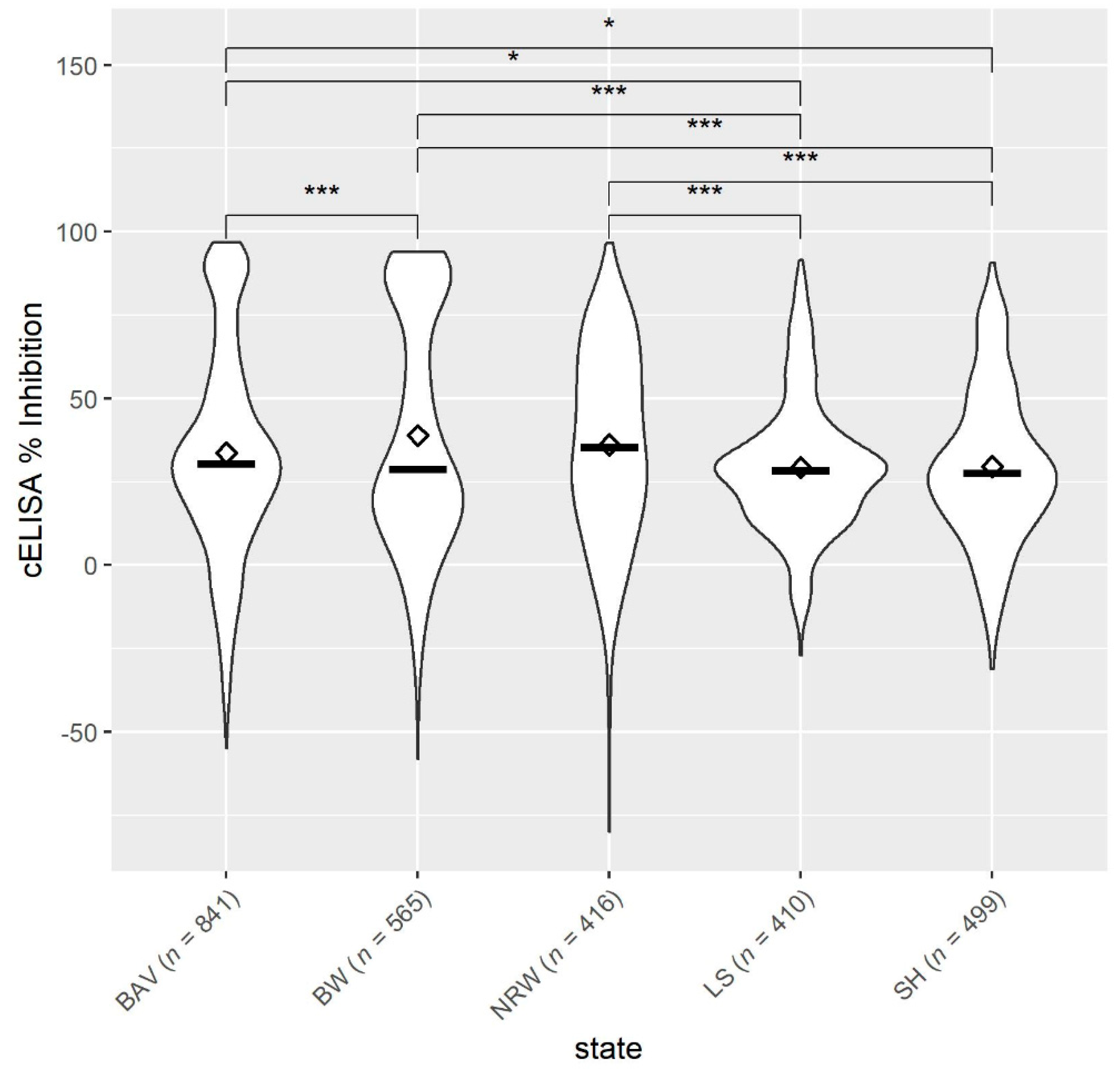
3.1. Occurrence of Anaplasma spp. in German Small Ruminant Flocks
3.2. Univariable Analysis
3.2.1. Risk Factors at Animal Level for Exposure to Anaplasma spp.
3.2.2. Risk Factors at Flock Level for Exposure to Anaplasma spp.
3.3. Multivariable Analysis
3.3.1. Risk Factors at Animal Level for Exposure to Anaplasma spp.
3.3.2. Risk Factors at Flock Level for Exposure to Anaplasma spp.
4. Discussion
4.1. Occurrence of Anaplasma spp. in German Small Ruminant Flocks
4.2. Risk Factor Analysis on Animal Level
4.3. Risk Factor Analysis on Flock Level
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuen, S. Haemoparasites in small ruminants in European countries: Challenges and clinical relevance. Small Rumin. Res. 2016, 142, 22–27. [Google Scholar] [CrossRef]
- Hornok, S.; Elek, V.; de la Fuente, J.; Naranjo, V.; Farkas, R.; Majoros, G.; Foldvari, G. First serological and molecular evidence on the endemicity of Anaplasma ovis and Anaplasma marginale in Hungary. Vet. Microbiol. 2007, 122, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Víchová, B.; Majláthová, V.; Nováková, M.; Stanko, M.; Hviščová, I.; Pangrácová, L.; Chrudimský, T.; Čurlík, J.; Peťko, B. Anaplasma infections in ticks and reservoir host from Slovakia. Infect. Genet. Evol. 2014, 22, 265–272. [Google Scholar] [CrossRef]
- Bauer, B.U.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Ambros, C.; Silaghi, C.; Ganter, M. Anaplasma phagocytophilum and Anaplasma ovis—Emerging pathogens in the German sheep population. Pathogens 2021. submitted. [Google Scholar]
- Mason, K.L.; Gonzalez, M.V.; Chung, C.; Mousel, M.R.; White, S.N.; Taylor, J.B.; Scoles, G.A. Validation of an improved Anaplasma antibody competitive ELISA for detection of Anaplasma ovis antibody in domestic sheep. J. Vet. Diagn. Investig. 2017, 29, 763–766. [Google Scholar] [CrossRef]
- Gorman, J.K.; Hoar, B.R.; Nieto, N.C.; Foley, J.E. Evaluation of Anaplasma phagocytophilum infection in experimentally inoculated sheep and determination of Anaplasma spp. seroprevalence in 8 free-ranging sheep flocks in California and Oregon. Am. J. Vet. Res. 2012, 73, 1029–1034. [Google Scholar] [CrossRef]
- de la Fuente, J.; Ruiz-Fons, F.; Naranjo, V.; Torina, A.; Rodriguez, O.; Gortazar, C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res. Vet. Sci. 2008, 84, 382–386. [Google Scholar] [CrossRef]
- Scharf, W.; Schauer, S.; Freyburger, F.; Petrovec, M.; Schaarschmidt-Kiener, D.; Liebisch, G.; Runge, M.; Ganter, M.; Kehl, A.; Dumler, J.S.; et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J. Clin. Microbiol. 2011, 49, 790–796. [Google Scholar] [CrossRef]
- Kauffmann, M.; Rehbein, S.; Hamel, D.; Lutz, W.; Heddergott, M.; Pfister, K.; Silaghi, C. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol. Cell. Probes 2017, 31, 46–54. [Google Scholar] [CrossRef]
- de la Fuente, J.; Atkinson, M.W.; Naranjo, V.; de Mera, I.G.F.; Mangold, A.J.; Keating, K.A.; Kocan, K.M. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet. Microbiol. 2007, 119, 375–381. [Google Scholar] [CrossRef]
- Dugat, T.; Lagrée, A.-C.; Maillard, R.; Boulouis, H.-J.; Haddad, N. Opening the black box of Anaplasma phagocytophilum diversity: Current situation and future perspectives. Front. Cell. Infect. Microbiol. 2015, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Estrada-Pena, A.; Cutler, S.J.; Taussat, M.V.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, A.; Grenda, S. Bacteria of the genus Anaplasma—Characteristics of Anaplasma and their vectors: A review. Vet. Med. 2008, 53, 573–584. [Google Scholar] [CrossRef]
- Friedhoff, K.T. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997, 39, 99–109. [Google Scholar] [PubMed]
- Hornok, S.; De La Fuente, J.; Biró, N.; de Mera, I.G.F.; Meli, M.L.; Elek, V.; Gönczi, E.; Meili, T.; Tánczos, B.; Farkas, R.; et al. First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector Borne Zoonotic Dis. 2011, 11, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Wang, Y.; Li, Y.; Han, S.Y.; Wang, B.; Yuan, G.H.; Zhang, P.Y.; Yang, Z.W.; Wang, S.L.; Chen, J.Y.; et al. Vector-borne pathogens with Veterinary and Public Health significance in Melophagus ovinus (Sheep Ked) from the Qinghai-Tibet Plateau. Pathogens 2021, 10, 249. [Google Scholar] [CrossRef]
- Henniger, T.; Henniger, P.; Grossmann, T.; Distl, O.; Ganter, M.; von Loewenich, F.D. Congenital infection with Anaplasma phagocytophilum in a calf in northern Germany. Acta Vet. Scand. 2013, 55, 38. [Google Scholar] [CrossRef]
- von Loewenich, F.D.; Stumpf, G.; Baumgarten, B.U.; Röllinghoff, M.; Dumler, J.S.; Bogdan, C. A case of Equine Granulocytic Ehrlichiosis provides molecular evidence for the presence of pathogenic Anaplasma phagocytophilum (HGE Agent) in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 303–305. [Google Scholar] [CrossRef]
- Silaghi, C.; Liebisch, G.; Pfister, K. Genetic variants of Anaplasma phagocytophilum from 14 equine granulocytic anaplasmosis cases. Parasites Vectors 2011, 4, 161. [Google Scholar] [CrossRef]
- Tegtmeyer, P.; Ganter, M.; von Loewenich, F.D. Simultaneous infection of cattle with different Anaplasma phagocytophilum variants. Ticks Tick Borne Dis. 2019, 10, 1051–1056. [Google Scholar] [CrossRef]
- Silaghi, C.; Nieder, M.; Sauter-Louis, C.; Knubben-Schweizer, G.; Pfister, K.; Pfeffer, M. Epidemiology, genetic variants and clinical course of natural infections with Anaplasma phagocytophilum in a dairy cattle herd. Parasites Vectors 2018, 11, 20. [Google Scholar] [CrossRef]
- Jensen, J.; Simon, D.; Escobar, H.M.; Soller, J.T.; Bullerdiek, J.; Beelitz, P.; Pfister, K.; Nolte, I. Anaplasma phagocytophilum in Dogs in Germany. Zoonoses Public Health 2007, 54, 94–101. [Google Scholar] [CrossRef]
- Silaghi, C.; Kohn, B.; Chirek, A.; Thiel, C.; Nolte, I.; Liebisch, G.; Pfister, K. Relationship of molecular and clinical findings on Anaplasma phagocytophilum involved in natural infections of dogs. J. Clin. Microbiol. 2011, 49, 4413–4414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamel, D.; Bondarenko, A.; Silaghi, C.; Nolte, I.; Pfister, K. Seroprevalence and bacteriamea of Anaplasma phagocytophilum in cats from Bavaria and Lower Saxony (Germany). Berl. Munch. Tierarztl. Wochenschr. 2012, 125. [Google Scholar] [CrossRef]
- Schäfer, I.; Kohn, B.; Müller, E. Anaplasma phagocytophilum in domestic cats from Germany, Austria and Switzerland and clinical/laboratory findings in 18 PCR-positive cats (2008–2020). J. Feline Med. Surg. 2021, 1098612X211017459. [Google Scholar] [CrossRef]
- Gokce, H.I.; Woldehiwet, Z. Differential Haematological Effects of Tick-borne Fever in Sheep and Goats. J. Vet. Med. Ser. B 1999, 46, 105–115. [Google Scholar] [CrossRef]
- Stuen, S.; Bergstrom, K.; Palmer, E. Reduced weight gain due to subclinical Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila) infection. Exp. Appl. Acarol. 2002, 28, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Grøva, L.; Olesen, I.; Steinshamn, H.; Stuen, S. Prevalence of Anaplasma phagocytophilum infection and effect on lamb growth. Acta Vet. Scand. 2011, 53, 30. [Google Scholar] [CrossRef]
- Sargison, N.; Edwards, G. Tick infestations in sheep in the UK. Practice 2009, 31, 58–65. [Google Scholar] [CrossRef]
- Overås, J.; Lund, A.; Ulvund, M.J.; Waldeland, H. Tick-borne fever as a possible predisposing factor in septicaemic pasteurellosis in lambs. Vet. Rec. 1993, 133, 398. [Google Scholar] [CrossRef]
- Daniel, R.G.; Carson, A.; Evans, C.; Cookson, R.; Wessels, M. Pathological observations of tick-borne fever and intercurrent bacterial infections in lambs. Vet. Rec. Case Rep. 2016, 4, e000357. [Google Scholar] [CrossRef]
- Van Miert, A.S.J.P.A.M.; Van Duin, C.T.M.; Schotman, A.J.H.; Franssen, F.F. Clinical, haematological and blood biochemical changes in goats after experimental infection with tick-borne fever. Vet. Parasitol. 1984, 16, 225–233. [Google Scholar] [CrossRef][Green Version]
- Gray, D.; Webster, K.; Berry, J.E. Evidence of louping ill and tick-borne fever in goats. Vet. Rec. 1988, 122, 66. [Google Scholar] [CrossRef]
- Jiménez, C.; Benito, A.; Arnal, J.L.; Ortín, A.; Gómez, M.; López, A.; Villanueva-Saz, S.; Lacasta, D. Anaplasma ovis in sheep: Experimental infection, vertical transmission and colostral immunity. Small Rumin. Res. 2019, 178, 7–14. [Google Scholar] [CrossRef]
- Pereira, A.; Parreira, R.; Nunes, M.; Casadinho, A.; Vieira, M.L.; Campino, L.; Maia, C. Molecular detection of tick-borne bacteria and protozoa in cervids and wild boars from Portugal. Parasites Vectors 2016, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031–1032. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Galindo, R.C.; Vicente, J.; Di Marco, V.; Russo, M.; Aronica, V.; Fiasconaro, M.; Scimeca, S.; Alongi, A.; Caracappa, S.; et al. Characterization of Anaplasma phagocytophilum and A. ovis infection in a naturally infected sheep flock with poor health condition. Trop. Anim. Health Prod. 2010, 42, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Alongi, A.; Naranjo, V.; Scimeca, S.; Nicosia, S.; Di Marco, V.; Caracappa, S.; Kocan, K.M.; de la Fuente, J. Characterization of anaplasma infections in Sicily, Italy. Ann. N. Y. Acad. Sci. 2008, 1149, 90–93. [Google Scholar] [CrossRef]
- Lacasta, D.; Ferrer, L.M.; Sanz, S.; Labanda, R.; González, J.M.; Benito, A.Á.; Ruiz, H.; Rodríguez-Largo, A.; Ramos, J.J. Anaplasmosis Outbreak in Lambs: First Report Causing Carcass Condemnation. Animals 2020, 10, 1851. [Google Scholar] [CrossRef]
- Yasini, S.; Khaki, Z.; Rahbari, S.; Kazemi, B.; Amoli, J.S.; Gharabaghi, A.; Jalali, S. Hematologic and clinical aspects of experimental ovine anaplasmosis caused by Anaplasma ovis in Iran. Iran. J. Parasitol. 2012, 7, 91–98. [Google Scholar]
- Stuen, S.; Longbottom, D. Treatment and control of chlamydial and rickettsial infections in sheep and goats. Vet. Clin. North. Am. Food Anim. Pr. 2011, 27, 213–233. [Google Scholar] [CrossRef]
- Hartelt, K.; Oehme, R.; Frank, H.; Brockmann, S.O.; Hassler, D.; Kimmig, P. Pathogens and symbionts in ticks: Prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int. J. Med. Microbiol. Suppl. 2004, 293, 86–92. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Krämer, A.; Sachse, S.; Straube, E. Detection of Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus ticks in a region of Middle Germany (Thuringia). Ticks Tick-Borne Dis. 2010, 1, 52–56. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Strube, C. Prevalence of Rickettsiales (Anaplasma phagocytophilum and Rickettsia spp.) in hard ticks (Ixodes ricinus) in the city of Hamburg, Germany. J. Parasitol. Res. 2014, 113, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Chirek, A.; Silaghi, C.; Pfister, K.; Kohn, B. Granulocytic anaplasmosis in 63 dogs: Clinical signs, laboratory results, therapy and course of disease. J. Small Anim. Pract. 2018, 59, 112–120. [Google Scholar] [CrossRef]
- Silaghi, C.; Fröhlich, J.; Reindl, H.; Hamel, D.; Rehbein, S. Anaplasma phagocytophilum and Babesia species of Sympatric Roe Deer (Capreolus capreolus), Fallow Deer (Dama dama), Sika Deer (Cervus nippon) and Red Deer (Cervus elaphus) in Germany. Pathogens 2020, 9, 968. [Google Scholar] [CrossRef]
- Langenwalder, D.B.; Schmidt, S.; Gilli, U.; Pantchev, N.; Ganter, M.; Silaghi, C.; Aardema, M.L.; von Loewenich, F.D. Genetic characterization of Anaplasma phagocytophilum strains from goats (Capra aegagrus hircus) and water buffalo (Bubalus bubalis) by 16S rRNA gene, ankA gene and multilocus sequence typing. Ticks Tick Borne Dis. 2019, 10, 101267. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Prufer, T.L.; Schoneberg, C.; Campe, A.; Runge, M.; Ganter, M.; Bauer, B.U. Risk factors for an infection with Coxiella burnetii in German sheep flocks. Epidemiol. Infect. 2020, 148, e260. [Google Scholar] [CrossRef]
- Wolf, A.; Prüfer, T.L.; Schoneberg, C.; Campe, A.; Runge, M.; Ganter, M.; Bauer, B.U. Prevalence of Coxiella burnetii in German sheep flocks and evaluation of a novel approach to detect an infection via preputial swabs at herd-level. Epidemiol. Infect. 2020, 148, e75. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.; de Echaide, S.T.; Palmer, G.; McGuire, T.; Stiller, D.; McElwain, T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 1996, 34, 2225–2230. [Google Scholar] [CrossRef]
- Shabana, I.I.; Alhadlag, N.M.; Zaraket, H. Diagnostic tools of caprine and ovine anaplasmosis: A direct comparative study. BMC Vet. Res. 2018, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.W. Generalized estimating equations (GEE). In Encyclopedia of Statistics in Behavioral Science, 1st ed.; Everitt, B.A.H., Ed.; John Wiley & Sons, Ltd: Chichester, England, 2005; pp. 47–89. [Google Scholar]
- Liang, K.-Y.; Zeger, S.L. Analysis of Longitudinal Data; Oxford Statistical Science Series; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Agresti, A. Categorial Data Analysis; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Silaghi, C.; Kauffmann, M.; Passos, L.M.; Pfister, K.; Zweygarth, E. Isolation, propagation and preliminary characterisation of Anaplasma phagocytophilum from roe deer (Capreolus capreolus) in the tick cell line IDE8. Ticks Tick Borne Dis. 2011, 2, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Vicente, J.; Alongi, A.; Scimeca, S.; Turlá, R.; Nicosia, S.; Di Marco, V.; Caracappa, S.; De La Fuente, J. Observed Prevalence of Tick-borne Pathogens in Domestic Animals in Sicily, Italy during 2003–2005. Zoonoses Public Health 2007, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Chitimia-Dobler, L.; Dautel, H.; Meyer-Kayser, E.; Kahl, O. Atlas of ticks (Acari: Argasidae, Ixodidae) in Germany. Exp. Appl. Acarol. 2021, 84, 183–214. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gethmann, J.; Hoffmann, B.; Kasbohm, E.; Süss, J.; Habedank, B.; Conraths, F.J.; Beer, M.; Klaus, C. Research paper on abiotic factors and their influence on Ixodes ricinus activity—observations over a two-year period at several tick collection sites in Germany. Parasitol. Res. 2020, 119, 1455–1466. [Google Scholar] [CrossRef]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany-Evidence of a continuing spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 578220. [Google Scholar] [CrossRef]
- Chochlakis, D.; Ioannou, I.; Sharif, L.; Kokkini, S.; Hristophi, N.; Dimitriou, T.; Tselentis, Y.; Psaroulaki, A. Prevalence of Anaplasma spp. in goats and sheep in Cyprus. Vector Borne Zoonotic Dis. 2008, 9, 457–463. [Google Scholar] [CrossRef]
- Lees, A.D. The sensory physiology of the sheep tick, Ixodes Ricinus L. J. Exp. Biol. 1948, 25, 145–207. [Google Scholar] [CrossRef]
- Osterkamp, J.; Wahl, U.; Schmalfuss, G.; Haas, W. Host-odour recognition in two tick species is coded in a blend of vertebrate volatiles. Comp. Physiol. 1999, 185, 59–67. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Gallois, M.; Fontugne, M.; Allain, E.; Denoual, M.; Moutailler, S.; Devillers, E.; Zientara, S.; Memmi, M.; Chauvin, A.; et al. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasites Vectors 2019, 12, 3. [Google Scholar] [CrossRef]
- Praskova, I.; Bezdekova, B.; Zeman, P.; Jahn, P. Seroprevalence of Anaplasma phagocytophilum in horses in the Czech Republic. Ticks Tick Borne Dis. 2011, 2, 111–115. [Google Scholar] [CrossRef]
- Barutzki, D.; De Nicola, A.; Zeziola, M.; Reule, M. Seroprevalence of Anaplasma phagocytophilum infection in dogs in Germany. Berl. Munch. Tierarztl. Wochenschr. 2006, 119, 342–347. [Google Scholar]
- Kang, J.-g.; Ko, S.; Kim, Y.-J.; Yang, H.-J.; Lee, H.; Shin, N.-S.; Choi, K.-S.; Chae, J.-S. New Genetic Variants of Anaplasma phagocytophilum and Anaplasma bovis from Korean Water Deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2011, 11, 929–938. [Google Scholar] [CrossRef]
- Jifei, Y.; Zhijie, L.; Qingli, N.; Jianxun, L.; Xiaolong, W.; Hong, Y. Molecular Detection of Anaplasma phagocytophilum in wild cervids and hares in China. J. Wildl. Dis. 2017, 53, 420–423. [Google Scholar] [CrossRef]
- Overzier, E.; Pfister, K.; Herb, I.; Mahling, M.; Bock, G., Jr.; Silaghi, C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), in questing ticks (Ixodes ricinus), and in ticks infesting roe deer in southern Germany. Ticks Tick Borne Dis. 2013, 4, 320–328. [Google Scholar] [CrossRef]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors 2014, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Pettersen, K.S.; Granquist, E.G.; Bergstrom, K.; Bown, K.J.; Birtles, R.J. Anaplasma phagocytophilum variants in sympatric red deer (Cervus elaphus) and sheep in southern Norway. Ticks Tick Borne Dis. 2013, 4, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.U.; Könenkamp, L.; Stöter, M.; Wolf, A.; Ganter, M.; Steffen, I.; Runge, M. Increasing awareness for tick-borne encephalitis virus using small ruminants as suitable sentinels: Preliminary observations. One Health 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Enemark, J.M.D.; Artursson, K.; Nielsen, B. Prophylactic treatment with flumethrin, a pyrethroid (Bayticol®, Bayer), against Anaplasma phagocytophilum infection in lambs. Acta Vet. Scand. 2012, 54, 31. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Variable | Category | Odds Ratio (OR) | 95% Confidence Interval | p-Value | Quasilikelihood under the Independence Model Criterion (QIC) |
|---|---|---|---|---|---|
| Species | Goat | 2.525 | 1.443–4.417 | 0.001 | 4342.598 |
| Sex | Male | 1.378 | 1.029–1.846 | 0.032 | |
| Age | >2 years | 0.739 | 0.970–0.970 | 0.029 |
| Variable | Category | Odds Ratio (OR) | 95% Confidence Interval | C- p-Value | LR- p-Value | Akaike Information Criterion (AIC) |
|---|---|---|---|---|---|---|
| Landscape conservation | No | 5.348 | 1.026–7.877 | 0.047 | 0.0002 | 49.614 |
| Deer | No | 0.149 | 0.015–1.461 | 0.102 | ||
| Cats | No | 10.731 | 1.681–68.514 | 0.012 | ||
| Dogs | No | 166.328 | 3.606–>999.999 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubel, W.; Schoneberg, C.; Wolf, A.; Ganter, M.; Bauer, B.U. Seroprevalence and Risk Factors of Anaplasma spp. in German Small Ruminant Flocks. Animals 2021, 11, 2793. https://doi.org/10.3390/ani11102793
Rubel W, Schoneberg C, Wolf A, Ganter M, Bauer BU. Seroprevalence and Risk Factors of Anaplasma spp. in German Small Ruminant Flocks. Animals. 2021; 11(10):2793. https://doi.org/10.3390/ani11102793
Chicago/Turabian StyleRubel, Wiebke, Clara Schoneberg, Annika Wolf, Martin Ganter, and Benjamin Ulrich Bauer. 2021. "Seroprevalence and Risk Factors of Anaplasma spp. in German Small Ruminant Flocks" Animals 11, no. 10: 2793. https://doi.org/10.3390/ani11102793
APA StyleRubel, W., Schoneberg, C., Wolf, A., Ganter, M., & Bauer, B. U. (2021). Seroprevalence and Risk Factors of Anaplasma spp. in German Small Ruminant Flocks. Animals, 11(10), 2793. https://doi.org/10.3390/ani11102793






