Scrapie at Abattoir: Monitoring, Control, and Differential Diagnosis of Wasting Conditions during Meat Inspection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Scrapie
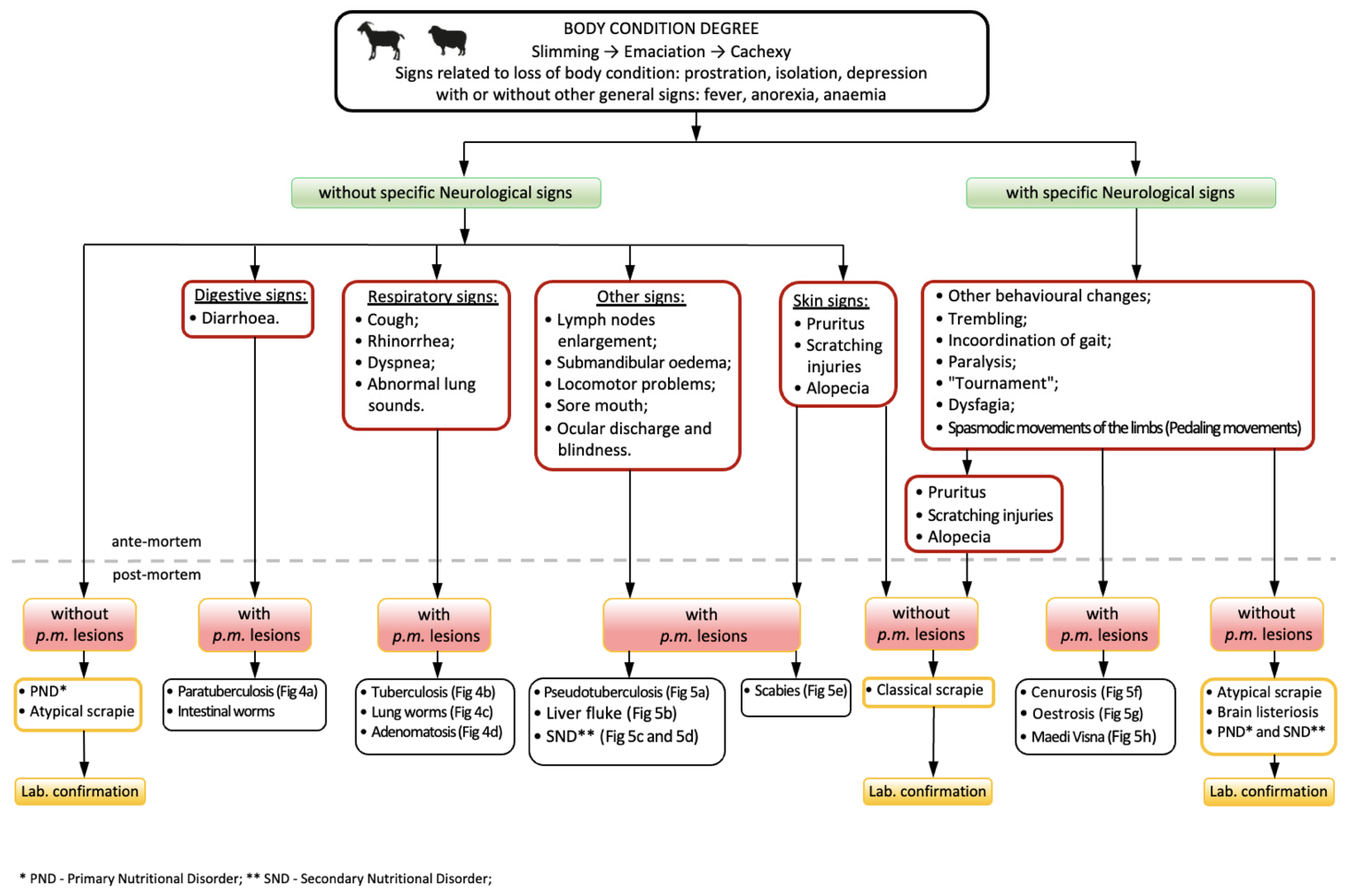
3. Scrapie—Differential Diagnosis and Judgment during Meat Inspection
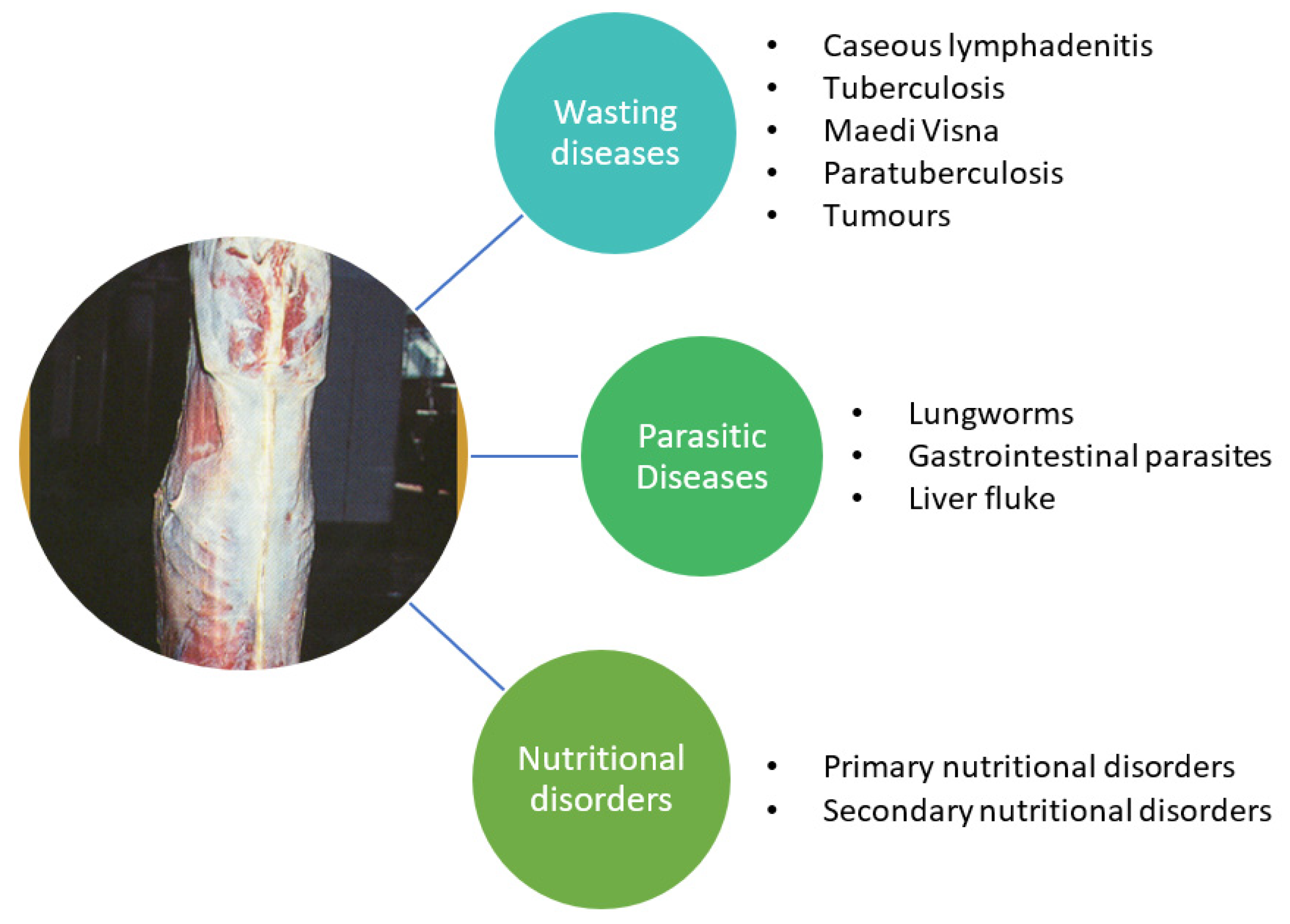
3.1. Other Wasting Diseases
3.2. Parasitic diseases
3.3. Nutritional Disorders
3.4. Laboratory Diagnosis of Scrapie
4. Differential Judgment of Wasting Carcasses during Meat Inspection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Official Journal of European Union. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls (Text with EEA Relevance). 2019, p. 51. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2019:131:FULL (accessed on 15 October 2021).
- Jensen, H.E.; Leifsson, P.S.; Nielsen, O.L.; Agerholm, J.S.; Iburg, T. Meat Inspection: The Pathoanatomic Basis, 1st ed.; Biofolia: Frederiksberg, Denmark, 2017; ISBN 978-87-91319-54-9. [Google Scholar]
- Lindén, J.; Pohjola, L.; Rossow, L.; Tognetti, D. Meat Inspection Lesions. In Meat Inspection and Control in the Slaughterhouse; Ninios, T., Lundén, J., Korkeala, H., Fredriksson-Ahomaa, M., Eds.; John Wiley & Sons Inc.: Chichester, UK, 2014; pp. 163–199. ISBN 978-1-118-52586-9. [Google Scholar]
- Collins, D.S.; Huey, R. Meat Inspection Protocols. In Gracey’s Meat Hygiene; Collins, D.S., Huey, R.J., Gracey, J.F., Eds.; John Wiley & Sons Inc.: Chichester, UK, 2015; pp. 185–222. ISBN 978-1-118-65002-8. [Google Scholar]
- Greenlee, J.J. Review: Update on Classical and Atypical Scrapie in Sheep and Goats. Vet. Pathol. 2019, 56, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Houston, F.; Andréoletti, O. Animal Prion Diseases: The Risks to Human Health: Animal Prion Diseases. Brain Pathol. 2019, 29, 248–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beringue, V.; Andreoletti, O. Classical and Atypical TSE in Small Ruminants. Anim. Front. 2014, 4, 33–43. [Google Scholar] [CrossRef]
- Benestad, S.L.; Arsac, J.-N.; Goldmann, W.; Nöremark, M. Atypical/Nor98 Scrapie: Properties of the Agent, Genetics, and Epidemiology. Vet. Res. 2008, 39, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benestad, S.L.; Sarradin, P.; Thu, B.; Schönheit, J.; Tranulis, M.A.; Bratberg, B. Cases of Scrapie with Unusual Features in Norway and Designation of a New Type, Nor98. Vet. Record. 2003, 153, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.; Kennedy, I.; Goldmann, W.; Green, A.; González, L.; Jeffrey, M.; Hunter, N. Archival Search for Historical Atypical Scrapie in Sheep Reveals Evidence for Mixed Infections. J. Gen. Virol. 2015, 96, 3165–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittelberger, R.; Chaplin, M.J.; Simmons, M.M.; Ramirez-Villaescusa, A.; McIntyre, L.; MacDiarmid, S.C.; Hannah, M.J.; Jenner, J.; Bueno, R.; Bayliss, D.; et al. Atypical Scrapie/Nor98 in a Sheep from New Zealand. J. Vet. Diagn. Investig. 2010, 22, 863–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, R.; Bingham, J.; Besier, A.; Bayley, C.; Hawes, M.; Shearer, P.; Yamada, M.; Bergfeld, J.; Williams, D.; Middleton, D. Atypical Scrapie in Australia. Aust. Vet. J. 2016, 94, 452–455. [Google Scholar] [CrossRef] [PubMed]
- OIE. Scrapie, Chapter 3.8.11. (Version Adopted in May 2016) in Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021. 2016. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 4 August 2021).
- Comoy, E.E.; Mikol, J.; Luccantoni-Freire, S.; Correia, E.; Lescoutra-Etchegaray, N.; Durand, V.; Dehen, C.; Andreoletti, O.; Casalone, C.; Richt, J.A.; et al. Transmission of Scrapie Prions to Primate after an Extended Silent Incubation Period. Sci. Rep. 2015, 5, 11573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassard, H.; Torres, J.-M.; Lacroux, C.; Douet, J.-Y.; Benestad, S.L.; Lantier, F.; Lugan, S.; Lantier, I.; Costes, P.; Aron, N.; et al. Evidence for Zoonotic Potential of Ovine Scrapie Prions. Nat. Commun. 2014, 5, 5821. [Google Scholar] [CrossRef] [PubMed]
- Huor, A.; Espinosa, J.C.; Vidal, E.; Cassard, H.; Douet, J.Y.; Lugan, S.; Aron, N.; Marμn-Moreno, A.; Lorenzo, P.; Aguilar-Calvo, P.; et al. The Emergence of Classical BSE from Atypical/Nor98 Scrapie. Proc. Natl. Acad. Sci. USA 2019, 116, 26853–26862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, S.; Lloyd, S.; Collinge, J. Genetic Factors in Mammalian Prion Diseases. Annu. Rev. Genet. 2019, 53, 117–147. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Calvo, P.; García, C.; Espinosa, J.C.; Andreoletti, O.; Torres, J.M. Prion and prion-like diseases in animals. Virus Res 2015, 207, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Konold, T.; Phelan, L. Clinical Examination Protocol to Detect Atypical and Classical Scrapie in Sheep. J. Vis. Exp. 2014, 83, e51101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, S.J.; Simmons, M.; Chaplin, M.; Spiropoulos, J. Neuroanatomical distribution of abnormal prion protein in naturally occurring atypical scrapie cases in Great Britain. Acta Neuropathol. 2008, 116, 547. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the Scrapie Situation in the EU after 10 Years of Monitoring and Control in Sheep and Goats. EFSA J. 2014, 12, 3781. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No. 999/2001 Laying down Rules for the Prevention, Control and Eradication of Certain Transmissible Spongiform Encephalopathies. Available online: https://www.ecolex.org/details/legislation/regulation-ec-no-9992001-laying-down-rules-for-the-prevention-control-and-eradication-of-certain-transmissible-spongiform-encephalopathies-lex-faoc030438/ (accessed on 6 August 2021).
- EUR-Lex—32016R0429-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2016/429/oj (accessed on 30 August 2021).
- European Food Safety Authority (EFSA). The European Union Summary Report on Surveillance for the Presence of Transmissible Spongiform Encephalopathies (TSE) in 2019. EFS2 2020, 18. [Google Scholar] [CrossRef]
- EUR-Lex—32013R0630-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX%3A32013R0630 (accessed on 6 August 2021).
- Official Journal of European Union. Commission Regulation (EU) 2018/969 of 9 July 2018 Amending Annex V to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Requirements for the Removal of Specified Risk Materials from Small Ruminants (Text with EEA Relevance). 2018, p. 12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0969&from=FR (accessed on 15 October 2021).
- Regulation (EC) No. 1069/2009 of the European Parliament and of the Council Laying down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No. 1774/2002 (Animal by-Products Regulation). Available online: https://www.ecolex.org/details/legislation/regulation-ec-no-10692009-of-the-european-parliament-and-of-the-council-laying-down-health-rules-as-regards-animal-by-products-and-derived-products-not-intended-for-human-consumption-and-repealing-regulation-ec-no-17742002-animal-by-products-regulation-lex-faoc091925/ (accessed on 4 August 2021).
- Al-Gaabary, M.H.; Osman, S.A.; Ahmed, M.S.; Oreiby, A.F. Abattoir Survey on Caseous Lymphadenitis in Sheep and Goats in Tanta, Egypt. Small Rumin. Res. 2010, 94, 117–124. [Google Scholar] [CrossRef]
- Baird, G.J.; Fontaine, M.C. Corynebacterium Pseudotuberculosis and Its Role in Ovine Caseous Lymphadenitis. J. Comp. Pathol. 2007, 137, 179–210. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.; Ferrer, L.M.; Ramos, J.J.; Baselga, C.; Alzuguren, O.; Tejedor, M.T.; de Miguel, R.; Lacasta, D. The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep. Animals 2020, 10, 1962. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Gutiérrez, C. Aspectos Epidemiologicos y Clinicos de La Tuberculosis Caprina. Ovis Tratado Patol. Prod. Ovina 1996, 46, 45–59. [Google Scholar]
- Garcia, M.; Gutiérrez, C. Diagnostico de La Tuberculosis Caprina. Ovis Tratado Patol. Prod. Ovina 1996, 46, 61–77. [Google Scholar]
- Minguijón, E.; Reina, R.; Pérez, M.; Polledo, L.; Villoria, M.; Ramírez, H.; Leginagoikoa, I.; Badiola, J.J.; García-Marín, J.F.; de Andrés, D.; et al. Small Ruminant Lentivirus Infections and Diseases. Vet. Microbiol. 2015, 181, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K. Genetic Diversity of Mycobacterium Avium Subspecies Paratuberculosis and the Influence of Strain Type on Infection and Pathogenesis: A Review. Vet. Res. 2015, 46, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanovic, S.; Zutic, I.; Pavlovic, I.; Zujovic, M. Caseous Lymphadenitis in Goats. Biotechnol. Anim. 2009, 25, 999–1007. [Google Scholar]
- Chapman, C.; Kennedy, M. Caseous Lymphadenitis Management in Goats; All Current Publications, 2017; Available online: https://digitalcommons.usu.edu/extension_curall/1647 (accessed on 15 October 2021).
- Osman, A.Y.; Nordin, M.L.; Kadir, A.A.; Saharee, A.A. The Epidemiology and Pathophysiology of Caseous Lymphadenitis: A Review. J. Vet. Med. Res. 2018, 5, 7. [Google Scholar]
- Yitagesu, E.; Alemnew, E.; Olani, A.; Asfaw, T.; Demis, C. Survival Analysis of Clinical Cases of Caseous Lymphadenitis of Goats in North Shoa, Ethiopia. Vet. Med. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oreiby, A.F. Diagnosis of Caseous Lymphadenitis in Sheep and Goat. Small Rumin. Res. 2015, 123, 160–166. [Google Scholar] [CrossRef]
- Bernabé, A.; Navarro, J.A.; Gómez, S.; Sánchez, J.; Sidrach, J.; Menchen, V.; Vera, A.; Sierra, M.A. Morphopathology of Caprine Tuberculosis. I. Pulmonary Tuberculosis. An. Vet. Murcia 1990, 6–7, 9–20. [Google Scholar] [CrossRef]
- Muñoz Mendoza, M.; de Juan, L.; Menéndez, S.; Ocampo, A.; Mourelo, J.; Sáez, J.L.; Domínguez, L.; Gortázar, C.; García Marín, J.F.; Balseiro, A. Tuberculosis Due to Mycobacterium Bovis and Mycobacterium Caprae in Sheep. Vet. J. 2012, 191, 267–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintas, H.; Reis, J.; Pires, I.; Alegria, N. Tuberculosis in Goats. Vet. Rec. 2010, 166, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Tomás, L.; Ortega, N.; Buendía, A.J.; del Rio, L.; Salinas, J.; Bezos, J.; Caro, M.R.; Navarro, J.A. Microscopical and Immunological Features of Tuberculoid Granulomata and Cavitary Pulmonary Tuberculosis in Naturally Infected Goats. J. Comp. Pathol. 2011, 145, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Plummer, P.; Plummer, K. Diseases of the Respiratory System. In Sheep and Goat Medicine; Pugh, D.G., Baird, A.N., Eds.; Veterinary Medicine; Elsevier/Saunders: Maryland Heights, MO, USA, 2012; pp. 143–149. ISBN 978-1-4377-2353-3. [Google Scholar]
- Buendía, A.J.; Navarro, J.A.; Salinas, J.; McNair, J.; de Juan, L.; Ortega, N.; Cámara, P.; Torreblanca, P.; Sanchez, J. Ante-Mortem Diagnosis of Caprine Tuberculosis in Persistently Infected Herds: Influence of Lesion Type on the Sensitivity of Diagnostic Tests. Res. Vet. Sci. 2013, 95, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Quintas, H.; Alegria, N.; Mendonça, A.; Botelho, A.; Alves, A.; Pires, I. Coexistence of Tuberculosis and Mammary Carcinoma in a Goat. Reprod. Dom. Anim. 2014, 49, 606–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luboya, L.W.; Malangu, M.; Kaleka, M.; Ngulu, N.; Nkokele, B.; Maryabo, K.; Pourrut, X.; Vincent, T.; Gonzalez, J.-P. An Assessment of Caprine Tuberculosis Prevalence in Lubumbashi Slaughterhouse, Democratic Republic of Congo. Trop. Anim. Health Prod. 2017, 49, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Quintas, H.; Pires, I.; Prada, J.; da Conceição Fontes, M.; Coelho, A.C. Diagnosis of Mycobacteriosis in Goats: Tuberculosis and Paratuberculosis. In Sustainable Goat Production in Adverse Environments: Volume I; Simões, J., Gutiérrez, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 247–266. ISBN 978-3-319-71854-5. [Google Scholar]
- Matthews, J.G. Chronic weight loss. In Diseases of the Goat; John Wiley & Sons Inc.: Chichester, UK; Ames, IA, USA, 2016; pp. 115–130. ISBN 978-1-119-07351-2. [Google Scholar]
- Hodgeman, R.; Mann, R.; Savin, K.; Djitro, N.; Rochfort, S.; Rodoni, B. Molecular Characterisation of Mycobacterium Avium Subsp. Paratuberculosis in Australia. BMC Microbiol. 2021, 21, 101. [Google Scholar] [CrossRef]
- Roller, M.; Hansen, S.; Knauf-Witzens, T.; Oelemann, W.M.R.; Czerny, C.-P.; Abd El Wahed, A.; Goethe, R. Mycobacterium Avium Subspecies Paratuberculosis Infection in Zoo Animals: A Review of Susceptibility and Disease Process. Front. Vet. Sci. 2020, 7, 572724. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; Gabric, D.M.; de Lisle, G.W. Identification of Two Groups of Mycobacterium Paratuberculosis Strains by Restriction Endonuclease Analysis and DNA Hybridization. J. Clin. Microbiol. 1990, 28, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, K.; Hughes, V.M.; de Juan, L.; Inglis, N.F.; Wright, F.; Sharp, J.M. Molecular Characterization of Pigmented and Nonpigmented Isolates of Mycobacterium Avium Subsp. Paratuberculosis. J. Clin. Microbiol. 2002, 40, 1798–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OIE. Paratuberculosis, Chapter 3.8.11. In Terrestrial Animal Health Code; OIE: Paris, France; Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/paratuberculosis.pdf (accessed on 4 August 2021).
- Kalogianni, A.I.; Bossis, I.; Ekateriniadou, L.V.; Gelasakis, A.I. Etiology, Epizootiology and Control of Maedi-Visna in Dairy Sheep: A Review. Animals 2020, 10, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayo, E.; Polledo, L.; Balseiro, A.; Martínez, C.P.; García Iglesias, M.J.; Preziuso, S.; Rossi, G.; García Marín, J.F. Inflammatory Lesion Patterns in Target Organs of Visna/Maedi in Sheep and Their Significance in the Pathogenesis and Diagnosis of the Infection. J. Comp. Pathol. 2018, 159, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Caswell, J.L.; Williams, K.J. Respiratory System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; p. 465. ISBN 978-0-7020-5322-1. [Google Scholar]
- Gelberg, H.B. Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity. In Pathologic Basis of Veterinary Disease; Zachary, J.F., Ed.; Elsevier: St. Louis, MO, USA, 2017; p. 324. ISBN 978-0-323-35775-3. [Google Scholar]
- Panayotova-Pencheva, M.S.; Alexandrov, M.T. Some Pathological Features of Lungs from Domestic and Wild Ruminants with Single and Mixed Protostrongylid Infections. Vet. Med. Int. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.E.; Kaplan, R.M.; Pugh, D.G. Internal Parasites. In Sheep and Goat Medicine; Pugh, D.G., Baird, A.N., Eds.; Veterinary Medicine; Elsevier/Saunders: Maryland Heights, MO, USA, 2012; pp. 106–125. ISBN 978-1-4377-2353-3. [Google Scholar]
- López, A.; Martinson, S.A. Respiratory System, Mediastinum, and Pleurae. In Pathologic Basis of Veterinary Disease; Zachary, J.F., Ed.; Elsevier: St. Louis, MO, USA, 2017; p. 471. ISBN 978-0-323-35775-3. [Google Scholar]
- Chartier, C.; Paraud, C. Coccidiosis Due to Eimeria in Sheep and Goats, a Review. Small Rumin. Res. 2012, 103, 84–92. [Google Scholar] [CrossRef]
- Roy, E.A.; Hoste, H.; Beveridge, I. The Effects of Concurrent Experimental Infections of Sheep with Trichostrongylus Colubriformis and T. Vitrinus on Nematode Distributions, Numbers and on Pathological Changes. Parasite 2004, 11, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, T.B.; Gasbarre, L.C. The Veterinary Importance of Nodular Worms (Olesophagostomum spp). Parasitol. Today 1989, 5, 209–213. [Google Scholar] [CrossRef]
- Uzal, F.A.; Plattner, B.L.; Hostetter, J.M. Alimentary System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, p. 1. ISBN 978-0-7020-5322-1. [Google Scholar]
- Khodakaram-Tafti, A.; Hashemnia, M. An Overview of Intestinal Coccidiosis in Sheep and Goats. Rev. Med. Vet. 2017, 167, 9–20. [Google Scholar]
- Rojo-Vázquez, F.A.; Meana, A.; Valcárcel, F.; Martínez-Valladares, M. Update on Trematode Infections in Sheep. Vet. Parasitol. 2012, 189, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Stalker, M.J. Liver and Biliary System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, p. 465. ISBN 978-0-7020-5322-1. [Google Scholar]
- Brown, D.L.; Wettere, A.J.; Cullen, J.M. Hepatobiliary System and Exocrine Pancreas. In Pathologic Basis of Veterinary Disease; Zachary, J.F., Ed.; Elsevier: St. Louis, MO, USA, 2017; p. 412. ISBN 978-0-323-35775-3. [Google Scholar]
- Löhr, C.V. One Hundred Two Tumors in 100 Goats (1987–2011). Vet. Pathol. 2013, 50, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Head, K. Tumours in Sheep. Practice 1990, 12, 68–80. [Google Scholar] [CrossRef]
- Hindson, J.C.; Winter, A.C. Manual of Sheep Diseases, 2nd ed.; Blackwell Science: Oxford, UK, 2002; ISBN 978-0-632-05999-7. [Google Scholar]
- Ortín, A.; De las Heras, M.; Borobia, M.; Ramo, M.A.; Ortega, M.; Ruíz de Arcaute, M. Ovine Pulmonary Adenocarcinoma: A Transmissible Lung Cancer of Sheep, Difficult to Control. Small Rumin. Res. 2019, 176, 37–41. [Google Scholar] [CrossRef]
- OIE Ovine Pulmonary Adecocarcinoma (Adenomatosis), Terrestrial Manual Chapter 3.7.8. 2018. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.07.08_OPA.pdf (accessed on 4 August 2021).
- De Las Heras, M.; González, L.; Sharp, J.M. Pathology of Ovine Pulmonary Adenocarcinoma. In Jaagsiekte Sheep Retrovirus and Lung Cancer; Fan, H., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2003; Volume 275, pp. 25–54. ISBN 978-3-642-62897-9. [Google Scholar]
- Palmarini, M.; Sharp, J.M.; de las Heras, M.; Fan, H. Jaagsiekte Sheep Retrovirus Is Necessary and Sufficient To Induce a Contagious Lung Cancer in Sheep. J. Virol. 1999, 73, 6964–6972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmarini, M.; Mura, M.; Spencer, T.E. Endogenous Betaretroviruses of Sheep: Teaching New Lessons in Retroviral Interference and Adaptation. J. Gen. Virol. 2004, 85, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asín, J.; Ramírez, G.A.; Navarro, M.A.; Nyaoke, A.C.; Henderson, E.E.; Mendonça, F.S.; Molín, J.; Uzal, F.A. Nutritional Wasting Disorders in Sheep. Animals 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.M. Unexplained Weight Loss in Sheep and Goats: A Guide to Differential Diagnosis, Therapy, and Management. Vet. Clin. N. Am. Large Anim. Pract. 1983, 5, 571–590. [Google Scholar] [CrossRef]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 148–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.G.; Young, P.B.; Blanchflower, W.J.; Scott, J.M.; Weir, D.G.; Molloy, A.M.; Kennedy, S. Cobalt-Vitamin B12 Deficiency Causes Lipid Accumulation, Lipid Peroxidation and Decreased Alpha-Tocopherol Concentrations in the Liver of Sheep. Int. J. Vitam. Nutr. Res. 1994, 64, 270–276. [Google Scholar] [PubMed]
- Johnson, E.H.; Muirhead, D.E.; Annamalai, K.; King, G.J.; Al-Busaidy, R.; Hameed, M.S. Hepatic Lipidosis Associated with Cobalt Deficiency in Omani Goats. Vet. Res. Commun. 1999, 23, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; McConnell, S.; Anderson, H.; Kennedy, D.G.; Young, P.B.; Blanchflower, W.J. Histopathologic and Ultrastructural Alterations of White Liver Disease in Sheep Experimentally Depleted of Cobalt. Vet. Pathol. 1997, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex—32001R0999-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32001R0999 (accessed on 5 August 2021).
- EUR-Lex—02001R0999-20201119-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02001R0999-20201119 (accessed on 5 August 2021).
- Codex Alimentarius. Carne y Productos Cárnicos Incluso Los “Bouillons” y Consomés. In Volumen 10, Programa Conjunto FAO/OMS Sobre Normas Alimentarias; Comision Del Codex Alimentarius: Roma, Italy, 1994; pp. 190–191. [Google Scholar]
- Gracey, J.; Collins, D.S.; Motzer, R. Pathology, in Meat Hygiene, 10th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1999. [Google Scholar]



| Classical Scrapie | Atypical Scrapie | |
|---|---|---|
| Epidemiology | Small ruminants between 2–5 years of age Several affected animals per flock Animal movement is a risk factor for the transmission | Small ruminants over 5 years of age Only one or two affected animals per flock Animal movement is not a risk factor |
Role of prnp | ARR/ARR Fully resistant ARR/AHQ, ARR/ARH, ARR/ARQ—Partially resistant AHQ/AHQ, AHQ/ARH, AHQ/ARQ, ARH/ARH, ARH/ARQ, ARQ/ARQ—Average risk of infectionARR/VRQ—High risk of infection AHQ/VRQ, ARH, VRQ, ARQ/VRQ, VRQ/VRQ—Highest risk of infection | Several genotypes including those related to resistance to classical scrapie AHQ/XXX, AFRQ/XXX—Highest risk of infection |
Role of prnp | H154, Q211, K222—Potentially resistant NN146—Highest risk of infection | H154-Highest risk of infection |
| Clinical signs | Behavioral changes (fixed stare, isolation, hyperexcitability, loss of inquisitiveness) Trembling Uncoordinated gait Weight loss or emaciation Pruritus (main symptom in sheep, usually leads to wool loss) Positive scratch response (“nibble reflex”) | Behavioral changes (fixed stare, isolation, hyperexcitability, loss of inquisitiveness) Trembling Incoordination of gait Weight loss or emaciation Visual impairment |
| Spongiform distribution | Medulla oblongata (obex) | Cortices of the cerebellum and the cerebrum |
| PrPsc types and distribution | PrPsc—Three-band profile on western blot between 27 and 19 kDa PrPsc types: Intracellular: intraneuronal Extracellular: stellate, perivascular, subpial, fine granular, aggregates, plaque-like, linear, perineuronal, vascular plaques, subependymal, ependymal Medulla oblongata (obex) (+++) Placenta and peripheral and lymphoid tissues (except ARR/xxx) | PrPsc—Multiple-band profile on western blot between 31 and 11 kDa PrPsc types: Extracellular: fine granular, aggregates, plaque-like, linear, perineuronal, Other: globular (white matter); punctate Medulla oblongata (obex) (+); Cerebellum (+++) No PrPsc in peripheral and lymphoid tissues (but infectivity demonstrated) |
| Caseous Lymphadenitis (CLA) or Pseudotuberculosis | References | |
| Etiology | Corynebacterium pseudotuberculosis | [28,29,30,35,36,37,38,39] |
| Clinical signs (Antemortem) | Swelling of affected superficial lymph node regions (e.g., retropharyngeal, submandibular, precrural, prescapular), emaciation and general deterioration of the animal. | |
| Gross lesions (Post-mortem) | Abscesses developing into pyogranulomatous lesions observed in one or more superficial lymph nodes (retropharyngeal, parotid, submandibular, popliteal, precrural, prescapular, or mammary). The spread of infection may extend to mediastinal and bronchial lymph nodes and multiple organs, such as the spleen, kidneys, and liver or mammary glands. Lesions may present as abscesses with a range of a few millimeters to centimeters in diameter, consisting of a creamy to thick, white-yellowish to pale-green purulent or caseated material surrounded by a thick fibrous wall; in mainly chronic cases, abscesses increase in size and develop a lamellated appearance (“onion ring”). | |
| Tuberculosis | References | |
| Etiology | Mycobacterium caprae, M. bovis, occasionally other mycobacteria from M. tuberculosis Complex | [31,32,40,41,42,43,44,45,46,47,48,49] |
| Clinical signs (Antemortem) | Depending on the affected organs. Respiratory symptoms in the final phase of the disease: deep and productive chronic cough, with tachypnea, dyspnea, and abnormal lung sounds. Emaciation and general deterioration of the animal. | |
| Gross lesions (Post-mortem) | Pulmonary lesions (more frequent): range from pale nodules, with caseification necrosis, mainly in primary complex lesions. Miliary nodules, extensive granulomas, areas of coalescent caseification with softening of the caseous are usually associated with post-primary lesions. Liquefaction leads to the destruction of bronchial walls and cave formation. Lymph nodes can present radial caseification, with necrotic and calcified nodules and fibrosis. Generalized process: hepatic miliary lesions or large nodules, with a fibrous capsule; in serous membranes, the most frequent lesion is granulomatous serositis, the so-called “pearl tuberculosis”. Small granulomas of different sizes are observed, with caseification and calcification; lymphoid tissue and Peyer’s plaques can present thicker and firmer plaques, miliary foci, or caseous ulcers. Abomasum, rumen, reticulum, and omasum rarely present lesions. | |
| Paratuberculosis | References | |
| Etiology | Mycobacterium avium subsp. paratuberculosis, allocated to two major strain types; type S (Sheep type with subtypes I and III) and type C (Cattle type or Type II; including type B: the USA and Indian Bison Type). | [33,50,51,52,53,54] |
| Clinical signs (Antemortem) | Initial signs can be subtle and become gradually more severe, leading to malnutrition, debilitation, and eventually death. Weight loss and exercise intolerance are prominent signs in sheep and goats. Diarrhea is less common compared with cattle, it is more likely to appear intermittently as soft feces. Some have submandibular edema, without other evidence of edema, with wool often damaged and easily shed. Anemia is also reported to be common. | |
| Gross lesions (Post-mortem) | Usually absent or subtle in subclinical carriers, and occasionally absent or minimal in clinical cases. Carcasses of animals with advanced disease are usually thin or emaciated, with loss of adipose tissue with serous atrophy of bone marrow (consistent with cachexia). Mesenteric, ileocolic, and colonic lymph nodes are moderate to severely enlarged and show a bulging cut surface, without evidence of necrosis or calcification. The intestine (distal part of the small intestine) exhibits mild to moderate diffuse thickening of the mucosa. There may be dependent edema and/or fluid in the body cavities. | |
| Maedi-Visna | References | |
| Etiology | Retrovirus from the genus Lentivirus, an RNA virus, present five groups of strains, that included about 21 subtypes. | [33,55,56] |
| Clinical signs (Antemortem) | Slow progressive disease with indications of pneumonia and mastitis are the predominant clinical signs. Respiratory distress, non-painful hard udder (decrease of milk production). Clinical signs of arthritis are progressive with lameness. Neurological signs include ataxia, paresis, incoordination, and, in most severe cases, total paralysis, as a consequence of meningoencephalitis. Emaciation and general deterioration of the animal. | |
| Gross lesions (Post-mortem) | Lungs presenting diffuse pneumonia, with zones firm, discolored and enlarged, with grey spots on the pleural surface. The mediastinal lymph nodes are often enlarged. Non-suppurative interstitial mastitis. Central nervous system with chronic inflammatory lesions. Meningoencephalitis. Arthritis: The most affected joints are the carpal and tarsal, even metatarsal and metacarpal joints. Vertebrae may also be affected. Several multi-organ infections may be affected as the disease progresses. These include liver, kidney, and heart, with lymphoid tissue hyperplasia. | |
| Nasal Carcinomas of Sheep and Goats | References | |
| Etiology | Betaretroviruses in sheep (ENTV-1) and goats (ENTV-2) | [64] |
| Clinical signs (Antemortem) | Variable but include stertor, inspiratory dyspnoea, open-mouth breathing, nasal discharge, nasal deformity, and weight loss. Tumors arise from surface epithelium and glands of the ethmoidal conchae, entire nasal cavity possibly occluded when unilateral there is the deviation of the nasal septum with tumor protruding from the nostril. | |
| Gross lesions (Post-mortem) | Tumour may be unilateral or bilateral occupying the entire affected nasal cavity. The neoplastic tissue is white, firm, and multinodular, or may contain brown-red areas of hemorrhage and necrosis. | |
| Ovine Pulmonary Adenocarcinoma (OPA) | References | |
| Etiology | Betaretrovirus, jaagsiekte sheep retrovirus (JSRV) | [63,65,66] |
| Clinical signs (Antemortem) | Afebrile respiratory illness, dyspnea, and moist respiratory sounds due to the accumulation of fluid in the respiratory airways and progressive weight loss. | |
| Gross lesions (Post-mortem) | Presence of grey or light-purple, firm coalescing nodules on lung, heavier lung. The cut surface of the lesion displays a granular appearance, is moist, and often exudes white frothy fluid as observed in respiratory passages, including the trachea. Intra and extrathoracic metastasis to lymph nodes and other tissues was also observed. In some countries, another form of OPA has been described (atypical OPA), in which non-coalescing, hard-white, and dry nodular lesions are a prominent feature. | |
| Lungworm Infection | References | |
| Etiology | Dictyocaulus filaria, Muellerius capillaris, Protostrongylus rufescens | [64,68,69,70] |
| Clinical signs (Antemortem) | Fever, cough, nasal discharge, moderate dyspnea, diarrhea, anorexia, tachypnea, weight loss, poor body condition, and chronic emaciation. | |
| Gross lesions (Post-mortem) | Presence of worms in bronchi or bronchiolar lumen, areas of atelectasis or consolidation secondary to bronchiolar obstruction, subpleural and pulmonary nodules (granulomas), and periodically in regional lymph nodes. | |
| Gastrointestinal Parasites | References | |
| Etiology | Abomasal parasites: Haemonchus contortus, Ostertagia (Teladorsagia) circumcincta Intestinal parasites: Trichostrongylus colubriformis, T. vitrinus, T. rugatus; Nematodirus spathiger, N. filicollis, N. abnormalis, Cooperia curticei, Bunostomum trigonocephalum, Oesophagostomum columbianum, O. venulosum, Eimeri ovinoidalis, E. weybridgensis/crandallis, E. ninakohlyakimovae, E. arloingi | [44,64,67,71,72,73,74] |
| Clinical signs (Antemortem) | Anemia, hypoproteinemia accompanied by edema most prominently in the intermandibular space (bottle jaw), diarrhea, dehydration, poor body condition, and chronic emaciation. | |
| Gross lesions (Post-mortem) | Pale carcass, generalized edema, hydrothorax, hydropericardium, and ascites, liver pale and friable. Abomasal parasites: Foci of mucosal hemorrhage, presence of nematodes, content fluid, and dark red-brown owing to the presence of blood (Haemonchus contortus); multinodular raised pale areas, often with a depressed center on thickened mucosa (Ostertagia). Intestinal parasites: villous atrophy of the cranial small intestine (Trichostrongylus); subserosal nodules (0.5–1 cm diameter with a caseous or mineralized center) in the large intestine, and occasionally observed in liver, lungs, mesentery and mesenteric lymph nodes, presence of nematodes in the intestine (Oesophagostomosis); proliferative enteritis of the caecum, colon and small intestine associated with the presence of small greyish-white mucosal multifocal lesions (nodules) (1–2 mm diameter) (Eimeria) | |
| Liver Fluke | References | |
| Etiology | Fasciola hepatica, Dicrocoelium dendriticum | [75,76,77] |
| Clinical signs (Antemortem) | Anemia, hypoalbuminemia, emaciation, submandibular edema (bottle jaw) and ascites, progressive loss of condition, and chronic emaciation. | |
| Gross lesions (Post-mortem) | Chronic cholangitis, bile duct obstruction owing to the presence of mature flukes, the liver visceral surface presents white, firm, branching cords resulting from periductular fibrosis and eventual mineralization, periportal and mesenteric lymph nodes markedly enlarged exhibiting brownish color, possible proliferative peritonitis, the appearance of fibrous tags with adhesions or diffuse fibrous thickening. | |
| By-Products Categorization | By-Product | Disposal and Use of Animal By-Products |
|---|---|---|
| C1 | - Carcasses, organs including hides and skins—wasting condition due suspected or confirmed scrapie | - Incineration or co-incineration |
| C2 | - Carcasses and organs—wasting condition not related to TSE diseases - Condemned organs or part of the carcass – presenting gross lesions. | - As C1, or: - Disposed of in an authorized landfill, following processing - Manufacturing of organic fertilizers or soil amendments following processing - Composted or transformed into biogas. |
| C3 | - Carcasses and organs—cachexia senile - Wasting carcass due to primary nutritional deficiencies. | - As C2, or: - Manufacturing of pet food. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, A.; Vieira-Pinto, M.; Quintas, H.; Orge, L.; Gama, A.; Alves, A.; Seixas, F.; Pires, I.; Pinto, M.d.L.; Mendonça, A.P.; et al. Scrapie at Abattoir: Monitoring, Control, and Differential Diagnosis of Wasting Conditions during Meat Inspection. Animals 2021, 11, 3028. https://doi.org/10.3390/ani11113028
Esteves A, Vieira-Pinto M, Quintas H, Orge L, Gama A, Alves A, Seixas F, Pires I, Pinto MdL, Mendonça AP, et al. Scrapie at Abattoir: Monitoring, Control, and Differential Diagnosis of Wasting Conditions during Meat Inspection. Animals. 2021; 11(11):3028. https://doi.org/10.3390/ani11113028
Chicago/Turabian StyleEsteves, Alexandra, Madalena Vieira-Pinto, Hélder Quintas, Leonor Orge, Adelina Gama, Anabela Alves, Fernanda Seixas, Isabel Pires, Maria de Lurdes Pinto, Ana Paula Mendonça, and et al. 2021. "Scrapie at Abattoir: Monitoring, Control, and Differential Diagnosis of Wasting Conditions during Meat Inspection" Animals 11, no. 11: 3028. https://doi.org/10.3390/ani11113028
APA StyleEsteves, A., Vieira-Pinto, M., Quintas, H., Orge, L., Gama, A., Alves, A., Seixas, F., Pires, I., Pinto, M. d. L., Mendonça, A. P., Lima, C., Machado, C. N., Silva, J. C., Tavares, P., Silva, F., Bastos, E., Pereira, J., Gonçalves-Anjo, N., Carvalho, P., ... Pires, M. d. A. (2021). Scrapie at Abattoir: Monitoring, Control, and Differential Diagnosis of Wasting Conditions during Meat Inspection. Animals, 11(11), 3028. https://doi.org/10.3390/ani11113028










