Free Faecal Water: Analysis of Horse Faecal Microbiota and the Impact of Faecal Microbial Transplantation on Symptom Severity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Comparison of Faecal Microbiota of FFW Horses and Healthy Control Horses
2.3. Assessment of the Effect of FMT on Horses Suffering from FFW
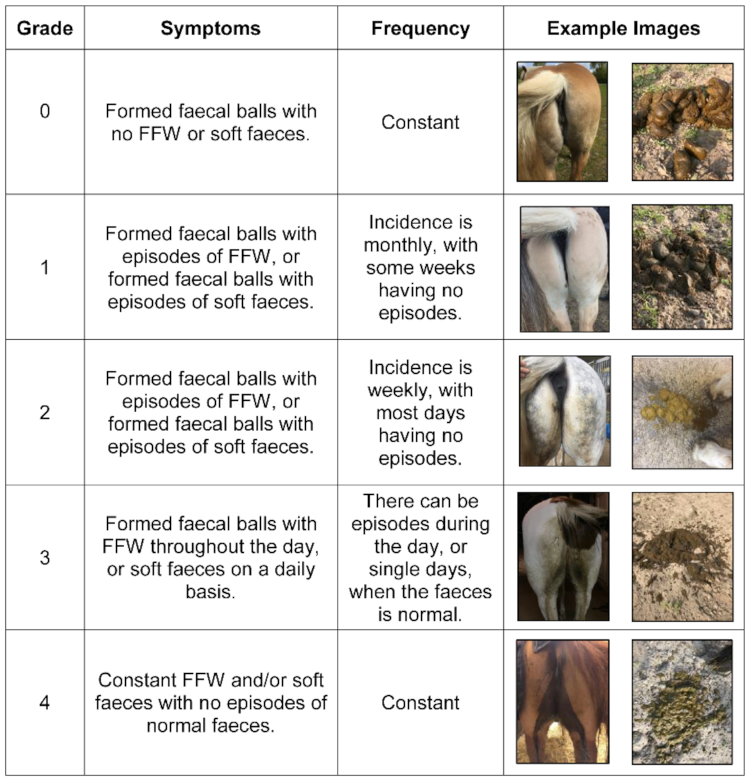
2.4. Data Collection
2.5. DNA Extraction from Faecal Samples and Donor Inocula
2.6. Prokaryotic Community Composition Profiling
2.7. Statistical Analysis
2.7.1. Comparison of Faecal Microbiota of FFW Horses and Healthy Control Horses
2.7.2. Assessment of the Effect of FMT on Horses Suffering from FFW
3. Results
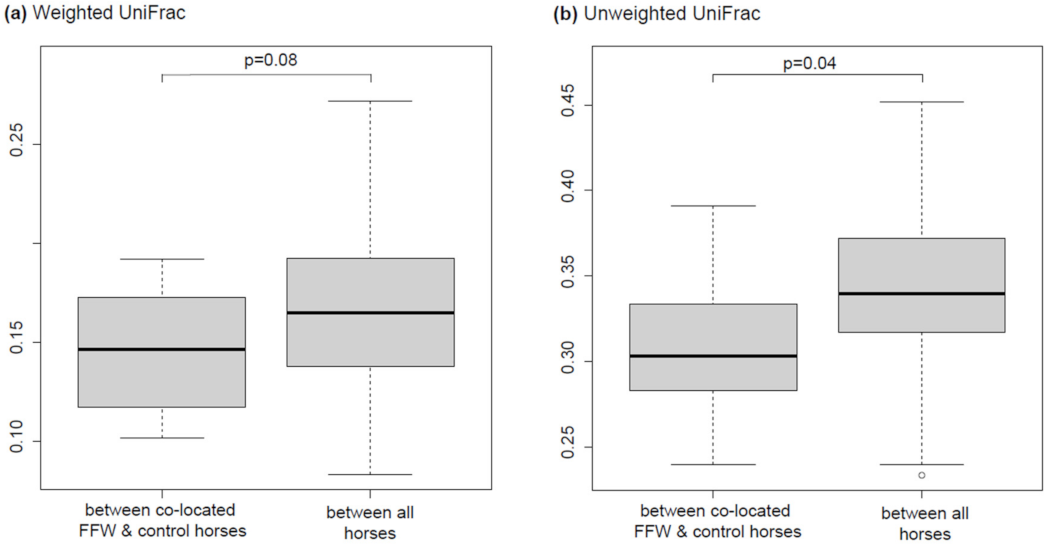
3.1. Comparison of Faecal Microbiota of FFW Horses and Healthy Control Horses
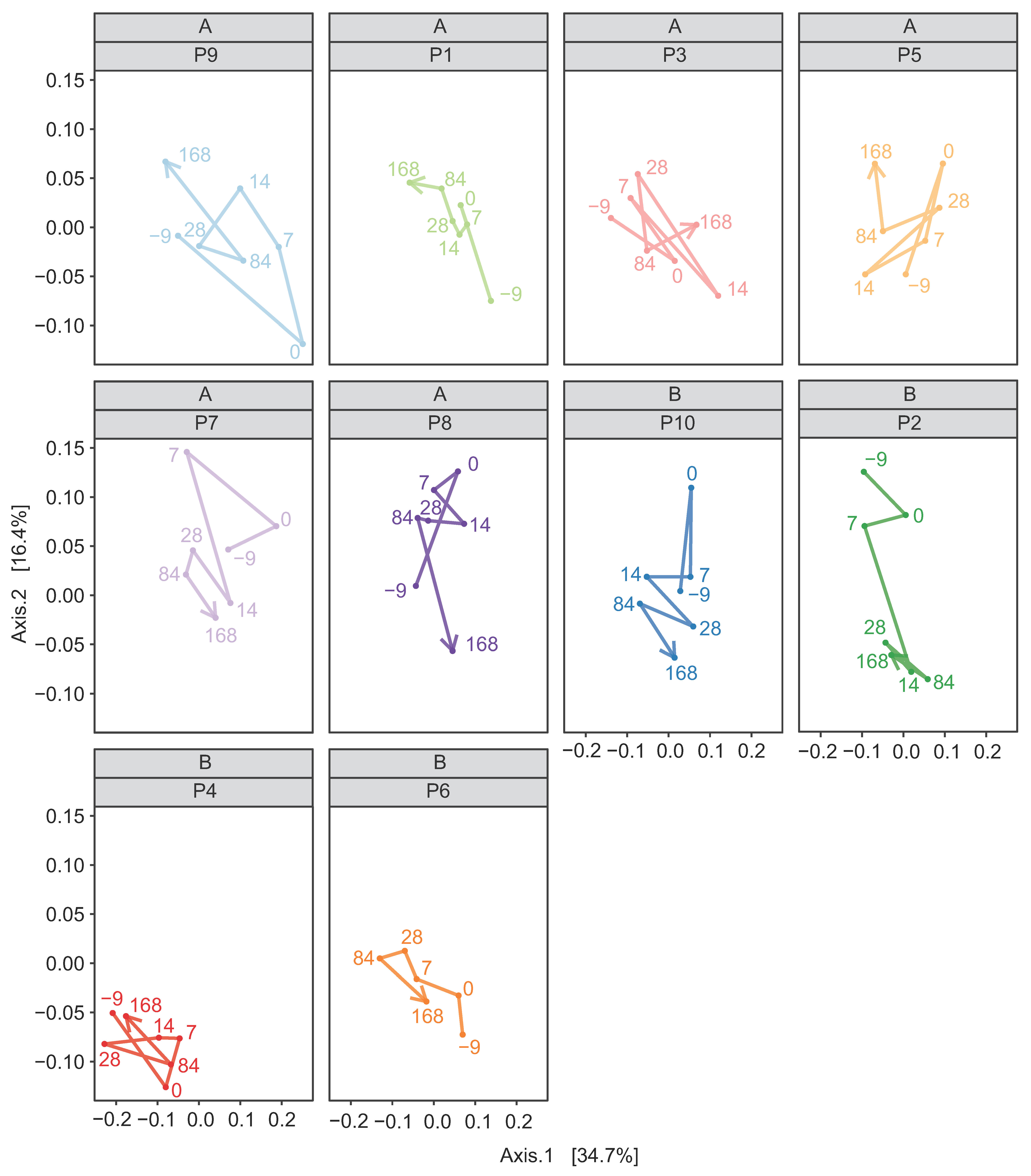
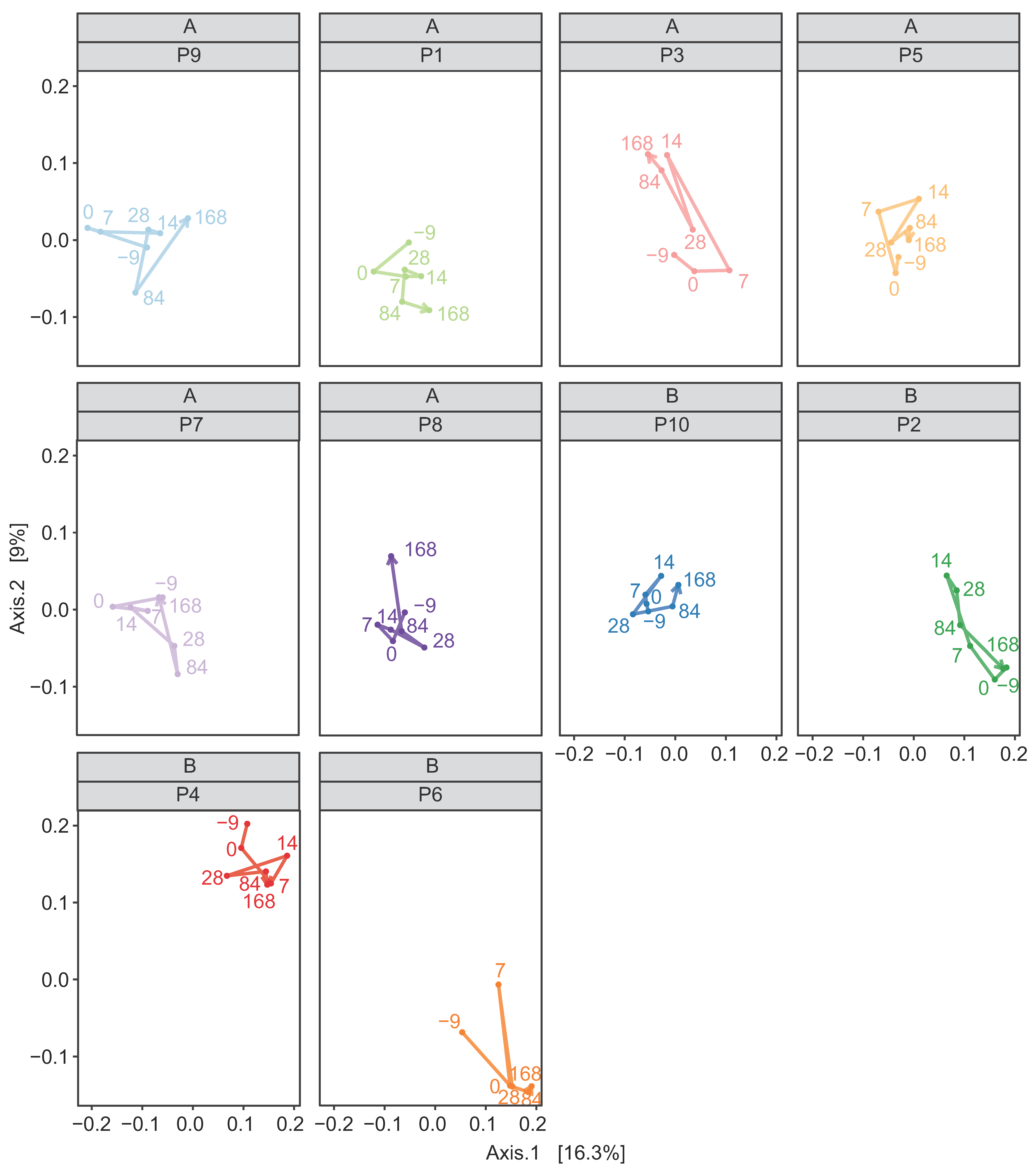
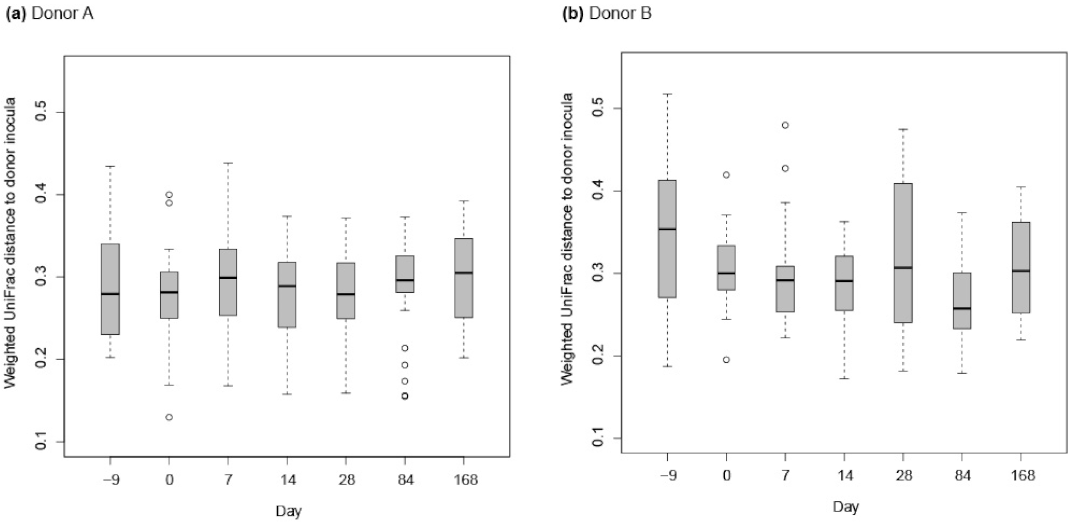
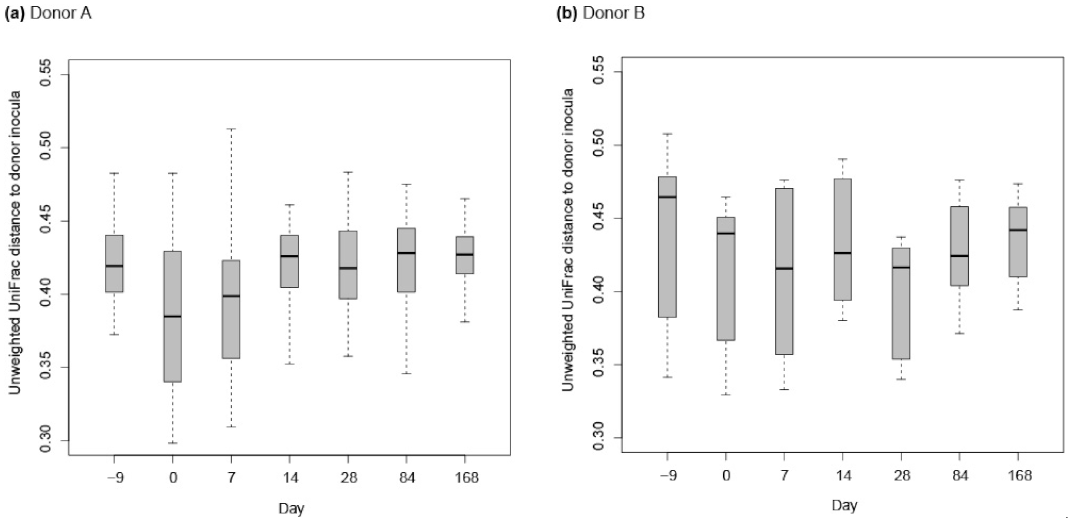
3.2. Assessment of the Effect of FMT on Horses Suffering from FFW
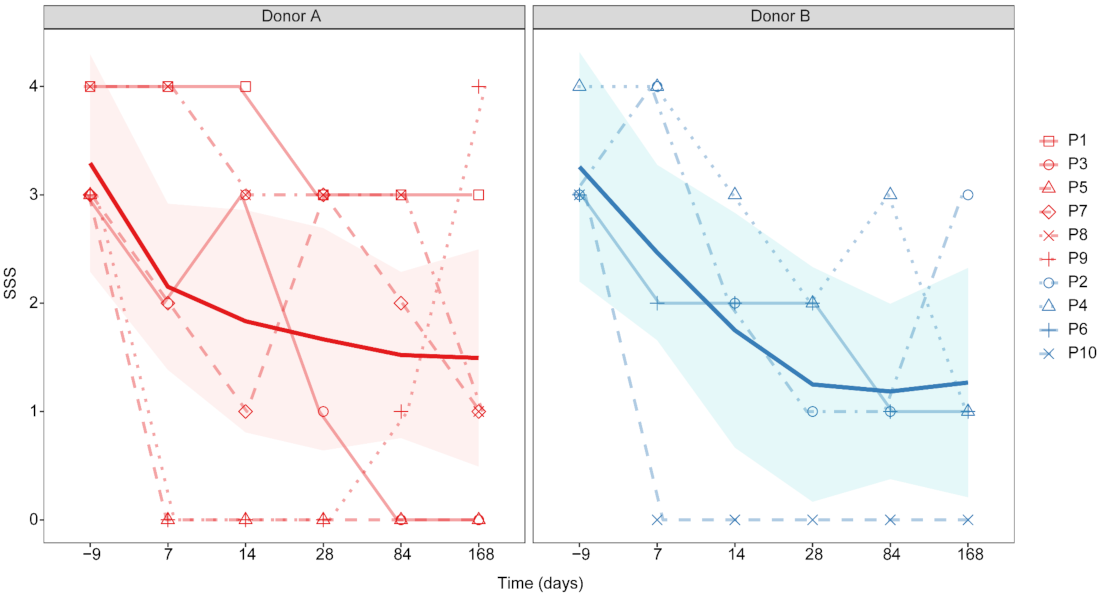
3.2.1. FFW Symptom Severity and BCS
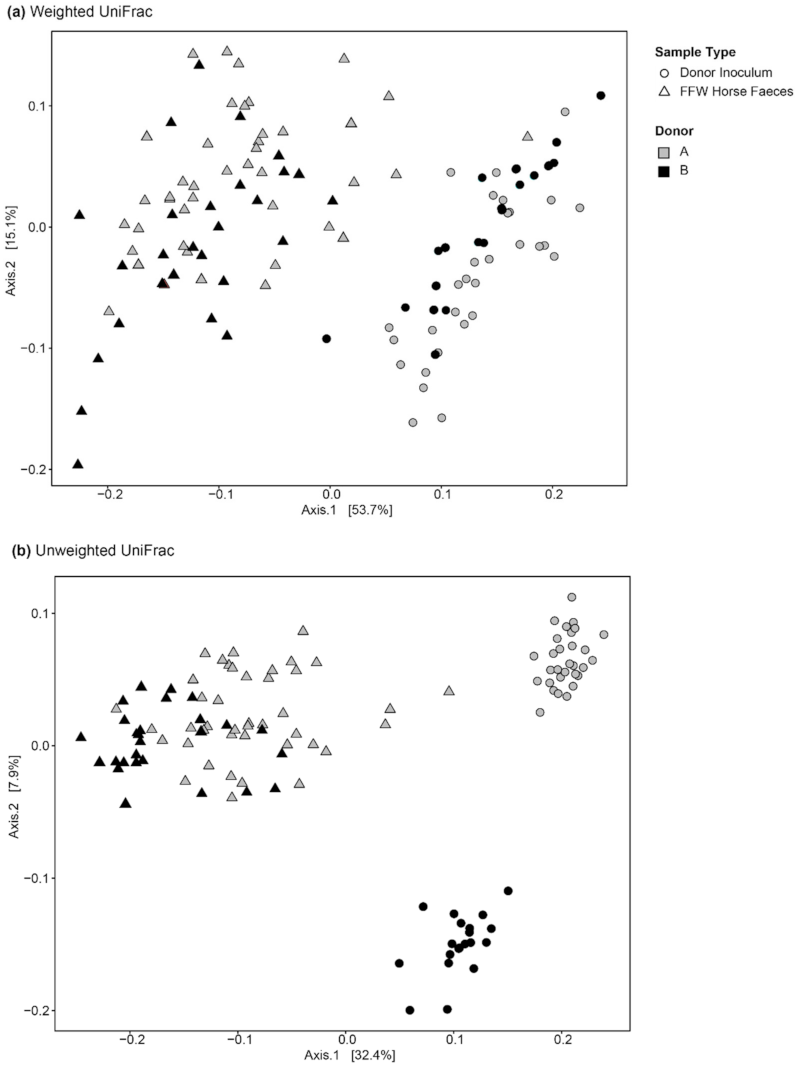
3.2.2. Donor Inocula and FFW Horse Faecal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kienzle, E.; Zehnder, C.; Pfister, K.; Gerhards, H.; Sauter-Louis, C.; Harris, P. Field Study on Risk Factors for Free Fecal Water in Pleasure Horses. J. Equine Vet. Sci. 2016, 44, 32–36. [Google Scholar] [CrossRef]
- Lindroth, K.M.; Johansen, A.; Båverud, V.; Dicksved, J.; Lindberg, J.E.; Müller, C.E. Differential defecation of solid and liquid phases in horses—A descriptive survey. Animals 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertelt, A.; Gehlen, H. Free fecal water in the horse—An unsolved problem. Pferdeheilkunde Equine Med. 2015, 31, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Sanchez-Labraca, N.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Schoster, A.; Weese, J.S.; Gerber, V.; Nicole Graubner, C. Dysbiosis is not present in horses with fecal water syndrome when compared to controls in spring and autumn. J. Vet. Intern. Med. 2020, 34, 1614–1621. [Google Scholar] [CrossRef]
- Costa, M.C.; Weese, J.S. Understanding the Intestinal Microbiome in Health and Disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Julliand, V.; Grimm, P. The Impact of Diet on the Hindgut Microbiome. J. Equine Vet. Sci. 2017, 52, 23–28. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. Horse species symposium: The microbiome of the horse hindgut: History and current knowledge. J. Anim. Sci. 2016, 94, 2262–2274. [Google Scholar] [CrossRef]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3–V5 Region of the 16S rRNA Gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Taminiau, B.; Brévers, B.; Avesani, V.; Van Broeck, J.; Leroux, A.; Gallot, M.; Bruwier, A.; Amory, H.; Delmée, M.; et al. Faecal microbiota characterisation of horses using 16 rDNA barcoded pyrosequencing, and carriage rate of Clostridium difficile at hospital admission. BMC Microbiol. 2015, 15, 181. [Google Scholar] [CrossRef] [Green Version]
- Steelman, S.M.; Chowdhary, B.P.; Dowd, S.; Suchodolski, J.; Janečka, J.E. Pyrosequencing of 16S rRNA genes in fecal samples reveals high diversity of hindgut microflora in horses and potential links to chronic laminitis. BMC Vet. Res. 2012, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Weese, J.S.; Holcombe, S.J.; Embertson, R.M.; Kurtz, K.A.; Roessner, H.A.; Jalali, M.; Wismer, S.E. Changes in the faecal microbiota of mares precede the development of post partum colic. Equine Vet. J. 2015, 47, 641–649. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.A.; Oliveira, B.C.M.; Bedenice, D.; Paradis, M.R.; Mazan, M.; Sage, S.; Sanchez, A.; Widmer, G. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS ONE 2020, 15, e0230148. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, K.M.; Dicksved, J.; Pelve, E.; Båverud, V.; Müller, C.E. Faecal bacterial composition in horses with and without free faecal liquid: A case control study. Sci. Rep. 2021, 11, 4745. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Shetty, S.A.; van den Berg, P.; Burden, F.; van Doorn, D.A.; Pellikaan, W.F.; Dijkstra, J.; Smidt, H. Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub)species. Anim. Microbiome 2020, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.E.; Schennink, A.; Burden, F.; Long, S.; van Doorn, D.A.; Pellikaan, W.F.; Dijkstra, J.; Saccenti, E.; Smidt, H. Domesticated equine species and their derived hybrids differ in their fecal microbiota. Anim. Microbiome 2020, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.C.; Stämpfli, H.R.; Arroyo, L.G.; Allen-Vercoe, E.; Gomes, R.G.; Weese, J. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 2015, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 321, 156–164. [Google Scholar] [CrossRef] [Green Version]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef]
- Holster, S.; Repsilber, D.; Geng, D.; Hyötyläinen, T.; Salonen, A.; Lindqvist, C.M.; Rajan, S.K.; Vos, W.M.; Brummer, R.J.; König, J. Correlations between microbiota and metabolites after faecal microbiota transfer in irritable bowel syndrome. Benef. Microbes 2021, 12, 17–30. [Google Scholar] [CrossRef]
- Korpela, K.; Helve, O.; Kolho, K.L.; Saisto, T.; Skogberg, K.; Dikareva, E.; Stefanovic, V.; Salonen, A.; Andersson, S.; de Vos, W.M. Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 2020, 183, 324–334.e5. [Google Scholar] [CrossRef] [PubMed]
- Brag, S.; Hansen, H.J. Treatment of ruminal indigestion according to popular belief in Sweden. Rev. Sci. Tech. 1994, 13, 529–535. [Google Scholar] [CrossRef] [PubMed]
- DePeters, E.J.; George, L.W. Rumen transfaunation. Immunol. Lett. 2014, 162, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.R.; Yasuda, K.; Divers, T.J.; Weese, J.S. Equine faecal microbiota transplant: Current knowledge, proposed guidelines and future directions. Equine Vet. Educ. 2018, 30, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Weese, J.S. The equine intestinal microbiome. Anim. Health Res. Rev. 2012, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Feary, D.J.; Hassel, D.M. Enteritis and Colitis in Horses. Vet. Clin. N. Am. Equine Pract. 2006, 22, 437–479. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, A.; Benes, V.; Costea, P.I.; Hercog, R.; Hildebrand, F.; Huerta-Cepas, J.; Nieuwdorp, M.; Salojärvi, J.; Voigt, A.Y.; et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016, 352, 586–589. [Google Scholar] [CrossRef]
- Kohnke, J. Feeding and Nutrition of Horses: The Making of a Champion; Birubi Pacific: Pymble, Australia, 1992; p. 197. [Google Scholar]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Ramiro-Garcia, J.; Hermes, G.D.A.; Giatsis, C.; Sipkema, D.; Zoetendal, E.G.; Schaap, P.J.; Smidt, H. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research 2018, 5, 1791. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 4 January 2019).
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan Community Ecology Package. R Package Version 2.5–3. 2018. Available online: https://github.com/vegandevs/vegan (accessed on 4 January 2019).
- Segal, J.P.; Mullish, B.H.; Quraishi, M.N.; Iqbal, T.H. Letter: Faecal microbiota transplantation for IBS. Aliment. Pharmacol. Ther. 2020, 52, 556–557. [Google Scholar] [CrossRef]
- Valle, E.; Gandini, M.; Bergero, D. Management of Chronic Diarrhea in an Adult Horse. J. Equine Vet. Sci. 2013, 33, 130–135. [Google Scholar] [CrossRef]
- Björnhag, G. Comparative aspects of digestion in the hindgut of mammals. The colonic separator mechanism (CSM) (a review). DTW Dtsch. Tierarztl. Wochenschr. 1987, 94, 33–36. [Google Scholar]
- Górka, P.; Kowalski, Z.M.; Zabielski, R.; Guilloteau, P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.P.B.; Navegantes-Lima, K.C.; Oliveira, A.L.; Rodrigues, D.V.; Gaspar, S.L.; Monteiro, V.V.S.; Moura, D.P.; Monteiro, M. Protective Mechanisms of Butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des. 2018, 24, 4154–4166. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Alagawany, M.; Elwan, H.A.M.; Fathi, M.A.; Farag, M.R. Effect of sodium butyrate on intestinal health of poultry—A review. Ann. Anim. Sci. 2020, 20, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Huxford, K.E.; Dart, A.J.; Perkins, N.R.; Bell, R.; Jeffcott, L.B. A pilot study comparing the effect of orally administered esomeprazole and omeprazole on gastric fluid pH in horses. N. Z. Vet. J. 2017, 65, 318–321. [Google Scholar] [CrossRef]
- Raidal, S.L.; Andrews, F.M.; Nielsen, S.G.; Trope, G. Pharmacokinetic and pharmacodynamic effects of two omeprazole formulations on stomach pH and gastric ulcer scores. Equine Vet. J. 2017, 49, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Sykes, B.W.; Hewetson, M.; Hepburn, R.J.; Luthersson, N.; Tamzali, Y. European College of Equine Internal Medicine Consensus Statement-Equine Gastric Ulcer Syndrome in Adult Horses. J. Vet. Intern. Med. 2015, 29, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Kopper, J.J.; Alexander, T.L.; Kogan, C.J.; Berreta, A.R.; Burbick, C.R. In Vitro Evaluation of the Effect of Storage at −20 °C and Proximal Gastrointestinal Conditions on Viability of Equine Fecal Microbiota Transplant. J. Equine Vet. Sci. 2021, 98, 103360. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.H.; Aziz, N.; Ghayur, M.N.; Gilani, A. Pharmacological Basis for the Medicinal Use of Psyllium Husk (Ispaghula) in Constipation and Diarrhea. Dig. Dis. Sci. 2011, 56, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Niinistö, K.E.; Ruohoniemi, M.O.; Freccero, F.; Raekallio, M.R. Investigation of the treatment of sand accumulations in the equine large colon with psyllium and magnesium sulphate. Vet. J. 2018, 238, 22–26. [Google Scholar] [CrossRef]
- Kaikkonen, R.; Niinistö, K.; Lindholm, T.; Raekallio, M. Comparison of psyllium feeding at home and nasogastric intubation of psyllium and magnesium sulfate in the hospital as a treatment for naturally occurring colonic sand (geosediment) accumulations in horses: A retrospective study. Acta Vet. Scand. 2016, 58, 73. [Google Scholar] [CrossRef] [Green Version]
- Henderson, G.; Cox, F.; Kittelmann, S.; Miri, V.H.; Zethof, M.; Noel, S.J.; Waghorn, G.C.; Janssen, P.H. Effect of DNA extraction methods and sampling techniques on the apparent structure of cow and sheep rumen microbial communities. PLoS ONE 2013, 8, e74787. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, J.D.; van den Bogert, B.; Edwards, J.E.; Boekhorst, J.; van Gastelen, S.; Saccenti, E.; Plugge, C.M.; Smidt, H. The effect of DNA extraction methods on observed microbial communities from fibrous and liquid rumen fractions of dairy cows. Front. Microbiol. 2018, 9, 92. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]



| Step | Description |
|---|---|
| Decrease gastric pH |
|
| Preparation of FMT inoculum |
|
| Administration of FMT |
|
| Horse Code | Duration/Onset of FFW Issue | Notes from Initial Evaluation for Study Recruitment | Donor Used | Status at d168 | Follow Up at d336 |
|---|---|---|---|---|---|
| P1 | >1 year | Constant FFW with a lot of faecal water produced and no regular faecal balls. Variation within a day, with sometimes regular faeces. | A | Limited improvement to faecal consistency. Diagnosed with Cushing during the study. | Limited improvement to faecal consistency. Very dependent on feeding management. Reacts to subtle feed changes. |
| P2 | >1 year | Periodic episodes of FFW daily, and able to form regular faecal balls. Summer symptoms less severe. A lot of abdominal gas and flatulence. Strong faecal odor. Cribbing behaviour. | B | Substantial improvement for 3 months, and then a return of symptoms between d84 and d168. | Occasional return of symptoms to same degree as before FMT. Stress induced. |
| P3 | >1 year—onset after purchase and relocation of the horse. | Constant FFW with no regular faecal balls. A lot of abdominal gas and flatulence. Strong faecal odor. Aggressive behavioral pattern. Rejected rider. Skin and hoof problems. | A | Complete resolution of FFW symptoms. Skin and hoof completely normal. | Only one occasion of return of symptoms that was grazing induced. Returned to complete resolution spontaneously. |
| P4 | >2 years—onset after feeding with wet haylage. | Constant FFW with no regular faecal balls. Extended abdomen with a lot of abdominal gas and flatulence. Recurring colic every two weeks. Colic surgery 12 months ago and associated antibiotic treatment afterwards. Very thin. | B | Many days with normal faeces or soft faeces. No colic for 5 months. Less flatulence. Weight gain and improved body condition. | Euthanized due to severe colic. Postmortem examination showed fibrino adhesions among organs in the abdominal cavity. |
| P5 | >1 year | Constant FFW with no regular faecal balls. Less severe in summer. Occasional normal faeces for short periods during the day. Hoof problems. | A | Complete resolution of FFW symptoms. | Only one occasion of return of symptoms, which was grazing induced. Returned to complete resolution spontaneously. |
| P6 | >1 year | Constant FFW with no regular faecal balls. Less severe in summer. Occasional normal faeces for short periods during the day. A lot of abdominal gas and flatulence. Strong faecal odor. Recuring colics. Antibiotic treatment worsened FFW symptoms. | B | FFW symptoms much improved. Only very few days with mild symptoms. No colic was reported after receiving the FMT. | No owner response to d336 questionnaire. |
| P7 | >3 years | Periodic episodes of FFW daily with no regular faecal balls. Worsens in autumn. | A | Symptoms much improved, with only a few days a month with FFW (which appeared to be associated with cold weather). | No owner response to d336 questionnaire. |
| P8 | >1 year | Constant FFW during autumn and winter. No regular faecal balls formed. Less severe in summer when grazing. | A | Symptoms improved only a little after FMT (which was performed in the winter). | Return of symptoms to same degree as before FMT. Induced by feeding of conserved forage (i.e., either hay or haylage). |
| P9 | >6 years—onset at weaning | Constant FFW with no regular faecal balls. Worse while grazing. | A | Complete resolution for three months but return of constant FFW symptoms when grazed. | Return of symptoms to same degree as before FMT. Grazing induced. |
| P10 | >3 years—onset at weaning | Constant FFW with no regular faecal balls. Worse in cold weather. | B | Complete resolution of FFW symptoms. | Occasional return of symptoms (associated with cold & damp weather) to a milder degree compared with before FMT. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laustsen, L.; Edwards, J.E.; Hermes, G.D.A.; Lúthersson, N.; van Doorn, D.A.; Okrathok, S.; Kujawa, T.J.; Smidt, H. Free Faecal Water: Analysis of Horse Faecal Microbiota and the Impact of Faecal Microbial Transplantation on Symptom Severity. Animals 2021, 11, 2776. https://doi.org/10.3390/ani11102776
Laustsen L, Edwards JE, Hermes GDA, Lúthersson N, van Doorn DA, Okrathok S, Kujawa TJ, Smidt H. Free Faecal Water: Analysis of Horse Faecal Microbiota and the Impact of Faecal Microbial Transplantation on Symptom Severity. Animals. 2021; 11(10):2776. https://doi.org/10.3390/ani11102776
Chicago/Turabian StyleLaustsen, Louise, Joan E. Edwards, Gerben D. A. Hermes, Nanna Lúthersson, David A. van Doorn, Supattra Okrathok, Theresa J. Kujawa, and Hauke Smidt. 2021. "Free Faecal Water: Analysis of Horse Faecal Microbiota and the Impact of Faecal Microbial Transplantation on Symptom Severity" Animals 11, no. 10: 2776. https://doi.org/10.3390/ani11102776
APA StyleLaustsen, L., Edwards, J. E., Hermes, G. D. A., Lúthersson, N., van Doorn, D. A., Okrathok, S., Kujawa, T. J., & Smidt, H. (2021). Free Faecal Water: Analysis of Horse Faecal Microbiota and the Impact of Faecal Microbial Transplantation on Symptom Severity. Animals, 11(10), 2776. https://doi.org/10.3390/ani11102776







