Trace Mineral Nutrition of Grazing Beef Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Essential Trace Minerals
2.1. Selenium
2.2. Copper
2.3. Zinc
2.4. Manganese
2.5. Cobalt
2.6. Iodine
3. Methods of Supplementation
3.1. Free-Choice, Salt-Based Supplements
3.2. Fortification of Energy/Protein Supplements
3.3. Trace Mineral Injections
3.4. Biofortification
3.5. Boluses and Drenches
4. Feed Ingredient Sources of Supplemental Trace Minerals
4.1. Inorganic Trace Minerals
4.2. Organic Trace Minerals
4.3. Hydroxychloride Trace Minerals
5. Trace Mineral Antagonists
5.1. Iron Antagonism
5.2. Molybdenum Antagonism
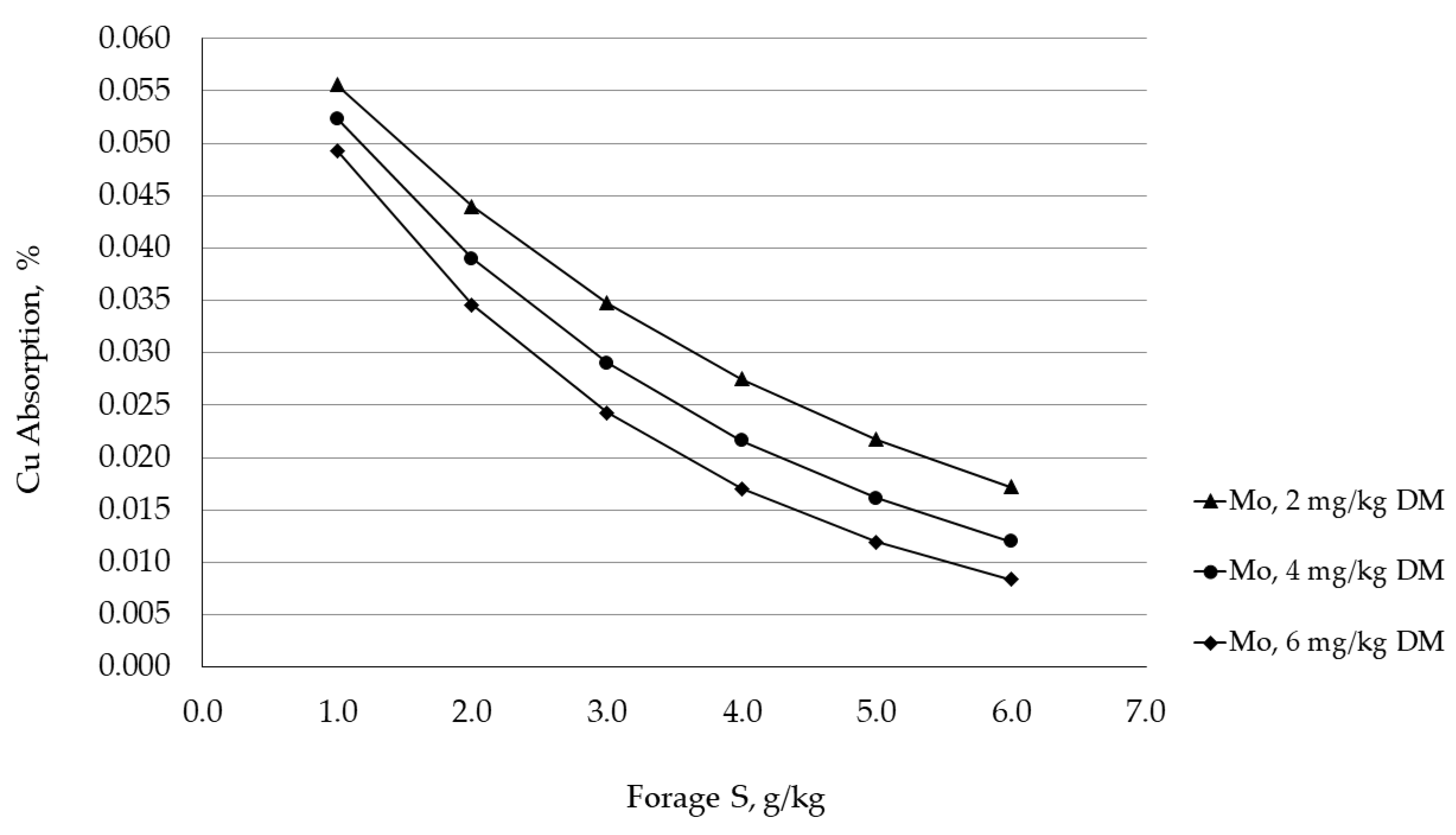
5.3. Sulfur Antagonism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDowell, L.R. Feeding minerals to cattle on pasture. Anim. Feed Sci. Technol. 1996, 60, 247–271. [Google Scholar] [CrossRef]
- Greene, L.W. Designing mineral supplementation of forage programs for beef cattle. J. Anim. Sci. 2000, 77, 1–9. [Google Scholar] [CrossRef]
- McDowell, L.R.; Arthington, J.D. Minerals for Grazing Ruminants in Tropical Regions, 4th ed.; Bulletin; University of Florida, Institute of Food and Agricultural Sciences, Department of Animal Sciences: Gainesville, FL, USA, 2005; pp. 1–86. [Google Scholar]
- Olson, K.C. Management of mineral supplementation programs for cow-calf operations. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 69–90. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Invited Review: Mineral and vitamin nutrition in ruminants. Prof. Anim. Sci. 2014, 30, 180–191. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Beef Cattle, 6th ed.; The National Academies Press: Washington, DC, USA, 1984. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Beef Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 1996; (revised 2020). [Google Scholar]
- USGS. NGS Geochemistry by County. 2008. Available online: https://mrdata.usgs.gov/geochem/doc/averages/countydata.htm (accessed on 17 July 2021).
- Hintze, K.J.; Lardy, G.P.; Marchello, M.J.; Finley, J.W. Selenium accumulation in beef: Effect of dietary selenium and geographical area of animal origin. J. Agric. Food Chem. 2002, 50, 3938–3942. [Google Scholar] [CrossRef]
- Shea, D.A.; Schierow, L.J.; Szymendera, S.D. Regulations of Fertilizers: Ammonium Nitrate and Anhydrous Ammonia. Congressional Research Service 7-5700. R43070. 2013. Available online: https://fas.org/sgp/crs/homesec/R43070.pdf (accessed on 17 July 2021).
- Arthington, J.D.; Rechcigl, J.E.; Yost, G.P.; McDowell, L.R.; Fanning, M.D. Effect of ammonium sulfate fertilization on bahiagrass quality and copper metabolism in grazing beef cattle. J. Anim. Sci. 2002, 80, 2507–2512. [Google Scholar] [CrossRef] [PubMed]
- Arthington, J.D.; Pate, F.M.; Spears, J.W. Effect of copper source and level on performance and copper status of cattle consuming molasses-based supplements. J. Anim. Sci. 2003, 81, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Arthington, J.D.; Corah, L.R.; Blecha, F. The effect of molybdenum-induced copper deficiency on acute-phase protein concentrations, superoxide dismutase activity, leukocyte numbers, and lymphocyte proliferation in beef heifers inoculated with bovine herpesvirus-1. J. Anim. Sci. 1996, 74, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, P.M.H.; Godson, D.L.; Toussaint, M.J.M.; Tjørnehøj, K.; Larsen, L.E.; Viuff, B.; Rønsholt, L. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 2000, 77, 151–159. [Google Scholar] [CrossRef]
- Stokka, G.L.; Edwards, A.J.; Spire, M.F.; Brandt, R.T.; Smith, J.E. Inflammatory response to clostridial vaccines in feedlot cattle. J. Am. Vet. Med. Assoc. 1994, 204, 415–419. [Google Scholar]
- Arthington, J.D.; Cooke, R.F.; Maddock, T.D.; Araujo, D.B.; Moriel, P.; Dilorenzo, N. Effects of vaccination on the acute-phase protein response and measures of performance in growing beef calves. J. Anim. Sci. 2013, 91, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Phillipo, M.; Humphries, W.R.; Atkinson, T.; Henderson, G.D.; Garthwaite, P.H. The effect of dietary molybdenum and iron on copper status, puberty, fertility and oestrus cycles in cattle. J. Agric. Sci. 1987, 109, 321–336. [Google Scholar] [CrossRef]
- Mason, J. Thiomolybdates: Mediators of molybdenum toxicity and enzyme inhibitors. Toxicology 1986, 42, 99–109. [Google Scholar] [CrossRef]
- Kendall, N.R.; Marsters, P.; Guo, L.; Scaramuzzi, R.J.; Campbell, B.K. Effect of copper and thiomolybdates on bovine theca cell differentiation In Vitro. J. Endocrinol. 2006, 189, 455–463. [Google Scholar] [CrossRef]
- Kendal, N.R.; Marsters, P.; Scaramuzzi, R.J.; Campbell, B.K. Expression of lysyl oxidase and effect of copper chloride and ammonium tetrathiomolybdate on bovine ovarian follicle granulosa cells cultured in serum-free media. Reproduction 2003, 125, 657–665. [Google Scholar] [CrossRef]
- Gould, L.; Kendall, N.R. Role of the rumen in copper and thiomolybdate absorption. Nutr. Res. Rev. 2011, 24, 176–182. [Google Scholar] [CrossRef]
- Clarkson, A.H.; Paine, S.; Martín-Tereso, J.; Kendall, N.R. Copper physiology in ruminants: Trafficking of systemic copper, adaptions to variation in nutritional supply and thiomolybdate challenge. Nutr. Res. Rev. 2020, 53, 1689–1699. [Google Scholar] [CrossRef]
- Spears, J.W. Micronutrients and immune function in cattle. Proc. Nutr. Soc. 2000, 59, 587–594. [Google Scholar] [CrossRef]
- Arthington, J.D.; Corah, L.R.; Blecha, F.; Hill, D.A. Effect of copper depletion and repletion on lymphocyte blastogenesis and neutrophil bactericidal function in beef heifers. J. Anim. Sci. 1995, 73, 2079–2085. [Google Scholar] [CrossRef]
- Arthington, J.D.; Spell, A.R.; Corah, L.R.; Blecha, F. Effect of molybdenum-induced copper deficiency on In Vivo and In Vitro measures of neutrophil chemotaxis both before and following an inflammatory stressor. J. Anim. Sci. 1996, 74, 2759–2764. [Google Scholar] [CrossRef]
- Mortimer, R.G.; Dargatz, D.; Corah, L.R. Forage Analyses from Cow/Calf Herds in 23 States; Report NAHMS Beef; U.S. Deptartment of Agriculture: Washington, DC, USA, 1999. Available online: https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef97/Beef97_dr_ForageAnal.pdf (accessed on 17 July 2021).
- Langova, L.; Novotna, I.; Nemcova, P.; Machacek, M.; Havlicek, Z.; Zemanova, M.; Chrast, V. Impact of nutrients on the hoof health in cattle. Animals 2020, 10, 1824. [Google Scholar] [CrossRef]
- Arthington, J.D.; Corah, L.R.; Hill, D.A. The effects of dietary zinc concentration and source on yearling bull growth and fertility. Prof. Anim. Sci. 2002, 18, 282–285. [Google Scholar] [CrossRef]
- Duff, G.C.; Galyean, M.L. Board-Invited Review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 2007, 85, 823–840. [Google Scholar] [CrossRef]
- Messersmith, E.M.; Smerchek, D.T.; Hansen, S.L. The crossroads between zinc and steroidal implant-induced growth of beef cattle. Animals 2021, 11, 1914. [Google Scholar] [CrossRef]
- McDowell, L. Mineral Nutrition History: The Early Years, 1st ed.; First Edition Design Publishing: Sarasota, FL, USA, 2017; pp. 1–722. [Google Scholar]
- Hansen, S.L.; Spears, J.W.; Lloyd, K.E.; Whisnant, C.S. Feeding a low manganese diet to heifers during gestation impairs fetal growth and development. J. Dairy Sci. 2006, 89, 4305–4311. [Google Scholar] [CrossRef]
- Hidiroglou, M. Zinc, copper, and manganese deficiencies and the ruminant skeleton: A review. Can. J. Anim. Sci. 1980, 60, 579–590. [Google Scholar] [CrossRef]
- Valero, G.; Alley, M.R.; Badcoe, L.M.; Manktelow, B.W.; Merrall, M. Chondrodystrophy in calves associated with manganese deficiency Chondrodystrophy in calves associated with manganese deficiency. N. Z. Vet. J. 1990, 38, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W. Boron, chromium, manganese, and nickel in agricultural animal production. Biol. Trace Elem. Res. 2019, 188, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Stangl, G.I.; Schwarz, F.J.; Muller, H.; Kirchgessner, M. Evaluation of the cobalt requirement of beef cattle based on vitamin B12, folate, homocysteine and methylmalonic acid. Br. J. Nutr. 2000, 84, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, M.E.; Fellner, V.; Spears, J.W. Influence of cobalt concentration on vitamin B12 production and fermentation of mixed ruminal microorganisms grown in continuous culture flow-through fermentors. J. Anim. Sci. 2006, 84, 635–640. [Google Scholar] [CrossRef]
- Stangl, G.I.; Schwarz, F.J.; Kirchgessner, M. Moderate long-term cobalt-deficiency affects liver, brain and erythrocyte lipids and lipoproteins of cattle. Nutr. Res. 1999, 19, 415–427. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 1–660. [Google Scholar]
- National Research Council. Mineral Tolerance of Animals, 2nd ed.; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Arthington, J.D.; Swenson, C.K. Effects of trace mineral source and feeding method on the productivity of grazing braford cows. Prof. Anim. Sci. 2004, 20, 155–161. [Google Scholar] [CrossRef]
- Cockwill, C.L.; McAllister, T.A.; Olson, M.E.; Milligan, D.N.; Ralston, B.J.; Huisma, C.; Hand, R.K. Individual intake of mineral and molasses supplements by cows, heifers and calves. Can. J. Anim. Sci. 2000, 80, 681–690. [Google Scholar] [CrossRef]
- Manzano, R.P.; Paterson, J.; Harbac, M.M.; Lima Filho, R.O. The effect of season on supplemental mineral intake and behavior by grazing steers. Prof. Anim. Sci. 2012, 28, 73–81. [Google Scholar] [CrossRef]
- Ranches, J.; De Oliveira, R.A.; Vedovatto, M.; Palmer, E.A.; Moriel, P.; Arthington, J.D. Use of radio-frequency identification technology to assess the frequency of cattle visits to mineral feeders. Trop. Anim. Health Prod. 2021, 53, 341. [Google Scholar] [CrossRef]
- Braghieri, A.; Pacelli, C.; Girolami, A.; Napolitano, F. Time budget, social and ingestive behaviours expressed by native beef cows in Mediterranean conditions. Livest. Sci. 2011, 141, 47–52. [Google Scholar] [CrossRef]
- Hammond, A.C.; Olson, T.A.; Chase, C.C.; Bowers, E.J.; Randel, R.D.; Murphy, C.N.; Vogt, D.W.; Tewolde, A. Heat tolerance in two tropically adapted bos taurus breeds, senepol and romosinuano, compared with brahman, angus, and hereford cattle in Florida. J. Anim. Sci. 1996, 74, 295–303. [Google Scholar] [CrossRef]
- Mccarthy, K.L.; Undi, M.; Becker, S.; Dahlen, C.R. Utilizing an electronic feeder to measure individual mineral intake, feeding behavior, and growth performance of cow-calf pairs grazing native range. Transl. Anim. Sci. 2021, 5, txab007. [Google Scholar] [CrossRef]
- Coppock, C.E.; Everett, R.W.; Merrill, W.G. Effect of ration on free choice consumption of calcium-phosphorus supplements by dairy cattle. J. Dairy Sci. 1972, 55, 245–256. [Google Scholar] [CrossRef]
- Muller, L.D.; Schaffer, L.V.; Ham, L.C.; Owens, M.J. Cafeteria style free-choice mineral feeder for lactating dairy cows. J. Dairy Sci. 1977, 60, 1574–1582. [Google Scholar] [CrossRef]
- Provenza, F.D. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. Rangel. Ecol. Manag. J. Range Manag. Arch. 1995, 48, 2–17. [Google Scholar] [CrossRef]
- Moriel, P.; Artioli, L.F.A.; Piccolo, M.B.; Miranda, M.; Ranches, J.; Ferreira, V.S.M.; Antunes, L.Q.; Bega, A.M.; Miranda, V.F.B.; Vieira, J.F.R.L.; et al. Effects of low-moisture, sugarcane molasses-based block supplementation on growth, physiological parameters, and liver trace mineral status of growing beef heifers fed low-quality, warm-season forage. Transl. Anim. Sci. 2019, 3, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Aubel, N.A.; Jaeger, J.R.; Drouillard, J.S.; Schlegel, M.D.; Pacheco, L.A.; Linden, D.R.; Bolte, J.W.; Higgins, J.J.; Olson, K.C. Effects of mineral-supplement delivery system on frequency, duration, and timing of supplement use by beef cows grazing topographically rugged, native rangeland in the Kansas Flint Hills. J. Anim. Sci. 2011, 89, 3699–3706. [Google Scholar] [CrossRef][Green Version]
- Bailey, D.W.; Welling, G.R. Evaluation of low-moisture blocks and conventional dry mixes for supplementing minerals and modifying cattle grazing patterns. Rangel. Ecol. Manag. 2007, 60, 54–64. [Google Scholar] [CrossRef]
- Dixon, R.M.; Smith, D.R.; Porch, I.; Petherick, J.C. Effects of experience on voluntary intake of supplements by cattle R. Aust. J. Exp. Agric. 2001, 40, 681–696. [Google Scholar] [CrossRef]
- Bowman, J.G.P.; Sowell, B.F. Delivery method and supplement consumption by grazing ruminants: A review. J. Anim. Sci. 1997, 75, 543–550. [Google Scholar] [CrossRef]
- Bohman, V.R.; Drake, E.L.; Behrens, W.C. Injectable copper and tissue composition of cattle. J. Dairy Sci. 1984, 67, 1468–1473. [Google Scholar] [CrossRef]
- Boila, R.J.; Devlin, T.J.; Drysdale, R.A.; Lillie, L.E. Injectable Cu complexes as supplementary Cu for grazing cattle. Can. J. Anim. Sci. 1984, 64, 365–378. [Google Scholar] [CrossRef]
- Pogge, D.J.; Richter, E.L.; Drewnoski, M.E.; Hansen, S.L. Mineral concentrations of plasma and liver after injection with a trace mineral complex differ among angus and simmental cattle. J. Anim. Sci. 2012, 90, 2692–2698. [Google Scholar] [CrossRef]
- Genther, O.N.; Hansen, S.L. A multielement trace mineral injection improves liver copper and selenium concentrations and manganese superoxide dismutase activity in beef steers. J. Anim. Sci. 2014, 92, 695–704. [Google Scholar] [CrossRef]
- Arthington, J.D.; Havenga, L.J. Effect of injectable trace minerals on the humoral immune response to multivalent vaccine administration in beef calves. J. Anim. Sci. 2012, 90, 1966–1971. [Google Scholar] [CrossRef]
- Palomares, R.A.; Hurley, D.J.; Bittar, J.H.J.; Saliki, J.T.; Woolums, A.R.; Moliere, F.; Havenga, L.J.; Norton, N.A.; Clifton, S.J.; Sigmund, A.B.; et al. Effects of injectable trace minerals on humoral and cell-mediated immune responses to Bovine viral diarrhea virus, Bovine herpes virus 1 and Bovine respiratory syncytial virus following administration of a modified-live virus vaccine in dairy calves. Vet. Immunol. Immunopathol. 2016, 178, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.L.; May, N.D.; Brauer, C.L.; Gentry, W.W.; Weiss, C.P.; Jennings, J.S.; Richeson, J.T. Effect of injectable trace mineral administration on health, performance, and vaccine response of newly received feedlot cattle. Prof. Anim. Sci. 2016, 32, 842–848. [Google Scholar] [CrossRef]
- Bittar, J.H.J.; Palomares, R.A.; Hurley, D.J.; Hoyos-Jaramillo, A.; Rodriguez, A.; Stoskute, A.; Hamrick, B.; Norton, N.; Adkins, M.; Saliki, J.T.; et al. Immune response and onset of protection from Bovine viral diarrhea virus 2 infection induced by modified-live virus vaccination concurrent with injectable trace minerals administration in newly received beef calves. Vet. Immunol. Immunopathol. 2020, 225, 110055. [Google Scholar] [CrossRef]
- Richeson, J.T.; Kegley, E.B. Effect of supplemental trace minerals from injection on health and performance of highly stressed, newly received beef heifers. Prof. Anim. Sci. 2011, 27, 461–466. [Google Scholar] [CrossRef]
- Mundell, L.R.; Jaeger, J.R.; Waggoner, J.W.; Stevenson, J.S.; Grieger, D.M.; Pacheco, L.A.; Bolte, J.W.; Aubel, N.A.; Eckerle, G.J.; Macek, M.J.; et al. Effects of prepartum and postpartum bolus injections of trace minerals on performance of beef cows and calves grazing native range. Prof. Anim. Sci. 2012, 28, 82–88. [Google Scholar] [CrossRef]
- Stokes, R.S.; Volk, M.J.; Ireland, F.A.; Gunn, P.J.; Shike, D.W. Effect of repeated trace mineral injections on beef heifer development and reproductive performance. J. Anim. Sci. 2018, 96, 3943–3954. [Google Scholar] [CrossRef]
- Springman, S.A.; Maddux, J.G.; Drewnoski, M.E.; Funston, R.N. Effects of injectable trace minerals on reproductive performance of beef heifers in adequate trace mineral status. Prof. Anim. Sci. 2018, 34, 649–652. [Google Scholar] [CrossRef]
- Preedy, G.W.; Hill, S.L.; Stevenson, J.S.; Weaber, R.L.; Olson, K.C. Injectable trace-mineral supplementation improves sperm motility and morphology of young beef bulls. Prof. Anim. Sci. 2017, 34, 1–9. [Google Scholar] [CrossRef]
- Puls, R. Mineral Levels in Animal Health: Diagnostic Data, 2nd ed.; Sherpa International: Quebec City, QC, Canada, 1994; pp. 1–356. [Google Scholar]
- Mäkelä, A.L.; Nänto, V.; Mäkela, P.; Wang, W. The effect of nationwide selenium enrichment of fertilizers on selenium status of healthy Finnish medical students living in the southwestern Finland. Biol. Trace Elem. Res. 1993, 36, 151–157. [Google Scholar] [CrossRef]
- Ranches, J.; Vendramini, J.M.B.; Arthington, J.D. Effects of selenium biofortification of hayfields on measures of selenium status in cows and calves consuming these forages. J. Anim. Sci. 2017, 95, 120–128. [Google Scholar] [CrossRef]
- Valle, G.; McDowell, L.R.; Wilkinson, N.S.; Wright, D. Selenium concentration of bermudagrass after spraying with sodium selenate 1. Commun. Soil Sci. Plant Anal. 1993, 24, 1763–1768. [Google Scholar] [CrossRef]
- Filley, S.J.; Peters, A.; Bouska, C.; Pirelli, G.; Oldfield, J. Selenium fertilization of pastures for improved forage selenium content. Prof. Anim. Sci. 2007, 23, 144–147. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Hunter, J.K.; Vorachek, W.R.; Stewart, W.C.; Vanegas, J.A.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J. Effect of feeding selenium-fertilized alfalfa hay on performance of weaned beef calves. PLoS ONE 2013, 8, e58188. [Google Scholar] [CrossRef]
- Hall, J.A.; Harwell, A.M.; Van Saun, R.J.; Vorachek, W.R.; Stewart, W.C.; Galbraith, M.L.; Hooper, K.J.; Hunter, J.K.; Mosher, W.D.; Pirelli, G.J. Agronomic biofortification with selenium: Effects on whole blood selenium and humoral immunity in beef cattle. Anim. Feed Sci. Technol. 2011, 164, 184–190. [Google Scholar] [CrossRef]
- Wallace, L.G.; Bobe, G.; Vorachek, W.R.; Dolan, B.P.; Estill, C.T.; Pirelli, G.J.; Hall, J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn calves. J. Anim. Sci. 2017, 95, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Isaiah, A.; Bobe, G.; Estill, C.T.; Bishop-Stewart, J.K.; Davis, T.Z.; Suchodolski, J.S.; Pirelli, G.J. Feeding selenium-biofortified alfalfa hay during the preconditioning period improves growth, carcass weight, and nasal microbial diversity of beef calves. PLoS ONE 2020, 15, e0242771. [Google Scholar] [CrossRef]
- Arthington, J.D.; Larson, R.L.; Corah, L.R. The effects of slow-release copper boluses on cow reproductive performance and calf growth. Prof. Anim. Sci. 1995, 11, 219–222. [Google Scholar] [CrossRef]
- Arthington, J.D. Effects of copper oxide bolus administration or high-level copper supplementation on forage utilization and copper status in beef cattle. J. Anim. Sci. 2005, 83, 2894–2900. [Google Scholar] [CrossRef]
- Sprinkle, J.E.; Schafer, D.W.; Peder Cuneo, S.; Tolleson, D.R.; Mark Enns, R. Effects of a long-acting trace mineral rumen bolus upon range cow productivity. Transl. Anim. Sci. 2021, 5, txaa232. [Google Scholar] [CrossRef]
- Jackson, T.D.; Carmichael, R.N.; Deters, E.L.; Messersmith, E.M.; VanValin, K.R.; Loy, D.D.; Hansen, S.L. Comparison of multiple single-use, pulse-dose trace mineral products provided as injectable, oral drench, oral paste, or bolus on circulating and liver trace mineral concentrations of beef steers. Appl. Anim. Sci. 2020, 36, 26–35. [Google Scholar] [CrossRef]
- AAFCO. Official Publication of the Association of American Feed Control Officials Incorporated; Bachman, P.M., Ed.; Association of American Feed Control Officials: Oxford, IN, USA, 1998; pp. 237–238. [Google Scholar]
- Gayathri, S.L.; Panda, N. Chelated minerals and its effect on animal production: A review. Agric. Rev. 2018, 39, 314–320. [Google Scholar] [CrossRef]
- Spears, J.W. Comparative trace element nutrition trace mineral bioavailability in ruminants. J. Nutr. 2003, 133, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Kabaija, E.; Smith, O.B. Trace element kinetics in the digestive tract of sheep fed diets with graded levels of dietary fibre. J. Anim. Physiol. Anim. Nutr. 1988, 59, 218–224. [Google Scholar] [CrossRef]
- Marques, R.S.; Cooke, R.F.; Rodrigues, M.C.; Cappellozza, B.I.; Mills, R.R.; Larson, C.K.; Moriel, P.; Bohnert, D.W. Effects of organic or inorganic cobalt, copper, manganese, and zinc supplementation to late-gestating beef cows on productive and physiological responses of the offspring. J. Anim. Sci. 2016, 94, 1215–1226. [Google Scholar] [CrossRef]
- Ahola, J.K.; Baker, D.S.; Burns, P.D.; Mortimer, R.G.; Enns, R.M.; Whittier, J.C.; Geary, T.W.; Engle, T.E. Effect of copper, zinc, and manganese supplementation and source on reproduction, mineral status, and performance in grazing beef cattle over a two-year period. J. Anim. Sci. 2004, 82, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Ahola, J.K.; Baker, D.S.; Burns, P.D.; Whittier, J.C.; Engle, T.E. Effect of copper, zinc, and manganese source on mineral status, reproduction, immunity, and calf performance in young beef females over a two-year period. Prof. Anim. Sci. 2005, 21, 297–304. [Google Scholar] [CrossRef]
- Kegley, E.B.; Pass, M.R.; Moore, J.C.; Larson, C.K. Supplemental trace minerals (zinc, copper, manganese, and cobalt) as Availa-4 or inorganic sources for shipping-stressed beef cattle 1. Prof. Anim. Sci. 2012, 28, 313–318. [Google Scholar] [CrossRef]
- Ryan, A.W.; Kegley, E.B.; Hawley, J.; Powell, J.G.; Hornsby, J.A.; Reynolds, J.L.; Laudert, S.B. Supplemental trace minerals (zinc, copper, and manganese) as sulfates, organic amino acid complexes, or hydroxy trace-mineral sources for shipping-stressed calves. Prof. Anim. Sci. 2015, 31, 333–341. [Google Scholar] [CrossRef]
- Rowe, M.P.; Powell, J.G.; Kegley, E.B.; Lester, T.D.; Rorie, R.W. Effect of supplemental tracemineral source on bull semen quality. Prof. Anim. Sci. 2014, 30, 68–73. [Google Scholar] [CrossRef]
- Behne, D.A.; Kyriakopoulos, A.; Scheid, S.; Gessner, H. Effects of chemical form and dosage on the incorporation of selenium into tissue proteins in rats. J. Nutr. 1991, 121, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.D.; O’Keefe, S.F.; Henry, P.R.; Ammerman, C.B.; Luo, X.G. The effect of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and dietary prooxidant activity. Poult. Sci. 1998, 77, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W.; Kegley, E.B.; Mullis, L.A. Bioavailability of copper from tribasic copper chloride and copper sulfate in growing cattle. Anim. Feed Sci. Technol. 2004, 116, 1–13. [Google Scholar] [CrossRef]
- Caldera, E.; Weigel, B.; Kucharczyk, V.N.; Sellins, K.S.; Archibeque, S.L.; Wagner, J.J.; Han, H.; Spears, J.W.; Engle, T.E. Trace mineral source influences ruminal distribution of copper and zinc and their binding strength to ruminal digesta. J. Anim. Sci. 2019, 97, 1852–1864. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Lippolis, K.D.; Ahola, J.K.; Wagner, J.J.; Spears, J.W.; Couch, D.; Engle, T.E. Influence of supplemental copper, manganese, and zinc source on reproduction, mineral status, and performance in a grazing beef cow-calf herd over a 2-year period. Appl. Anim. Sci. 2020, 36, 745–753. [Google Scholar] [CrossRef]
- Roura, E.; Humphrey, B.; Tedó, G.; Ipharraguerre, I. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can. J. Anim. Sci. 2008, 88, 535–558. [Google Scholar] [CrossRef]
- Moriel, P.; Arthington, J.D. Effects of trace mineral-fortified, limit-fed preweaning supplements on performance of pre- and postweaned beef calves. J. Anim. Sci. 2013, 91, 1371–1380. [Google Scholar] [CrossRef]
- Epke, E.M.; McClure, S.T.; Lawless, H.T. Effects of nasal occlusion and oral contact on perception of metallic taste from metal salts. Food Qual. Prefer. 2009, 20, 133–137. [Google Scholar] [CrossRef]
- Caramalac, L.S.; Saran Netto, A.; Martins, P.G.M.A.; Moriel, P.; Ranches, J.; Fernandes, H.J.; Arthington, J.D. Effects of hydroxychloride sources of copper, zinc, and manganese on measures of supplement intake, mineral status, and pre- and postweaning performance of beef calves. J. Anim. Sci. 2017, 95, 1739–1750. [Google Scholar] [CrossRef]
- Ranches, J.; De Oliveira, R.A.; Vedovatto, M.; Palmer, E.A.; Moriel, P.; Silva, L.D.; Zylberlicht, G.; Drouillard, J.S.; Arthington, J.D. Low moisture, cooked molasses blocks: A limited intake method for supplementing trace minerals to pre-weaned calves. Anim. Feed Sci. Technol. 2021, 273, 114793. [Google Scholar] [CrossRef]
- Arthington, J.D.; Silveira, M.L.; Caramalac, L.S.; Fernandes, H.J.; Heldt, J.S.; Ranches, J. Effects of varying sources of Cu, Zn, and Mn on mineral status and preferential intake of salt-based supplements by beef cows and calves and rainfall-induced metal loss. Transl. Anim. Sci. 2021, 5, txab046. [Google Scholar] [CrossRef] [PubMed]
- Arthington, J.D. Trace mineral supplementation of grazing beef cattle. In Proceedings of the Applied Reproductive Strategies in Beef Cattle, Manhattan, KS, USA, 29–30 August 2017; pp. 136–148. [Google Scholar]
- Mills, C.F. Dietary interactions involving the trace elements. Annu. Rev. Nutr. 1985, 5, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Bremner, I.W.; Humphries, R.; Phillippo, M.; Walker, M.J.; Morrice, P.C. Iron-induced copper deficiency in calves: Dose–response relationships and interactions with molybdenum and sulfur. Anim. Prod. 1987, 45, 403–414. [Google Scholar] [CrossRef]
- Mullis, L.A.; Spears, J.W.; Mccraw, R.L. Effects of breed (Angus vs. Simmental) and copper and zinc source on mineral status of steers fed high dietary iron. J. Anim. Sci. 2003, 81, 318–322. [Google Scholar] [CrossRef]
- Suttle, N.F.; Abrahams, P.; Thornton, I. The role of a soil X dietary sulphur interaction in the impairment of copper absorption by ingested soil in sheep. J. Agric. Sci. 1984, 103, 81–86. [Google Scholar] [CrossRef]
- Dias, R.S.; López, S.; Montanholi, Y.R.; Smith, B.; Haas, L.S.; Miller, S.P.; France, J. A meta-analysis of the effects of dietary copper, molybdenum, and sulfur on plasma and liver copper, weight gain, and feed conversion in growing-finishing cattle. J. Anim. Sci. 2013, 91, 5714–5723. [Google Scholar] [CrossRef]
- Agricultural Research Council. The Nutrient Requirements of Ruminant Livestock; Technical Review; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1980. [Google Scholar]
- Ward, J.D.; Spears, J.W. Long-term effects of consumption of low-copper diets with or without supplemental molybdenum on copper status, performance, and carcass characteristics of cattle. J. Anim. Sci. 1997, 75, 3057–3065. [Google Scholar] [CrossRef]
- Thorndyke, M.P.; Guimaraes, O.; Tillquist, N.M.; Zervoudakis, J.T.; Engle, T.E. Molybdenum exposure in drinking water vs. feed impacts apparent absorption of copper differently in beef cattle consuming a high-forage diet. Biol. Trace Elem. Res. 2021, 199, 2913–2918. [Google Scholar] [CrossRef]
- Arthington, J.D. Effects of supplement type and selenium source on measures of growth and selenium status in yearling beef steers. J. Anim. Sci. 2008, 86, 1472–1477. [Google Scholar] [CrossRef]
- Ranches, J.; Alves, R.; Vedovatto, M.; Palmer, E.A.; Moriel, P.; Arthington, J.D. Differences in copper and selenium metabolism between Angus (Bos taurus) and Brahman (Bos indicus) cattle. J. Anim. Sci. 2021, 99, skab048. [Google Scholar] [CrossRef]
- Van Metre, D.C.; Callan, R.J. Selenium and vitamin E. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 373–402. [Google Scholar] [CrossRef]
| Mineral, mg/kg | 6th NRC, 1984 [7] | 7th NRC, 1996–2000 [8] | 8th NRC, 2016 [6] | Maximum Tolerable Concentration 2 |
|---|---|---|---|---|
| Chromium | - | - | - | 1000 |
| Cobalt | 0.10 | 0.10 | 0.15 | 25 |
| Copper | 8 | 10 | 10 | 40 |
| Iodine | 0.50 | 0.50 | 0.50 | 50 |
| Iron | 50 | 50 | 50 | 500 |
| Manganese 3 | 40 | 40 | 40 | 1000 |
| Molybdenum | - | - | - | 5 |
| Nickel | - | - | - | 50 |
| Selenium | 0.20 | 0.10 | 0.10 | 5 |
| Zinc | 30 | 30 | 30 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthington, J.D.; Ranches, J. Trace Mineral Nutrition of Grazing Beef Cattle. Animals 2021, 11, 2767. https://doi.org/10.3390/ani11102767
Arthington JD, Ranches J. Trace Mineral Nutrition of Grazing Beef Cattle. Animals. 2021; 11(10):2767. https://doi.org/10.3390/ani11102767
Chicago/Turabian StyleArthington, John D., and Juliana Ranches. 2021. "Trace Mineral Nutrition of Grazing Beef Cattle" Animals 11, no. 10: 2767. https://doi.org/10.3390/ani11102767
APA StyleArthington, J. D., & Ranches, J. (2021). Trace Mineral Nutrition of Grazing Beef Cattle. Animals, 11(10), 2767. https://doi.org/10.3390/ani11102767





