Innovative Seafood Preservation Technologies: Recent Developments
Abstract
Simple Summary
Abstract
1. Seafood: Definitions, Structure and Composition
2. Seafood Spoilage
2.1. Spoilage Due to Autolytic Enzyme Activity
2.2. Microbial Spoilage
2.3. Oxidation and Hydrolysis
3. Innovative Seafood Preservation Methods
3.1. Use of Natural Preservatives
3.1.1. Organic Acids
Use of Organic Acids in Fish Preservation
3.1.2. Essential Oils and Plant/Algal Extracts
Use of Essential Oils and Plant/Algal Extracts in Fish Preservation
Use of Essential Oils and Plant/Algal Extracts in Fishery Products Preservation
3.1.3. Biopreservation (Lactic Acid Bacteria, Bacteriocins)
Use of Lactic Acid Bacteria and Bacteriocins in Fish and Fishery Product Preservation
3.1.4. Chitosan
Use of Chitosan in Fish Preservation
Use of Chitosan in Fishery Products Preservation
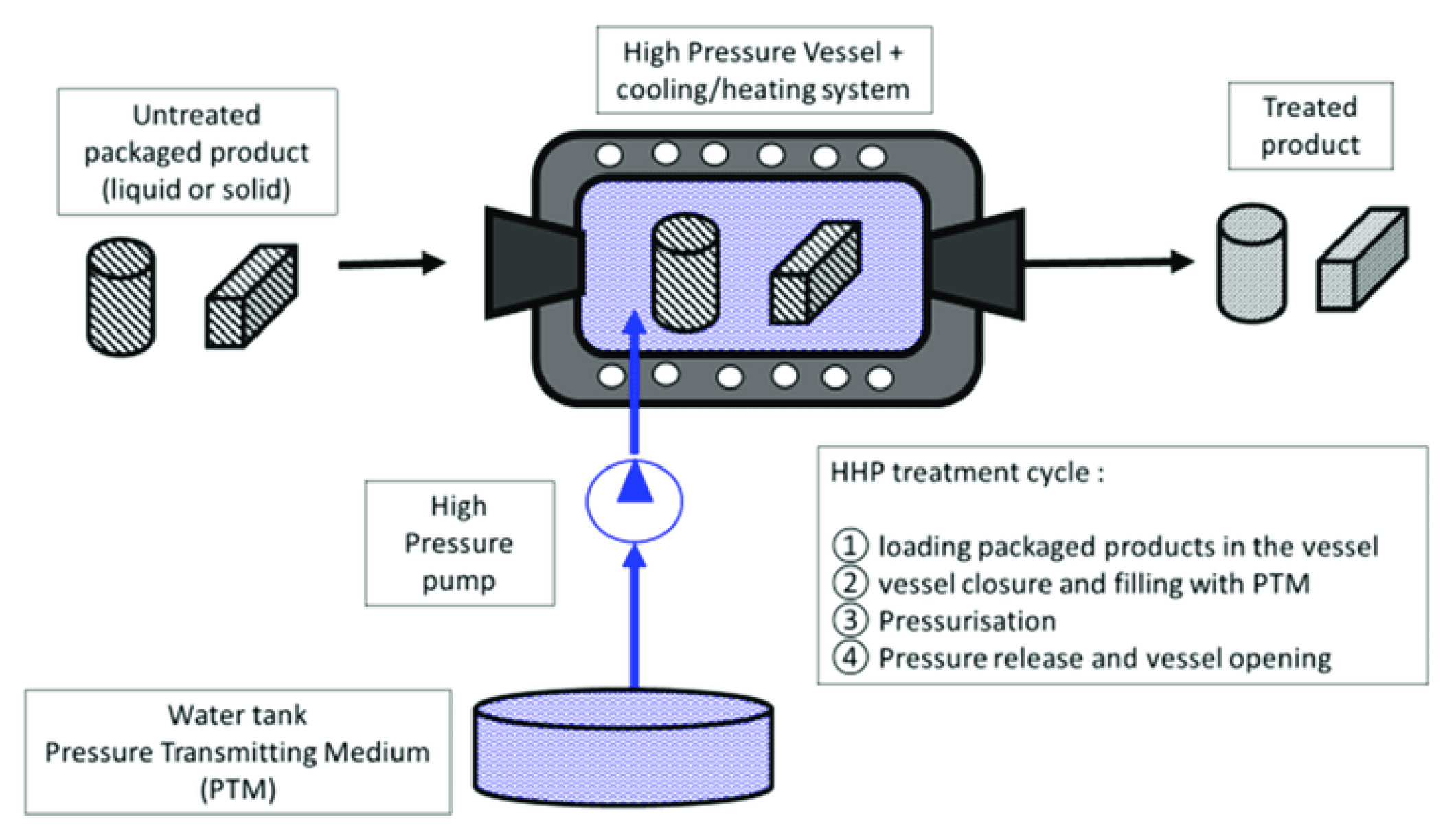
3.2. High Hydrostatic Pressure
3.2.1. Use of High Hydrostatic Pressure (HHP) in Fishery Products Preservation
- (i)
- At 250 MPa, with 10 or 5 min holding at 7 and 15 °C, respectively;
- (ii)
- At 220 MPa, with 5 min holding at 15 or 25 °C;
- (iii)
- At 330 MPa, with 10 min holding at 25 °C.
3.2.2. Use of HHP in Controlling Pathogens in Seafood
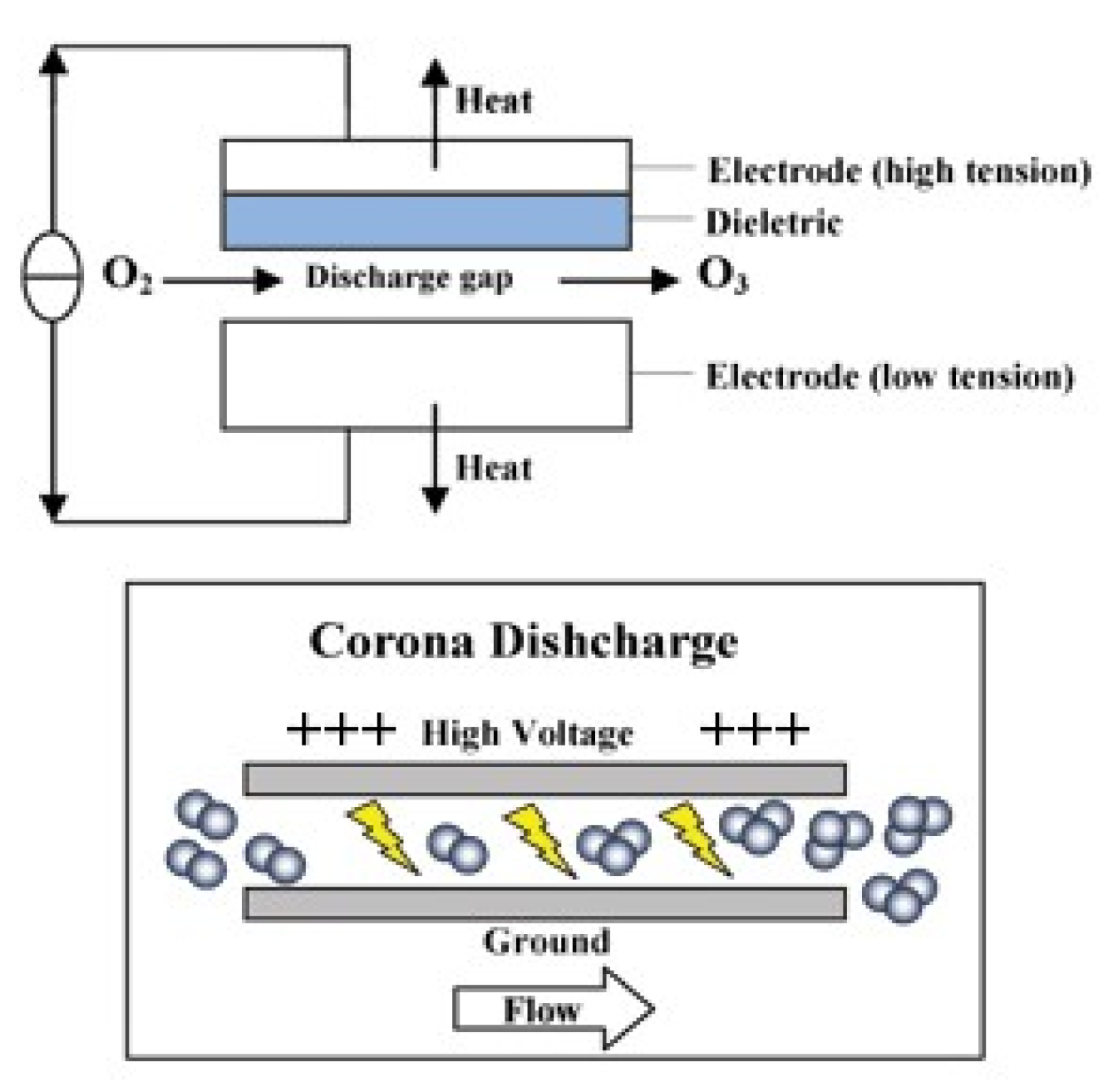
3.3. Ozonation of Seafood
3.3.1. Use of Ozone in Fish Preservation
3.3.2. Use of Ozone in Fishery Products Preservation
3.3.3. Use of Ozone for the Decontamination of Seafood
3.4. Irradiaton of Seafood
3.4.1. Use of Irradiation in Fish Preservation
3.4.2. Use of Irradiation in Fishery Products Preservation
3.4.3. Use of Irradiation for the Decontamination of Sea Food Products
3.5. Pulsed Electric Field Processing
Use of Pulsed Light Technology to Fishery Products Preservation
3.6. Retort Pouch Processing (RPP)
- The polyester layer provides excellent strength and printability.
- The aluminum protects from exposure to light, gases, moisture and odors and prolongs product shelf life.
- The nylon layer protects from abrasion.
- The polypropylene layer acts as a heat seal surface and provides strength and flexibility.
- The specific construction of the pouch provides rapid heat transfer for sterilization during processing. A 30–40% reduction in processing time is possible, with energy savings.
- Reduced heat exposure maintains product taste, color and flavor while resulting in fewer nutrient losses.
- Preparation of products that need to be heated to serving temperature can be accomplished in 3–5 min by immersing the pouch in boiling water or placing the plastic container in a microwave oven.
- Shelf life of retort pouch products is equivalent to that of foods in metal cans.
- Refrigeration or freezing is not required by packers, retailers or consumers.
- Pouches and containers do not corrode externally and there is a minimum of product–container interaction.
- Easy opening of the pouch.
- Empty retort pouches and nesting containers offer processors a reduction in storage space and lighter weight. Compared to empty cans, an equal number of retort pouches use 85% less space and are significantly lighter.
- Production of pouches uses less energy compared to metal containers.
3.6.1. Use of Retort Pouch Processing in Fish Preservation
3.6.2. Use of Retort Pouch Processing in Fishery Product Preservation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, M.R.; Moss, M.O. Microbiology of primary food commodities. In Food Microbiology; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 103–135. [Google Scholar]
- Shewan, J.M. The microbiology of fish and fishery products—A progress report. J. Appl. Bacteriol. 1971, 34, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Beuchat, L.R.; Montville, T.J. Food Microbiology: Fundamentals and Frontiers, 2nd ed.; ASM Press: Washington, DC, USA, 2001; pp. 91–105. [Google Scholar]
- Watt, B.K.; Merrill, A.L. Composition of Foods: Raw, Processed, Prepared. Agriculture Handbook No. 8; U.S. Department of Agriculture: Beltsville, MD, USA, 1963.
- Olafsdottir, G.; Lauzon, H.L.; Martinsdottir, E.; Kristbergsson, K. Influence of storage temperature on microbial spoilage characteristics of haddock fillets (Melanogrammus aeglefinus) evaluated by multivariate quality prediction. Int. J. Food Microbiol. 2006, 111, 112–125. [Google Scholar] [CrossRef] [PubMed]
- FAO. Post-Harvest Changes in Fish; Fisheries and Aquaculture Department, Food and Agriculture Organization: Rome, Italy, 2005; Available online: http://www.fao.org/fishery/topic/12320/en (accessed on 15 October 2019).
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Fraser, O.; Sumar, S. Compositional changes and spoilage in fish. Nutr. Food Sci. 1998, 5, 275–279. [Google Scholar] [CrossRef]
- Liston, J. Microbiology in fishery science. In Advances in Fish Science and Technology; Connell, J.J., Ed.; Fishing News Books Ltd.: Farnham, UK, 1980; pp. 138–157. [Google Scholar]
- Cheng, J.H.; Sun, D.W.; Pu, H.; Zhu, Z. Development of hyperspectral imaging coupled with chemometric analysis to monitor K-value for evaluation of chemical spoilage in fish fillets. Food Chem. 2015, 185, 245–253. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Sumpavapol, P.; Nirmal, N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012, 155, 171–178. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Fresh and processed fish and shellfish. In The Microbiological Safety and Quality of Foods; Lund, B.M., Baird-Parker, A.C., Gould, G.W., Eds.; Chapman and Hall: London, UK, 2000; pp. 472–506. [Google Scholar]
- Dalgaard, P.; Madsen, H.L.; Samieian, N.; Emborg, J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone) effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 2006, 101, 80–95. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria-problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Connell, J.J. Control of Fish Quality, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Boziaris, I.S. Current trends on the study of microbiological spoilage of fresh fish. Fish Aquac. J. 2014, 6, 1000e115. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatiles profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef]
- Abbas, K.A.; Saleh, A.M.; Mohamed, A.; Lasekan, O. The relationship between water activity and fish spoilage during cold storage: A review. J. Food Agric. Environ. 2009, 7, 86–90. [Google Scholar]
- Hultin, H.O. Oxidation of lipids in seafoods. In Seafoods Chemistry, Processing Technology and Quality, 1st ed.; Shahidi, F., Botta, J.R., Eds.; Blackie Academic and Professional: London, UK, 1994; pp. 49–74. [Google Scholar]
- Huis in’t Veld, J.H.J. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Undeland, I.; Hall, G.; Wendin, K.; Gangby, I.; Rutgersson, A. Preventing lipid oxidation during recovery of functional proteins from herring (Clupea harengus) fillets by an acid solubilization process. J. Agric. Food Chem. 2005, 53, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- Lampila, L.E.; McMillin, K.W. Major microbial hazards associated with packaged seafood. In Advances in Meat, Poultry and Seafood Packaging; Kerry, J.P., Ed.; Woodhead Publ. Ltd.: Cambridge, UK, 2012; pp. 59–80. [Google Scholar]
- Schwarz, J.R. Rapid chilling of oyster shell stock: A postharvest process to reduce Vibrio. In Proceedings of the 25th Annual Meeting of the Seafood Science & Technology Society of the Americas, Longboat, FL, USA, 9–11 October 2000. [Google Scholar]
- Papadopoulou, C.; Economou, E.; Zakas, G.; Salamoura, C.; Dontorou, C.; Apostolou, J. Microbiological and pathogenic contaminants of seafood in Greece. J. Food Qual. 2007, 30, 28–42. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Abdu, M.; Bereket, A.; Melake, S.; Hamada, M.; Winta, A.; Elham, M. Fish preservation: A multi-dimensional approach. MOJ Food Process. Technol. 2018, 6, 303–310. [Google Scholar]
- Samples, S. The effects of storage and preservation technologies on the quality of fish products: A review. J. Food Process. Preserv. 2015, 39, 1206–1215. [Google Scholar] [CrossRef]
- Nagarajarao, R.C. Recent advances in processing and packaging of fishery products: A review. Aquat. Procedia 2016, 7, 201–213. [Google Scholar] [CrossRef]
- Wu, C.-H.; Yuan, C.-H.; Ye, X.-Q.; Hu, Y.-Q.; Chen, S.-G.; Liu, D. A critical review on superchilling preservation technology in aquatic product. J. Integr. Agric. 2014, 13, 2788–2806. [Google Scholar] [CrossRef]
- Erkan, N.; Ali Gunlu, A.; Genη, I.Y. Alternative seafood preservation technologies: Ionizing radiation and high pressure processing. J. FisheriesSciences.com 2014, 8, 238–251. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Mirzapour-Kouhdasht, A.; Oliyaei, N. Application of essential oils for shelf-life extension of seafood products. IntechOpen 2019. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf life of seafood: A review. Food Res. Int. 2020, 108762. [Google Scholar] [CrossRef] [PubMed]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 2017, 12, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Gonzαlez, M.P.; Murado, M.A. Effects of lactic acid bacteria cultures on pathogenic microbiota from fish. Aquacult 2005, 245, 149–161. [Google Scholar] [CrossRef]
- Garcia-Soto, B.; Barros-Velazquez, J.; Aubourg, S.P. Use of citric and lactic acids in ice to enhance quality of two fish species during on-board chilled storage. Int. J. Refrig. 2014, 40, 390–397. [Google Scholar] [CrossRef]
- Bou, R.; Claret, A.; Stamatakis, A.; Martinez, B.; Guerrero, L. Quality changes and shelf-life extension of ready-to-eat fish patties by adding encapsulated citric acid. J. Sci. Food Agric. 2017, 97, 5352–5360. [Google Scholar] [CrossRef]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef]
- Gokoglu, N.; Cengz, E.; Yerlkaya, P. Determination of the shelf life of marinated sardine (Sadinia pilchardus) stored at 4 °C. Food Control 2004, 15, 1–4. [Google Scholar] [CrossRef]
- Rey, M.S.; García-Soto, B.; Fuertes-Gamundi, J.R.; Aubourg, S.; Barros-Velázquez, J. Effect of a natural organic acid-icing system on the microbiological quality of commercially relevant chilled fish species. LWT 2012, 46, 217–223. [Google Scholar] [CrossRef]
- Kin, S.; Schilling, M.W.; Smith, B.S.; Silva, J.L.; Kim, T.; Pham, A.J.; Campano, S.G. Potassium acetate and potassium lactate enhance the microbiological and physical properties of marinated catfish fillets. J. Food Sci. 2011, 76, S242–S250. [Google Scholar] [CrossRef]
- Schirmer, B.; Ragnhild, H.; Thomas, E.; Trond, M.; Tove, M.; Mats, C.; Solveig, L. A novel packaging methods with dissolving CO2 headspace combined with organic acids prolongs the self-life of fresh salmon. Int. J. Food Microbiol. 2009, 133, 154–160. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Melanosis and quality changes of pacific white shrimp (Litopenaeus vannamei) treated with catechin during iced storage. J. Agric. Food Chem. 2009, 57, 3578–3586. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Martinez-Alvarez, O.; Gómez-Guillén, M.C.; Montero, P. Quality of thawed deep-water pink shrimp (Parapenaeus longirostris) treated with melanosis -inhibiting formulations during chilled storage. Int. J. Food Sci. Technol. 2007, 42, 1029–1038. [Google Scholar] [CrossRef]
- Monirul, I.; Yang, F.; Niaz, M.; Qixing, J.; Wenshui, X. Effectiveness of combined acetic acid and ascorbic acid spray on fresh silver carp (Hypophthalmichthys molitrix) fish to increase shelf-life at refrigerated temperature. Curr. Res. Nutr. Food Sci. 2019, 7, 415–426. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems. A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef]
- Hac-Wydro, K.; Flasinski, M.; Romanczuk, K. Essential oils as food eco-preservatives: Model system studies on the effect of temperature on limonene antibacterial activity. Food Chem. 2017, 235, 127–135. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Infect. Dis. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Freeman, B.C.; Beattie, G.A. An overview of plant defenses against pathogens and herbivores. Plant Health Instr. 2008. [Google Scholar] [CrossRef]
- Davidson, P.M. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers, 2nd ed.; Doyle, M.P., Beuchat, L.R., Molntville, T.J., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 593–627. [Google Scholar]
- Aubourg, S.P.; Trigo, M.; Martínez, B.; Rodríguez, A. Effect of prior chilling period and alga-extract packaging on the quality of a canned underutilised fish species. Foods 2020, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- European Council Regulation (14/02/1997). European Community (EC), No 258/97, 27 January 1997. Concerning novel foods and novel food ingredients. CELEX-EUR Off. J. 1997, L-43, 1–7. [Google Scholar]
- García-Soto, B.; Miranda, J.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Rodríguez-Martínez, A.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Pattipeilohy, F.; Moniharapon, T.; Nelce Mailoa, M.; Bonan, R.; Sormin, D. Antibacterial activity of seaweed (Gymnogongrus sp.) extract against Salmonella typhimurium, Escherichia coli and Bacillus subtilis. Int. J. Sci. Res. Publ. 2017, 7, 433–439. [Google Scholar]
- Goulas, A.E.; Kontominas, M.G. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chem. 2007, 100, 287–296. [Google Scholar] [CrossRef]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiol. 2009, 26, 598–605. [Google Scholar] [CrossRef]
- Attouchi, M.; Sadok, S. The effect of powdered thyme sprinkling on quality changes of wild and farmed gilthead sea bream fillets stored in ice. Food Chem. 2010, 119, 1527–1534. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López de Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef]
- Özogul, F.; Kuley, E.; Kenar, M. Effects of rosemary and sage tea extract on biogenic amines formation of sardine (Sardina pilchardus) fillets. Int. J. Food Sci. Technol. 2011, 46, 761–766. [Google Scholar] [CrossRef]
- Attouchi, M.; Sadok, S. The effects of essential oils addition on the quality of wild and farmed sea bream (Sparus aurata) stored in ice. Food Bioproc. Technol. 2012, 5, 1803–1816. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W.; Zhang, X.; Li, X.; Zhao, J. Shelf-life extension of crucian carp (Carassius auratus) using natural preservatives during chilled storage. Food Chem. 2012, 135, 140–145. [Google Scholar] [CrossRef]
- Su, H.; Chen, W.; Fu, S.; Wu, C.; Li, K.; Huang, Z.; Wu, T.; Li, J. Antimicrobial effect of bayberry leaf extract for the preservation of large yellow croaker (Pseudosciaena crocea). J. Sci. Food Agric. 2014, 94, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Houicher, A.; Kuley, E.; Bendeddouche, B.; Ozogul, F. Effect of Mentha spicata L. and Artemisia campestris extracts on the shelf life and quality of vacuum-packed refrigerated sardine (Sardina pilchardus) fillets. J. Food Prot. 2013, 76, 1719–1725. [Google Scholar] [CrossRef]
- Eskandari, S.; Hosseini, H.; Gholamzadeh, M.; Khaneghah, A.M.; Hosseini, E. The effects of black cumin, black caraway extracts and their combination on shelf life extension of silver carp (Hypophthalmichthys molitrix) during refrigerated storage. J. Food Saf. 2015, 35, 154–160. [Google Scholar] [CrossRef]
- Ga, I.F.; Ortoffi, M.; Giancotti, V.; Medana, C.; Peiretti, P.G. Effect of red grape pomace extract on the shelf life of refrigerated rainbow trout (Oncorhynchus mykiss) minced muscle. J. Aquat. Food Prod. Technol. 2015, 24, 468–480. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Haute, S.V.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and MAP/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Baccarin Dargelio, M.D.; Pedroso-Yoshida, C.M.; Ribeiro Junior, E.E.; Massarioli, A. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 125. [Google Scholar] [CrossRef]
- El-Sayed, M.H.; El-Aziz, Z.K.A.; Elbadawy, H.H. Evaluation of the microbial spoilage of Atlantic salmon (Salmo salar) fillets during the packaging processes and its control by preservatives. IJSRSET 2015, 1, 134–141. [Google Scholar]
- Hasani, S.; Alizadeh, E.; Elahi, M.Y. Effects of grape pomace extract on the quality and shelf life of silver carp (Hypophthalmicthys molitrix) fillets during chill storage. Int. J. Aquat. Biol. 2015, 3, 108–113. [Google Scholar]
- Maghami, W.; Motalebi, A.; Anvar, S.A. Influence of chitosan nanoparticles and fennel essential oils (Foeniculum vulgare) on the shelf life of Huso huso fish fillets during the storage. Food Sci. Nutr. 2019, 7, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M.; Hasani, M. Nano-encapsulation of lemon essential oil approach to reducing the oxidation process in fish burger during refrigerated storage. J. Food Biosci. Technol. 2020, 10, 35–46. [Google Scholar]
- Ahmed, E.S.S.; Shehata, M.G.; Abd-Rabou, H.S.; El-Menshawy, H. Extend shelf-life of vacuum-packaged herring fish fillets using garlic and ginger extracts. J. Pure Appl. Microbiol. 2019, 13, 1571–1581. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Campos, C.A.; Augourg, S.P. Preservative effect of algae Extracts on lipid composition and rancidity development in brine-canned Atlantic chub mackerel (Scomber colias). Eur. J. Lipid Sci. Technol. 2019, 212, 1900129. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Shirahigue, L.D.; de Arruda Sucasas, L.F.; Anbe, L.; da Cruz, P.G.; Cláudio Rosa Gallo, C.R.; Carpes, S.T.; Marques, M.J.; Oetterer, M. Shelf life and quality study of minced tilapia with nori and hijiki seaweeds as natural additives. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Atrea, I.; Papavergou, A.; Amvrosiadis, I.; Savvaidis, I.N. Combined effect of vacuum-packaging and oregano essential oil on the shelf-life of Mediterranean octopus (Octopus vulgaris) from the Aegean Sea stored at 4 °C. Food Microbiol. 2009, 26, 166–172. [Google Scholar] [CrossRef]
- Xi, D.; Liu, C.; Su, Y. Effects of green tea extract on reducing Vibrio parahaemolyticus and increasing shelf life of oyster meats. Food Control 2012, 25, 368–373. [Google Scholar] [CrossRef]
- Ozogul, I.; Polat, A.; Ozogul, Y.; Boga, E.K.; Ozogul, F.; Ayas, D. Effects of laurel and myrtle extracts on the sensory, chemical and microbiological properties of vacuum-packed and refrigerated European eel (Anguilla anguilla) fillets. Int. J. Food Sci. Technol. 2014, 49, 847–853. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Benjakul, S.; Sae-leaw, T. Effect of chamuang (Garcinia cowa Roxb.) leaf extract on inhibition of melanosis and quality changes of pacific white shrimp during refrigerated storage. Food Chem. 2019, 270, 554–561. [Google Scholar] [CrossRef]
- Garcia, P.; Rodriguez, L.; Rodriguez, A.; Martinez, B. Food biopreservation: Promising strategies using bacteriocins, bacteriophage and endolysins. Trends Food Sci. Technol. 2010, 21, 373–382. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Gaudu, P.; Lechardeur, D.; Petit, M.-A.; Gruss, A. Aerobic respiration metabolism in lactic acid bacteria and uses in biotechnology. Rev. Food Sci. Technol. 2012, 3, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Francoise, L. Occurance and role of lactic acid bacteria in seafood products. Food Microbiol. 2010, 27, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Suskovic, J.; Kos, B.; Beganovic, J.; Pavunc, A.L.; Habjanic, K.; Matosic, S. Antimicrobial activity—The most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Holzapfel, W.H.; Schillinger, U.; Geisen, R.; Lucke, F.K. Starter and protective cultures. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Kluwer Academic/Plenum Publ.: New York, NY, USA, 2003; pp. 291–320. [Google Scholar]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 10, 777–788. [Google Scholar] [CrossRef]
- EU. Commission Regulation No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. Available online: https://www.fsai.ie/uploadedFiles/Reg1129_2011.pdf (accessed on 10 November 2020).
- Matamoros, S.; Leroi, F.; Cardinal, M.; Gigout, F.; Kasbi Chadli, F.; Cornet, J.; Prevost, H.; Pilet, M.F. Psychrotrophic lactic acid bacteria used to improve the safety and quality of vacuum-packaged cooked and peeled tropical shrimp and cold-smoked salmon. J. Food Prot. 2009, 72, 365–374. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Balkiza, F.; Gad, E.; Alexandraki, V.; Avramiotis, S.; Georgalaki, M.; Xenakis, A. Reverse micelles as nano-carriers of nisin against foodborne pathogens. Part II: The case of essential oils. Food Chem. 2019, 278, 415–423. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Gómez-Sala, B.; Herranz, C.; Díaz-Freitas, B.; Hernández, P.E.; Sala, A.; Cintas, L.M. Strategies to increase the hygienic and economic value of fresh fish: Biopreservation using lactic acid bacteria of marine origin. Int. J. Food Microbiol. 2016, 223, 41–49. [Google Scholar] [CrossRef]
- Anacarso, I.; Messi, P.; Condò, C.; Iseppi, R.; Bondi, M.; Sabia, C.; De Niederhäusern, S. A bacteriocin-like substance produced from Lactobacillus pentosus 39 is a natural antagonist for the control of Aeromonas hydrophila and Listeria monocytogenes in fresh salmon fillets. LWT 2014, 55, 604–611. [Google Scholar] [CrossRef]
- Ibrahim, F.; Vesterlund, S. Lactococcus lactis ssp. lactis as protective culture in vacuum-packed raw salmon (Salmo salar). J. Aquat. Food Prod. Technol. 2014, 23, 601–607. [Google Scholar]
- Sarika, A.R.; Lipton, A.P.; Aishwarya, M.S.; Dhivya, R.S. Isolation of a bacteriocin-producing Lactococcus lactis and application of its bacteriocin to manage spoilage bacteria in high-value marine fish under different storage temperatures. Appl. Biochem. Biotechnol. 2012, 167, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Shelf life definition for Italian anchovies inoculated with Lactobacillus plantarum and Bifidobacterium animalis subsp. lactis. Innov. Food Sci. Emerg. Technol. 2012, 16, 171–180. [Google Scholar] [CrossRef]
- Leroi, F.; Cornet, J.; Chevalier, F.; Cardinal, M.; Coeuret, G.; Chaillou, S.; Joffrauda, J.J. Selection of bioprotective cultures for preventing cold-smoked salmon spoilage. Int. J. Food Microbiol. 2015, 213, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Rodríguez, M.; Garriga, M.; Bover-Cid, S. Assessment of the bioprotective potential of lactic acid bacteria against Listeria monocytogenes on vacuum-packed cold-smoked salmon stored at 8 °C. Food Microbiol. 2019, 83, 64–70. [Google Scholar] [CrossRef] [PubMed]
- da Silva Vieira, G.R.A.; Soares, M.; Boliva Ramírez, N.C.; Schleder, D.D.; Corre da Silva, B.; Pedreira Mouriño, J.L.; Andreata, E.R.; do Nascimento Vieira, F. Lactic acid bacteria used as preservative in fresh feed for marine shrimp maturation. Pesq. Agropec. Bras. 2016, 5, 1799–1805. [Google Scholar] [CrossRef][Green Version]
- Saraoui, T.; Cornet, J.; Guillouet, E.; Piletb, M.F.; Chevalier, F.; Joffraud, J.J.; Leroi, F. Improving simultaneously the quality and safety of cooked and peeled shrimp using a cocktail of bioprotective lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 69–77. [Google Scholar] [CrossRef]
- Fall, P.A.; Leroi, F.; Cardinal, M.; Chevalier, F.; Pilet, M.F. Inhibition of Brochothrix thermosphacta and sensory improvement of tropical peeled cooked shrimp by Lactococcus piscium CNCM I-4031. Lett. Appl. Microbiol. 2010, 50, 357–361. [Google Scholar] [CrossRef]
- Wiernasz, N.; Leroi, F.; Chevalier, F.; Cornet, J.; Cardinal, M.; Rohloff, J.; Passerini, D.; Skırnisdóttir, S.; Pilet, M.-F. Salmon gravlax biopreservation with lactic acid bacteria: A polyphasic approach to assessing the impact on organoleptic properties, microbial ecosystem and volatilome composition. Front. Microbiol. 2020, 10, 3103. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterization and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, Y.; Cherian, G.; Zhao, Y. Effect of combined chitosan-krill oil coating and modified atmosphere packaging on the storability of cold-stored lingcod (Ophiodon elongates) fillets. Food Chem. 2010, 122, 1035–1042. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Kwakye, K.; Fell, J.T.; Kipo, S.L. Effects of pH of medium and molecular weight on polyelectrolyte complex formation between pectin and chitosan. J. Sci. Technol. 2004, 26, 66–73. [Google Scholar] [CrossRef][Green Version]
- Kong, M.; Guang, X.; Xing, K.; Jin, H. Microbiology antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Feng, T.; Du, Y.; Li, J.; Hu, Y.; Kennedy, J.F. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr. Polym. 2008, 73, 126–132. [Google Scholar] [CrossRef]
- Soares, N.; Silva, P.; Barbosa, C.; Pinheiro, R.; Vicente, A.A. Comparing the effects of glazing and chitosan-based coating applied on frozen salmon on its organoleptic and physicochemical characteristics over six-months storage. J. Food Eng. 2017, 194, 79–86. [Google Scholar] [CrossRef]
- Tayel, A.A. Macromolecules microbial chitosan as a biopreservative for fish sausages. Int. J. Biol. Macromol. 2016, 93, 41–46. [Google Scholar] [CrossRef]
- Saloko, S.; Darmadji, P.; Setiaji, B.; Pranoto, Y. Antioxidative and antimicrobial activities of liquid smoke nanocapsules using chitosan and maltodextrin and its application on tuna fish preservation. Food Biosci. 2014, 7, 71–79. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Gopal, T.K.S. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocol. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Vatavali, K.; Karakosta, L.; Nathanailides, C.; Georgantelis, D.; Kontominas, M.G. Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food Bioproc. Technol. 2013, 6, 3510–3521. [Google Scholar] [CrossRef]
- Alak, G. The effect of chitosan prepared in different solvents on the quality parameters of brown trout fillets (Salmo trutta fario). Food Nutr. Sci. 2012, 3, 1303–1306. [Google Scholar]
- Fan, W.; Sun, J.; Chen, Y.; Qiu, J.; Zhang, Y.; Chi, Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009, 115, 66–70. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Gómez-Guillén, M.C.; Pérez-Mateos, M.; Montero, P. A chitosan—Gelatin blend as a coating for fish patties. Food Hydrocol. 2005, 19, 303–311. [Google Scholar] [CrossRef]
- Cao, X.; Islam, M.N.; Chitrakar, B.; Duan, Z.; Xu, W.; Zhong, S. Effect of combined chlorogenic acid and chitosan coating on antioxidant, antimicrobial, and sensory properties of snakehead fish in cold storage. Food Sci. Nutr. 2020, 8, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Chouljenko, A.; Chotiko, A.; Bonilla, F.; Moncada, M.; Reyes, V.; Sathive, S. Effects of vacuum tumbling with chitosan nanoparticles on the quality characteristics of cryogenically frozen shrimp. LWT 2017, 75, 114–123. [Google Scholar] [CrossRef]
- Carrión-granda, X.; Fernández-pan, I.; Jaime, I.; Rovira, J.; Maté, J.I. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int. J. Food Microbiol. 2016, 232, 144–149. [Google Scholar] [CrossRef]
- Chantarasataporn, P.; Yoksan, R.; Visessanguan, W. Water-based nano-sized chitin and chitosan as seafood additive through a case study of Pacific white shrimp (Litopenaeus vannamei). Food Hydrocol. 2013, 32, 341–348. [Google Scholar] [CrossRef]
- Yanar, Y.; Küçükgülmez, A.; Gökçin, M.; Gelibolu, S. Antioxidant effects of chitosan in European eel (Anguilla anguilla L.) fillets during refrigerated storage. CyTA J. Food 2013, 11, 328–333. [Google Scholar] [CrossRef]
- Cao, R.; Xue, C.; Liu, Q. Changes in microbial flora of pacific oysters (Crassostrea gigas) during refrigerated storage and its shelf-life extension by chitosan. Int. J. Food Microbiol. 2009, 131, 272–276. [Google Scholar] [CrossRef]
- Kuçukgulmez, A.; Yanar, Y.; Gerçek, G.; Gulnaz, O.; Celik, M. Effects of chitosan on color, sensory and microbiological properties of European eel (Anguilla Anguilla) fillets during refrigerated storage. J. Food Process. Preserv. 2013, 37, 766–771. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.; Balasubramaniam, V.M. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Sarika, K.; Bindou, J. Non thermal processing of seafoods. In Non Thermal Processing of Foods; Chauhan, O.P., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 374–389. [Google Scholar]
- Hogan, E.; Kelly, A.; Sun, D.W. High pressure processing of foods: An overview. In Emerging Technologies for Food Processing; Sun, D.W., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 3–32. [Google Scholar]
- Kaur, B.P.; Rao, P.S. Modeling the combined effect of pressure and mild heat on the inactivation kinetics of Escherichia coli, Listeria innocua, and Staphylococcus aureus in black tiger shrimp (Penaeus monodon). Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Matejkováa, K.; Krízeka, M.; Váchab, F.; Dadáková, E. Effect of high-pressure treatment on biogenic amines formation in vacuum-packed trout flesh (Oncorhynchus mykiss). Food Chem. 2013, 137, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Munoz, A.; Chevalier, D.; LeBail, A.; Ramaswamy, H.S.; Simpson, B.K. Physicochemical changes induced in carp (Cyprinus carpio) fillets by high pressure processing at low temperature. Innov. Food Sci. Emerg. Technol. 2006, 7, 13–18. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.; Balaban, M.O.; Marshall, M.R. Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and mahi mahi (Coryphaena hippurus). J. Food Sci. 2007, 72, C509–C515. [Google Scholar] [CrossRef]
- Erkan, N.; Uretener, G.; Alpas, H. Effect of high pressure (HP) on the quality and shelf life of red mullet (Mullus surmelutus). Innovative Food Sci. Emerg. Technol. 2010, 11, 259–264. [Google Scholar] [CrossRef]
- Erkan, N.; Gonza, U.; Alpas, H.; Selçuk, A.; Özkan, Ö.; Buzrul, S. Effect of high hydrostatic pressure (HHP) treatmenton physicochemical properties of horse mackerel (Trachurustrachurus). Food Bioproc. Technol. 2011, 4, 1322–1429. [Google Scholar] [CrossRef]
- Gunlu, A.; Sipahioğlu, S.; Alpas, H. The effect of chitosan-based edible film and high hydrostatic pressure process on the microbiological and chemical quality of rainbow trout (Oncorhynchus mykiss Walbaum) fillets during cold storage (4 ± 1 °C). High Pres. Res. 2014, 34, 110–121. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hsieh, C.-Y.; Chen, M.-L.; Wang, C.-Y.; Lin, C.-S.; Tsai, Y.-H. High-pressure inactivation of histamine-forming bacteria Morganella morganii and Photobacterium phosphoreum. J. Food Prot. 2020, 83, 621–627. [Google Scholar] [CrossRef]
- Lopez-Caballero, M.E.; Perez-Mateos, M.; Montero, P.; Borderias, A.J. Oyster preservation by high-pressure treatment. J. Food Prot. 2000, 63, 196–201. [Google Scholar] [CrossRef]
- Linton, M.; Mc Clements, J.M.J.; Patterson, M.F. Changes in the microbiological quality of shellfish, brought about by treatment with high hydrostatic pressure. Int. J. Food Sci. Technol. 2003, 38, 713–727. [Google Scholar] [CrossRef]
- Bindu, J.; Ginson, J.; Kamalakanth, C.K.; Gopal, T.K.S. High pressure treatment of green mussel Perna viridis Linnaeus, 1758: Effect on shucking and quality changes in meat during chill storage. Indian J. Fish. 2015, 62, 70–76. [Google Scholar]
- Narwankar, S.P.; Flimlin, G.E.; Schaffner, D.W.; Tepper, B.J.; Karwe, M.V. Microbial safety and consumer acceptability of high-pressure processed hard clams (Mercenaria Mercenaria). J. Food Sci. 2011, 76, M375–M380. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Jahncke, M.L.; Paul, W.; Reno, P.W.; Hu, X.; Mallikarjunan, P. Inactivation of Vibrio parahaemolyticus and Vibrio vulnificus in phosphate-buffered saline and in inoculated whole oysters by high-pressure processing. J. Food Prot. 2006, 69, 596–601. [Google Scholar] [CrossRef][Green Version]
- Cruz-Romero, M.; Kerry, J.P.; Kelly, A.L. Changes in the microbiological and physicochemical quality of high-pressure-treated oysters (Crassostrea gigas) during chilled storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Ginson, J.; Panda, S.K.; Bindu, J.; Kamalakanth, C.K.; Srinivasa Gopal, T.K. Effect of high pressure treatment on microbiological quality of Indian white prawn (Fenneropenaeus indicus) during chilled storage. Food Microbiol. 2015, 46, 596–603. [Google Scholar] [CrossRef]
- Hughes, B.H.; Perkins, L.B.; Yang, T.C.; Skonberg, D.I. Impact of post-rigor high pressure processing on the physicochemical and microbial shelf-life of cultured red abalone (Haliotis rufescens). Food Chem. 2016, 194, 487–494. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Perez-Won, M.; Zamarca, M.; Aguilera-Radic, J.M.; Tabilo-Munizaga, G. Effects of high hydrostatic pressure on microstructure, texture, colour and biochemical changes of red abalone (Haliotis rufecens) during cold storage time. Innovative Food Sci. Emerg. Technol. 2012, 13, 42–50. [Google Scholar] [CrossRef]
- Ritz, M.H.; Jugiau, F.; Federighi, M.; Chapleau, N.; de Lamballerie, M. Effects of high pressure, subzero temperature, and pH on survival of Listeria monocytogenes in buffer and smoked salmon. J. Food Prot. 2008, 71, 1612–1618. [Google Scholar] [CrossRef]
- Ye, M.; Huang, Y.; Chen, H. Inactivation of Vibrio parahaemolyticus and Vibrio vulnificus in oysters by high-hydrostatic pressure and mild heat. Food Microbiol. 2012, 32, 179–184. [Google Scholar] [CrossRef]
- Terio, V.; Tantillo, G.; Martella, V.; Pinto, P.D.; Buonavoglia, C.; Kingsley, D.H. High pressure inactivation of HAV within mussels. Food Environ. Virol. 2010, 2, 83–88. [Google Scholar] [CrossRef]
- Phuvasate, S.; Su, Y.C. Efficacy of low-temperature high hydrostatic pressure processing in inactivating Vibrio parahaemolyticus in culture suspension and oyster homogenate. Int. J. Food Microbiol. 2015, 196, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mootian, G.K.; Flimlin, G.E.; Karwe, M.V.; Schaffner, D.W. Inactivation of Vibrio parahaemolyticus in hard clams (Mercanaria mercanaria) by high hydrostatic pressure (HHP) and the effect of HHP on the physical characteristics of hard clam meat. J. Food Sci. 2013, 78, E251–E257. [Google Scholar] [CrossRef]
- Ma, L.; Su, Y.C. Validation of high pressure processing for inactivating Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 2011, 144, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.S.; Kingsley, D.H.; Montes, J.S.; Richards, G.P.; Lyon, G.M.; Abdulhafid, G.M.; Seitz, S.R.; Fernandez, M.L.; Teunis, P.F.; Flick, G.J.; et al. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl. Environ. Microbiol. 2011, 77, 5476–5482. [Google Scholar] [CrossRef]
- Calci, K.R.; Meade, G.K.; Tezloff, R.C.; Kingsley, D.H. High-pressure inactivation of hepatitis. A virus within oysters. Appl. Environ. Microbiol. 2005, 71, 339–343. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Calci, K.; Holliman, S.; Dancho, B.; Flick, G. High pressure inactivation of HAV within oysters: Comparison of shucked oysters with whole-in-shell meats. Food Environ. Virol. 2009, 1, 137–140. [Google Scholar] [CrossRef]
- Rollex Australia Ltd., Leaflet on ozone. P.O., Box 13655, Johnsonville, Ngauranga, Wellington, New Zealand. 1999. Available online: http://www.rollex.com.au/ (accessed on 10 November 2020).
- Goche, l.; Cox, B. Ozone Treatment of fresh H&G Alaska salmon. Report to Alaska Science and Technology Foundation and Alaska Department of Environmental Conservation; Surefish: Seattle, WA, USA, 1999. [Google Scholar]
- Manousaridis, G.; Nerantzaki, A.; Paleologos, E.K.; Tsiotsias, A.; Savvaidis, I.N.; Kontominas, M.G. Effect of ozone on microbial, chemical and sensory attributes of shucked mussels. Food Microbiol. 2005, 22, 1–9. [Google Scholar] [CrossRef]
- Nerantzaki, A.; Tsiotsias, A.; Paleologos, E.K.; Savvaidis, I.N.; Bezirtzoglou, E.; Kontominas, M.G. Effects of ozonation on microbilogical, chemical and sensory attributes of vacuum-packaged rainbow trout at 4 ± 0.5 °C. Eur. Food Res. Technol. 2005, 221, 675–683. [Google Scholar] [CrossRef]
- Goncalves, A.A. Ozone, An emerging technology for the seafood industry. Braz. Arch. Biol. Technol. 2009, 52, 1527–1539. [Google Scholar] [CrossRef]
- Powell, A.; Scolding, J.W.S. Direct application of ozone in aquaculture systems. Rev. Aquacult. 2018, 10, 424–438. [Google Scholar] [CrossRef]
- Feng, l.; Zhang, K.; Gao, M.; Shi, C.; Ge, C.; Qu, D.; Zhu, J.; Shi, Y.; Han, J. Inactivation of Vibrio parahaemolyticus by aqueous ozone. J. Microbiol. Biotechnol. 2018, 28, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.K.; Mukhimm, K.; Swer, T.; Dutta, D.; Verma, N.; Deka, B.C.; Gangwar, B.A. Review on application of ozone in the food processing and packaging. J. Food Prod. Dev. Packag. 2014, 1, 7–21. [Google Scholar]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological aspects of ozone application in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Graham, D.M. Use of ozone for food processing. Food Technol. 1997, 6, 72–75. [Google Scholar]
- Food and Drug Administration (FDA). Amendment of the Food Additive Regulations to Provide for the Safe Use of Ozone in Gaseous and Aqueous Phases as an Antimicrobial Agent on Food; FDA Federal Register 66 No.123; FDA: Boston, MA, USA, 2001; pp. 33829–33830.
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Gelman, A.; Sachs, O.; Khanin, Y.; Drabkin, V.; Glatman, L. Effect of ozone pretreatment on fish storage life at low temperature. J. Food Prot. 2005, 68, 778–784. [Google Scholar] [CrossRef]
- Campos, C.A.; Losada, V.; Rodriguez, O.; Aubourg, S.P.; Barros-Velazquez, J. Evaluation of an ozone slurry ice combined with refrigeration system for the storage of farmed turbot (Psetta maxima). Food Chem. 2006, 97, 223–230. [Google Scholar] [CrossRef]
- Campos, C.A.; Rodríguez, O.; Losada, V.; Aubourg, S.P.; Barros-Velázquez, J. Effects of storage in ozonised slurry ice on the sensory and microbial quality of sardine (Sardina Pilchardus). Int. J. Food Microbiol. 2005, 103, 121–130. [Google Scholar] [CrossRef]
- Sopher, C.D.; Battles, G.T.; Knueve, E.A. Ozone: Science & engineering. J. Int. Ozone Assoc. 2007, 29, 221–228. [Google Scholar]
- Dehkordi, B.M.; Zokaie, N. Extension of fish shelf life by ozone treatment. Int. J. Environ. Chem. Ecolog. Geolog. Geophys. Eng. 2010, 4, 108–110. [Google Scholar]
- Lu, F.; Liu, S.-L.; Liu, R.; Ding, Y.-C.; Ding, Y.-T. Combined effect of ozonized water pretreatment and ozonized flake ice on maintaining quality of Japanese sea bass (Lateolabrax japonicus). J. Aquat. Food Prod. Technol. 2012, 21, 160–180. [Google Scholar] [CrossRef]
- Pastoriza, L.; Bernárdez, M.; Sampedro, G.; Cabo, M.L.; Herrera, J.J.R. Use of sterile and ozonized water as a strategy to stabilize the quality of stored refrigerated fresh fish. Food Control 2008, 19, 772–780. [Google Scholar] [CrossRef]
- Chawla, A.S.; Bell, J.W.; Marlene, E.J. Optimization of ozonated water treatment of wild-caught and mechanically peeled shrimp meat. J. Aquat. Food Prod. Technol. 2007, 16, 41–56. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored Pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Crapo, C.; Himelbloom, B.; Vitt, S.; Pedersen, L. Ozone efficacy as a bactericide in seafood processing. J. Aquat. Food Prod. Technol. 2004, 13, 111–123. [Google Scholar] [CrossRef]
- Blogoslawski, W.J.; Stewart, M.E. Ozone: Science & engineering. J. Int. Ozone Assoc. 2011, 33, 368–373. [Google Scholar]
- Louppis, A.P.; Katikou, P.; Georgantelis, D.; Badeka, A.V.; Kontominas, M.G. Effect of ozonation and γ-irradiation on post-harvest decontamination of mussels (Mytillus galloprovincialis) containing diarrhetic shellfish toxins. Food Addit. Contam. Part A 2011, 28, 1735–1744. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. High-Dose Irradiation: Wholesomeness of Food Irradiated with Doses above 10 kGy; Report of a Joint FAO/IAEA/WHO Study Group; WHO Technical Report Series No. 890; WTO: Geneva, Switzerland, 1999. [Google Scholar]
- EFSA. Scientific opinion on the chemical safety of irradiation of food. EFSA J. 2011, 9, 1930. [Google Scholar] [CrossRef]
- FDA. Center for Food Safety and Applied Nutrition. Irradiated Food & Packaging—Food Irradiation: What You Need to Know. Available online: www.fda.gov (accessed on 14 April 2018).
- Munir, Μ.Τ.; Federighi, Μ. Control of foodborne biological hazards by ionizing radiations. Foods 2020, 9, 878. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–280. [Google Scholar]
- Roberts, P. Food irradiation: Standards, regulations, and world-wide trade. Radiat. Phys. Chem. 2016, 129, 30–34. [Google Scholar] [CrossRef]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World market development and consumer acceptance of irradiation technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Approves Irradiation for Shellfish to Prevent Food Poisoning Outbreaks; Food and Drug Administration: Washington, DC, USA, 11 April 2014.
- Chouliara, I.; Savvaidis, I.N.; Riganakos, K.A.; Kontominas, M.G. Shelf-life extension of vacuum-packaged sea bream (Sparus aurata) fillets by combined γ-irradiation and refrigeration: Microbiological, chemical and sensory changes. J. Sci. Food Agric. 2005, 85, 779–784. [Google Scholar] [CrossRef]
- Mendes, R.; Silva, E.A.; Nunes, M.L.; Empis, J.M.A. Effect of low-dose irradiation on the microflora, sensory, characteristics and biogenic amines of Atlantic horse mackerel (trachurus trachurus). Eur. Food Res. Technol. 2005, 221, 329–335. [Google Scholar] [CrossRef]
- Silva, H.A.; Mendes, R.; Nunes, M.L.; Empis, J. Protein changes after irradiation and ice storage of horse mackerel (Trachurus trachurus). Eur. Food Res. Technol. 2006, 224, 83–90. [Google Scholar] [CrossRef]
- Ozden, O.; Inugur, M.; Erkan, N. Preservation of iced refrigerated sea bream (Sparus aurata) by irradiation: Microbiological, chemical and sensory attributes. Eur. Food Res. Technol. 2007, 225, 797–805. [Google Scholar] [CrossRef]
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M.; Erikson, U.; Rustad, T. Effect of irradiation on properties and storage stability of Som-fug produced from bigeye snapper. Food Chem. 2007, 103, 274–286. [Google Scholar] [CrossRef]
- Mbarki, R.; Sadok, S.; Barkallah, I. Influence of gamma irradiation on microbiological, biochemical, and textural properties of bonito (Sarda sarda) during chilled storage. Food Sci. Technol. Int. 2008, 14. [Google Scholar] [CrossRef]
- Mbarki, R.; Sadok, S.; Barkallah, I. Quality changes of the Mediterranean horse mackerel (Trachurus mediterraneus) during chilled storage: The effect of low-dose gamma irradiation. Radiat. Phys. Chem. 2009, 78, 288–292. [Google Scholar] [CrossRef]
- Altan, C.O.; Turan, H. Synergistic effect of freezing and irradiation on bonito fish (Sarda sarda Bloch, 1793). J. Food Prot. 2016, 79, 2136–2142. [Google Scholar] [CrossRef]
- Ozden, O.; Inugur, M.; Erkan, N. Effect of different dose gamma radiation and refrigeration on the chemical and sensory properties and microbiological status of aquacultured sea bass (Dicentrarchus labrax). Radiat. Phys. Chem. 2007, 76, 1169–1178. [Google Scholar] [CrossRef]
- Lee, K.H.; Ahn, H.J.; Jo, C.; Yook, H.S.; Byun, M.W. Production of low salted and fermented shrimp by irradiation. J. Food Sci. 2002, 67, 1772–1777. [Google Scholar] [CrossRef]
- Sharma, S.K.; Basu, S.; Gholap, A.S. Effect of irradiation on the volatile compounds of shrimp (Solenocera choprii). J. Food Sci. Technol. 2007, 44, 267–271. [Google Scholar]
- Sinanoglou, V.J.; Batrinou, A.; Konteles, S.; Sflomos, K. Microbial population, pjysicochemical quality and allergenicity of mollusks and shrimp treated with cobalt-60 gamma radiation. J. Food Prot. 2007, 70, 958–966. [Google Scholar] [CrossRef]
- Kim, J.-H.; Seo, H.-J.; Kim, K.-S. Analysis of radiolytic products of lipid in irradiated dried squids (Todarodes pacificus). J. Food Prot. 2004, 67, 1731–1735. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chawla, S.P.; Chander, R.; Sharma, A. Development of shelf-stable, ready-to-eat shrimps (Penaeus indicus) using γ-radiation as one of the hurdles. LWT 2006, 39, 621–626. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23920, 399–411. [Google Scholar] [CrossRef]
- USFDA. Irradiation in the Production, Processing and Handling of Food; Federal Register/Vol. 79, No. 71/Monday, April 14, 2014/Rules and Regulations; USFDA: Boston, MA, USA, 2014; pp. 20771–22779.
- Monti, L. New process from Mississippi Company Helps Remove Bacteria from Oysters; Crystal Seas Seafood, LLC: Christian, MS, USA, 2014. [Google Scholar]
- Komolprasert, V. Packaging for foods treated by ionizing radiation. In Packaging for Non Thermal Processing of Food; Han, J.H., Ed.; IFT Press/Blackwell Publ.: Ames, IA, USA, 2007; pp. 87–116. [Google Scholar]
- Bauer, N.; Cook, V.; Disney, T.; Ebel, E.; Guo, C.; Johnston, J.; La Barre, D.; Lee, J.; McCoy, E.; Morrison, J.; et al. DRAFT Risk Assessment of the Potential Human Health Effect of Applying Continuous Inspection to Catfish; The Risk Assessment Division Office of Public Health Science Food Safety and Inspection Service, United States Department of Agriculture: Washington, DC, USA, 2010.
- FDA. Guidance for the Industry: Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Food Safety: Boston, MA, USA, 2011; pp. 245–292.
- Reed, C. Import Risk Analysis: Frozen, Skinless and Boneless Fillet Meat of Pangasius spp. Fish from Vietnam for Human Consumption; MAF Biosecurity: Wellington, New Zealand, 2008.
- FAO. Report of the FAO Expert Workshop on the Application of Biosecurity Measures to Control Salmonella Contamination. In Sustainable Aquaculture; Fisheries and Aquaculture Report No. 937 FIPM/R937; FAO: Mangalore, India, 2010. [Google Scholar]
- Dent, F. Fishery Industry Officer, Fish Products, Trade & Marketing Branch (FIPM): Global Outlook for Shrimp Markets and Demand. 2013. Available online: www.globefish.org (accessed on 19 April 2020).
- Norhana, M.N.W.; Poole, S.E.; Deeth, H.C.; Dykes, G.A. Prevalence, persistence and control of Salmonella and Listeria in shrimp and shrimp products: A review. Food Control 2010, 21, 343–361. [Google Scholar] [CrossRef]
- Pinu, F.R.; Yeasmin, S.; Bar, B.L.; Rahman, M.M. Microbiological conditions of frozen shrimp in different food markets of Dhaka City. Food Sci. Technol. Res. 2007, 13, 362–365. [Google Scholar] [CrossRef][Green Version]
- Asai, Y.; Kaneko, M.; Ohtsuka, K.; Morita, Y.; Kaneko, S.; Noda, H.; Furukawa, I.; Takatori, K.; Hara-Kudo, Y. Salmonella prevalence in seafood imported into Japan. J. Food Prot. 2008, 71, 1460–1464. [Google Scholar] [CrossRef]
- Olgunoglu, I.A. Salmonella in fish and fishery products. In Salmonella—A Dangerous Foodborne Pathogen; Barakat, S.M.M., Ed.; InTech: Rijaka, Croatia, 2012; Available online: http://www.intechopen.com/books/salmonella-a-dangerous-foodbornepathogen/salmonella-in-fish-and-fishery-products (accessed on 20 March 2020).
- EFSA: Scientific Report of EFSA and ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013 European Food Safety Authority, European Centre for Disease Prevention and Control European Food Safety Authority (EFSA), Parma, Italy, European Centre for Disease Prevention and Control (ECDC), Stockholm, Sweden. EFSA J. 2015, 13, 3991.
- Bakr Wafaa, M.K.; Hazzah Walaa, A.; Abaza Amani, F. Detection of Salmonella and Vibrio spp. in some seafood in Alexandria. J. Am. Sci. 2011, 7, 663–668. [Google Scholar]
- Brands, D.A.; Inman, A.E.; Gerba, C.P.; Maré, C.J.; Billington, S.J.; Saif, L.A.; Levine, J.F.; Joens, L.A. Prevalence of Salmonella spp. in oysters in the United States. Appl. Environ. Microbiol. 2005, 71, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Jakabi, M.; Gelli, D.S.; Torre, J.C.; Rodas, M.A.; Franco, B.D.; Destro, M.T.; Landgrafi, M. Inactivation by ionizing radiation of Salmonella Enteritidis, Salmonella Infantis, and Vibrio parahaemolyticus in oysters (Crassostrea brasiliana). J. Food Prot. 2003, 66, 1025–1029. [Google Scholar] [CrossRef]
- Sommers, C.H.; Rajkowski, K.T. Radiation inactivation of foodborne pathogens on frozen seafood products. J. Food Prot. 2011, 74, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Duan, J.; Morrissey, M.T. Electron beam irradiation for reducing Listeria monocytogenes contamination on cold-smoked salmon. J. Aquat. Food Prod. Technol. 2004, 13, 3–11. [Google Scholar] [CrossRef]
- Robertson, C.B.; Andrews, L.S.; Marshall, D.L.; Coggins, P.; Schilling, M.W.; Martin, R.E.; Collette, R. Effect of x-ray irradiation on reducing the risk of listeriosis in ready-to-eat vacuum-packaged smoked mullet. J. Food Prot. 2006, 69, 1561–1564. [Google Scholar] [CrossRef]
- Jo, C.; Lee, N.Y.; Kang, H.J.; Hong, S.P.; Kim, Y.H.; Kim, J.K.; Woo, M.; Byun, M.W. Inactivation of pathogens inoculated into prepared seafood products for manufacturing kimbab, steamed rice rolled in dried seaweed, by gamma irradiation. J. Food Prot. 2005, 68, 396–402. [Google Scholar] [CrossRef]
- Collins, M.V.; Flick, G.J.; Smith, S.A.; Fayer, R.; Rubendall, E.; Linday, D.S. The effects of e-beam irradiation and microwave energy on eastern oysters (Crassostrea virginica) experimentally infected with Cryptosporidium parvum. J. Eukaryot. Microbiol. 2005, 52, 484–488. [Google Scholar] [CrossRef]
- Klonowski, I.; Heinz, V.; Toepfl, S.; Gunnarsson, G.; Þorkelsson, G. Applications of Pulsed Electric Field Technology for the Food Industry; Icelandic Fisheries Laboratories Publ. Report 06: Reykjavík, Iceland, 2006. [Google Scholar]
- Nowosad, K.; Sujka, M.; Pankkiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Bastianello Campagnol, P.C.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef]
- Borderias, J.; Moreno, H.M. Recent advances in seafood technology: An overview. In Trends in Fish Processing Technologies; Borda, D., Nicolau, A.I., Raspor, P., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–25. [Google Scholar]
- Oziembłowski, M.; Kopeć, W. Pulsed electric fields (PEF) as an unconventional method of food preservation. Polish J. Food Nutr. Sci. 2005, 14, 31–35. [Google Scholar]
- Pourzaki, A.; Mirzaee, H. Pulsed electric field generators in food processing. In Proceedings of the 18th National Congress on Food Technology, Mashhad, Iran, 15–16 October 2008; pp. 1–7. [Google Scholar]
- Zhou, Y.; He, Q.; Zhou, D. Optimization extraction of protein from mussel by high-intensity pulsed electric fields. J. Food Process. Preserv. 2017, 41, e12962. [Google Scholar] [CrossRef]
- Zhou, Y.; Sui, S.; Huang, H.; He, G.; Wang, S.; Yin, Y.; Ma, Z. Process optimization for extraction of fishbone calcium assisted by high intensity pulsed electric fields. Trans. Chin. Soc. Agric. Eng. 2012, 28, 265–270. [Google Scholar]
- He, G.; Yin, Y.; Yan, X.; Yu, Q. Optimisation extraction of chondroitin sulfate from fish bone by high intensity pulsed electric fields. Food Chem. 2014, 164, 205–210. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Yin, Y.; Yan, X.; Wang, Y. Semi-bionic extraction of effective ingredient from fishbone by high intensity pulsed electric fields. J. Food Process. Eng. 2017, 40, e12392. [Google Scholar] [CrossRef]
- Li, M.; Lin, J.; Chen, J.; Fang, T. Pulsed electric field-assisted enzymatic extraction of protein from abalone (Haliotis Discus Hannai Ino) viscera. J. Food Process. Eng. 2016, 39, 702–710. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Farhoosh, R.; Javidnia, K.; Shahidi, F. Extraction of essential oils from Mentha piperita using advanced techniques: Microwave versus ohmic assisted hydrodistillation. Food Bioprod. Process. 2015, 94, 50–58. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.M.; Farid, M.M. Pulsed electric field treatment of red wine: Inactivation of Brettanomyces and potential hazard caused by metal ion dissolution. Innov. Food Sci. Emerg. Technol. 2019, 52, 57–65. [Google Scholar] [CrossRef]
- Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of pulsed electric fields for obtaining antioxidant extracts from fish residues. Antioxidants 2020, 9, 90. [Google Scholar] [CrossRef]
- Ravishankar, C.N.; Mohan, C.O.; Yathavamoorthi, R.; Srinivasa Gopal, T.K.; Shashidhar, K. Retort Pouch Processing of Fishery Products; ICAR-Central Institute of Fisheries Technology: Cochin, India, 2013. [Google Scholar]
- Bindu, J.; Mallick, A.K.; Gopal, T.K.S. Thermal processing of fishery products in flexible and rigid containers. Fish. Technol. 2014, 51, 137–148. [Google Scholar]
- Ninan, G. Fish Processing and Value Addition—A Global Scenario; Fish Processing Division, ICAR-Central Institute of Fisheries Technology: Cochin, India, 2018. [Google Scholar]
- Holdsworth, D.; Simpson, R. Introduction. In Thermal Processing of Packaged Foods, 2nd ed.; Holdsworth, D., Simpson, R., Eds.; Springer: New York, NY, USA, 2007; pp. 1–13. [Google Scholar]
- Featherstone, S. Retortable Flexible Containers for Food Packaging. In A Complete Course in Canning and Related Processes, 14th ed.; Featherstone, S., Ed.; Woodhead Publ.: Cambridge, UK, 2015; pp. 137–146. [Google Scholar]
- Simpson, R.; Almonacid, S.; Mitchell, M. Mathematical model development, experimental validation and process optimization: Retortable pouches packed with seafood in cone frustum shape. J. Food Eng. 2004, 63, 153–162. [Google Scholar] [CrossRef]
- Manju, S.; Sonaji, E.R.; Leema, J.; Srinivasa Gopal, T.K.; Ravishankar, C.N.; Vijayan, R.K. Heat penetration characteristics and shelf life studies of seer fish moilee packed in retort pouches. Fish. Technol. 2004, 41, 37–44. [Google Scholar]
- Ali, A.; Sudhir, B.; Gopal, T.K.S. Effect of heat processing on the texture profile of canned and retort pouch packed oil sardine (Sardinella longiceps) in oil medium. J. Food Sci. 2005, 70, S350–S354. [Google Scholar] [CrossRef]
- Kuda, T.; Fujita, M.; Goto, H.; Yano, T. Effects of retort conditions on ATP-related compounds in pouched fish muscle. LWT 2008, 41, 469–473. [Google Scholar] [CrossRef]
- Byun, Y.; Bae, H.; Cooksey, K.; Whiteside, S. Comparison of the quality and storage stability of salmon packaged in various retort pouches. LWT 2010, 43, 551–555. [Google Scholar] [CrossRef]
- Bindu, J.; Ravishankar, C.N.; Srinivasa Gopal, T.K.; Mallick, A.K. Investigation of shelf life and heat penetration attributes of ready-to-eat “fish peera” from anchovy (Stolephorous commersoni) in retort pouches. J. Food Process. Preserv. 2010, 34, 207–222. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Dhar, B.; Roy, D.; Saha, A. Optimization of process conditions for Rohu fish in curry medium in retortable pouches using instrumental and sensory characteristics. J. Food Sci. Technol. 2015, 52, 5671–5680. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Dhar, B.; Saha, A.; Parhi, J.; Sanjit, A.; Singh, A.S. Evaluation of textural quality as a parameter to optimize thermal process during retort pouch processing of boneless rohu balls in curry medium. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Bindu, J.; Srinivasa Gopal, T.K.; Unnikrishnan Nair, T.S.U. Ready-to-eat mussel processed in retort pouches for the retail and export market. Packag. Technol. Sci. 2004, 17, 113–117. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Gopal, T.K.S.; Bindu, J. Thermal processing of prawn ‘kuruma’ in retortable pouches and alluminium cans. Int. J. Food Sci. Technol. 2008, 43, 200–207. [Google Scholar] [CrossRef]
- Mallick, A.K.; Srinivasa Gopal, T.K.S.; Ravishankar, C.N.; Geethalakshmi, V. Changes in instrumental and sensory properties of Indian white shrimp in curry medium during retort pouch processing at different F0 values. J. Texture Stud. 2010, 41, 611–632. [Google Scholar] [CrossRef]
- Tribuzi, G.; Schmidt, F.C.; Laurindo, J.B. Operational diagrams for salting-marination processes and quality of cooked mussels. LWT 2014, 59, 746–753. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Deepayan, R.; Saha, A. Textural and sensory characteristics of retort-processed freshwater prawn (Macrobrachium rosenbergii) in curry medium. Int. J. Food Propert. 2017, 20, 2487–2498. [Google Scholar] [CrossRef]
- Sreelakshmi, K.R.; Manjusha, L.; Nagalakshmi, K.; Chouksey, M.K.; Venkateshwarlu, G. Ready-to-serve-crab sandwich spread in retort pouch: Product development and process optimization. J. Aquat. Food Prod. Technol. 2013, 24, 315–329. [Google Scholar]
- Bindu, J.; Ravishankar, C.; Srinivasa Gopal, T. Shelf life evaluation of a ready-to-eat black clam (Villorita cyprinoides) product in indigenous retort pouches. J. Food Eng. 2007, 78, 995–1000. [Google Scholar] [CrossRef]


| Seafood | Moisture | Carbohydrates | Proteins | Fat | Ash |
|---|---|---|---|---|---|
| Bony fish | |||||
| Bluefish | 74.6 | 0 | 20.5 | 4.0 | 1.2 |
| Cod | 82.6 | 0 | 16.5 | 0.4 | 1.2 |
| Haddock | 80.7 | 0 | 18.2 | 0.1 | 1.4 |
| Atlantic halibut | 75.4 | 0 | 18.6 | 5.2 | 1.0 |
| Atlantic herring | 67.2 | 0 | 18.3 | 12.5 | 2.7 |
| Atlantic mackerel | 68.1 | 0 | 18.7 | 12.0 | 1.2 |
| Pacific salmon | 63.4 | 0 | 17.4 | 16.5 | 1.0 |
| Swordfish | 75.8 | 0 | 19.2 | 4.0 | 1.3 |
| Crustaceans | |||||
| Crab | 80.0 | 0.6 | 16.1 | 1.6 | 1.7 |
| Lobster | 79.2 | 0.5 | 16.2 | 1.9 | 2.2 |
| Shrimp | 72.5 | 0.9 | 20.5 | 5.5 | 0.8 |
| Crayfish | 80.0 | 0.5 | 17.0 | 1.5 | 0.9 |
| Mollusks | |||||
| Clams, meat | 80.3 | 3.4 | 12.8 | 1.4 | 2.1 |
| Oysters | 80.5 | 5.6 | 9.8 | 2.1 | 2.0 |
| Scallops | 80.3 | 3.4 | 14.8 | 0.1 | 1.4 |
| Squid/mantle | 83.5 | 1.4 | 13.5 | 0.8 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Innovative Seafood Preservation Technologies: Recent Developments. Animals 2021, 11, 92. https://doi.org/10.3390/ani11010092
Kontominas MG, Badeka AV, Kosma IS, Nathanailides CI. Innovative Seafood Preservation Technologies: Recent Developments. Animals. 2021; 11(1):92. https://doi.org/10.3390/ani11010092
Chicago/Turabian StyleKontominas, Michael G., Anastasia V. Badeka, Ioanna S. Kosma, and Cosmas I. Nathanailides. 2021. "Innovative Seafood Preservation Technologies: Recent Developments" Animals 11, no. 1: 92. https://doi.org/10.3390/ani11010092
APA StyleKontominas, M. G., Badeka, A. V., Kosma, I. S., & Nathanailides, C. I. (2021). Innovative Seafood Preservation Technologies: Recent Developments. Animals, 11(1), 92. https://doi.org/10.3390/ani11010092







