Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Chemical and Reagents
2.3. Culture Media Used for Bacterial Growth
2.4. Instrumentation and Equipment
2.5. Experimental Animal Design
2.6. Egg Productivity
2.7. Egg Quality
2.8. Microbiological Determinations
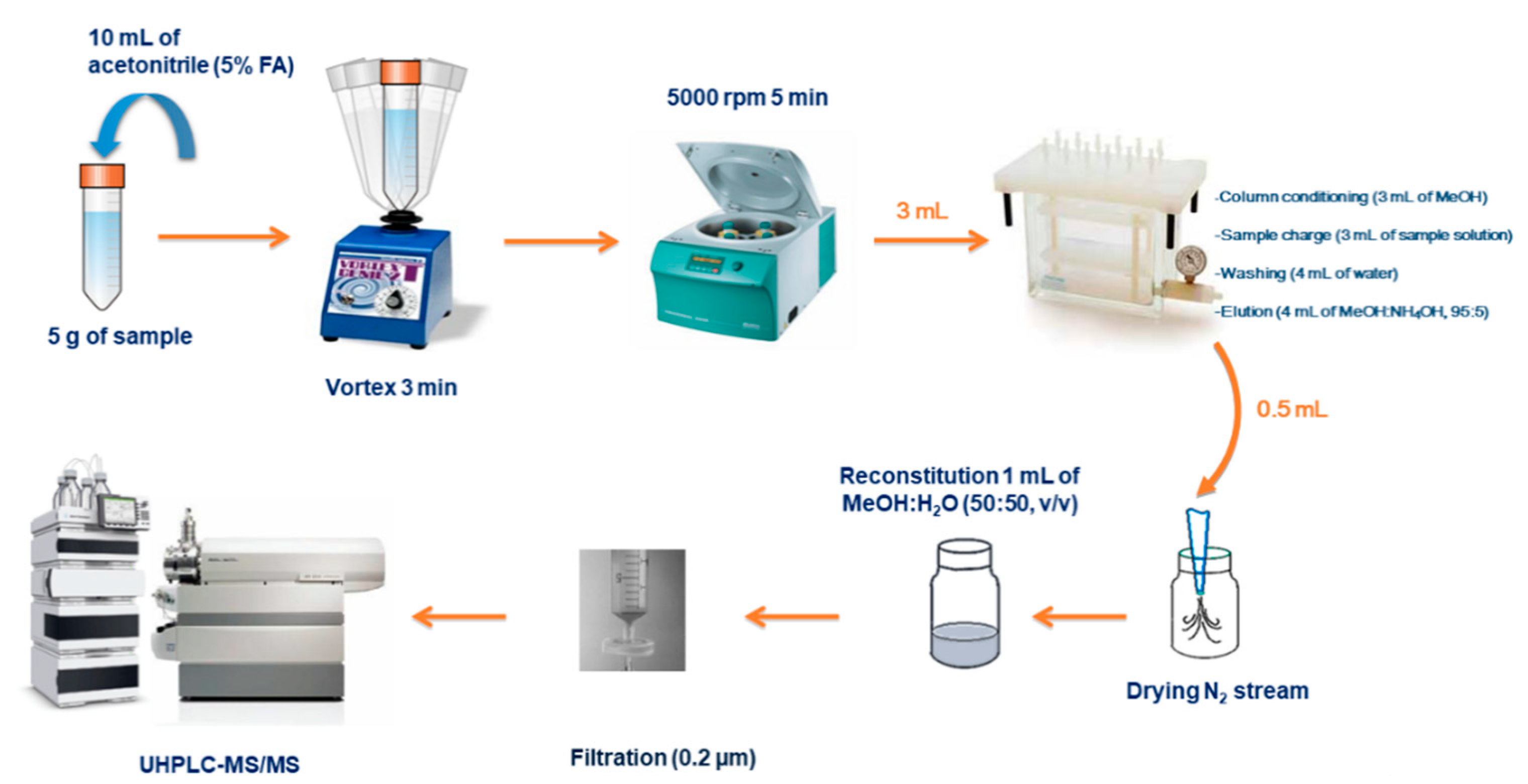
2.9. Procedure for the Monitoring of PTSO Residues in Egg Samples
2.10. Statistical Analysis
3. Results and Discussion
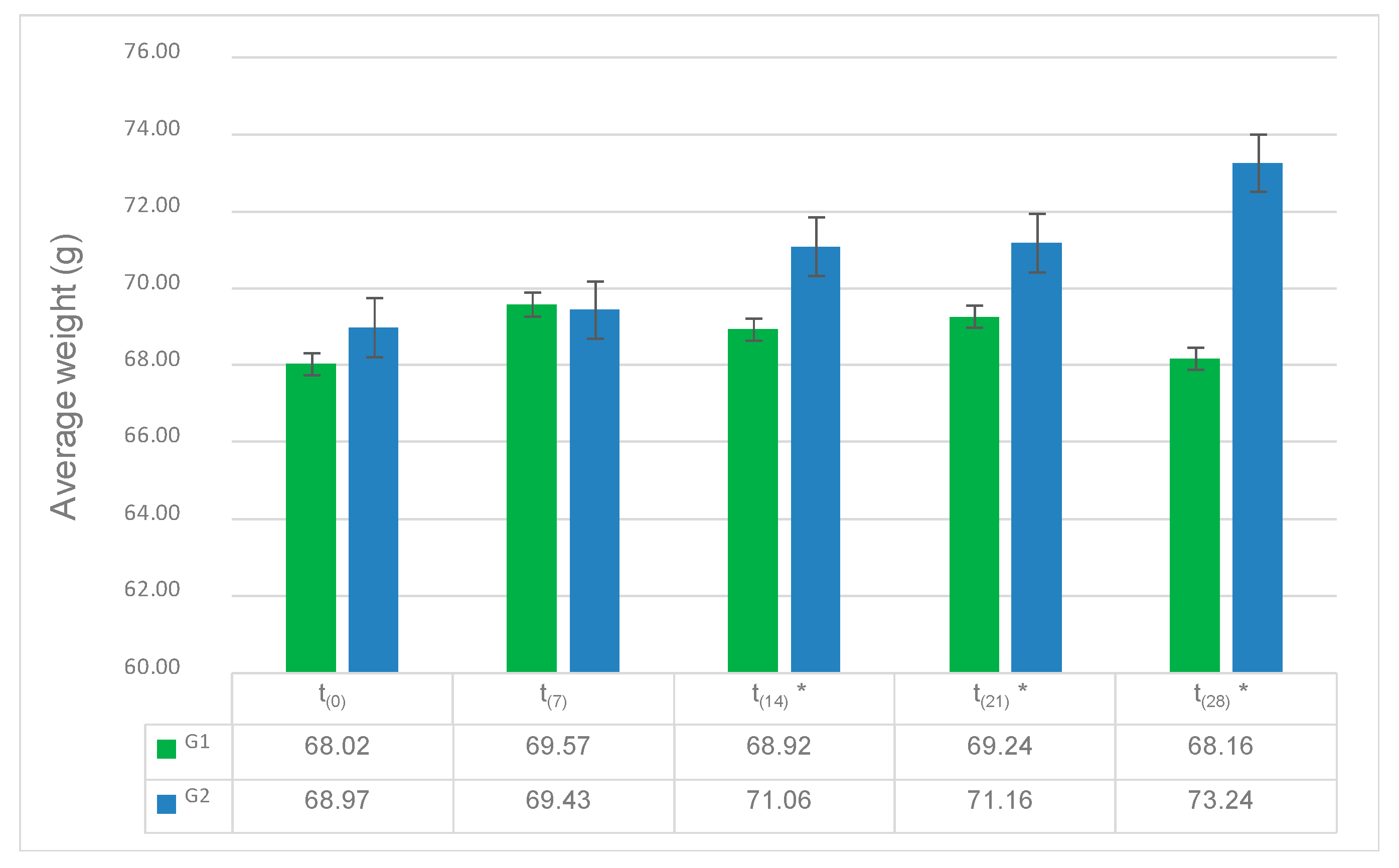
3.1. Influence of Allium Extract on Egg Productivity
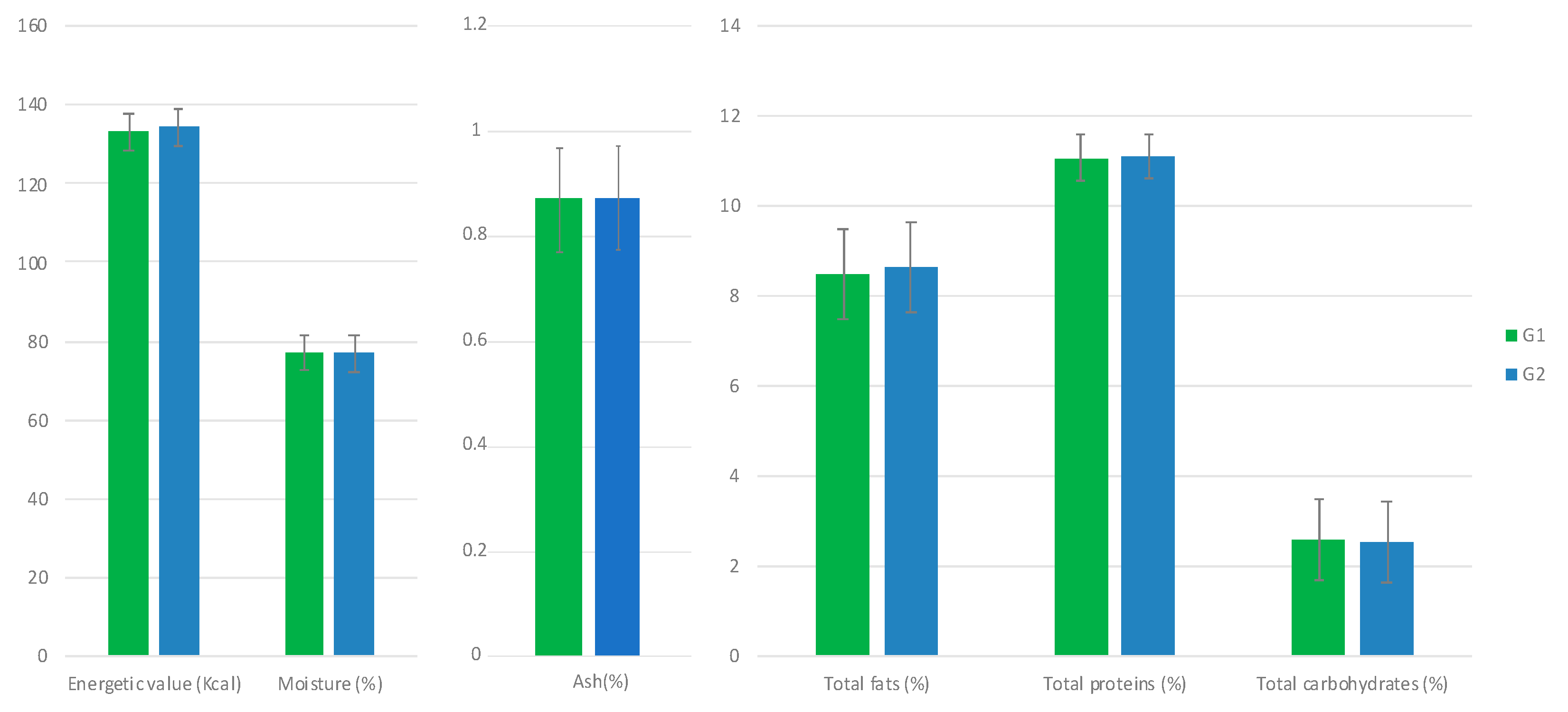
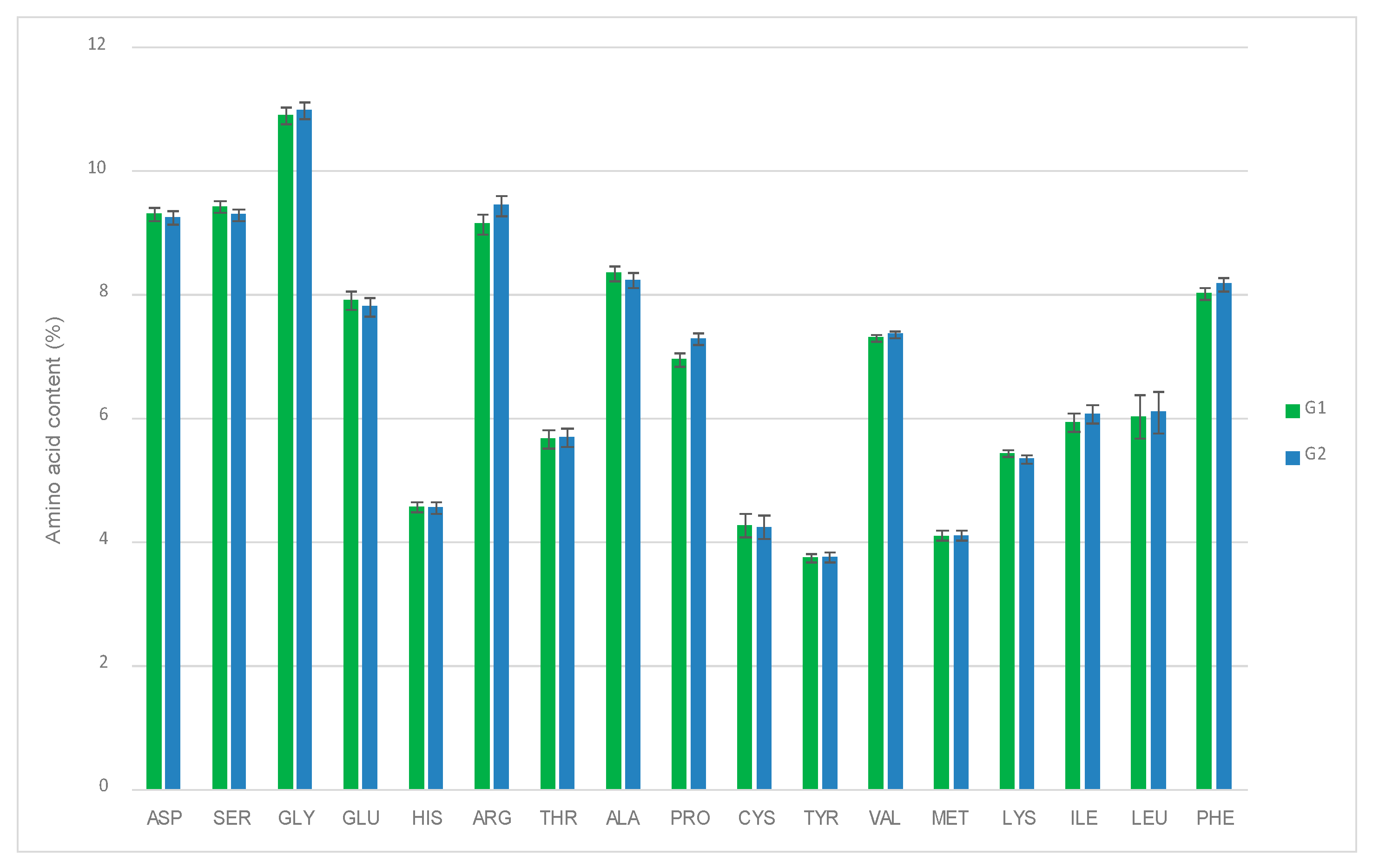
3.2. Influence of Allium Extract on Egg Quality
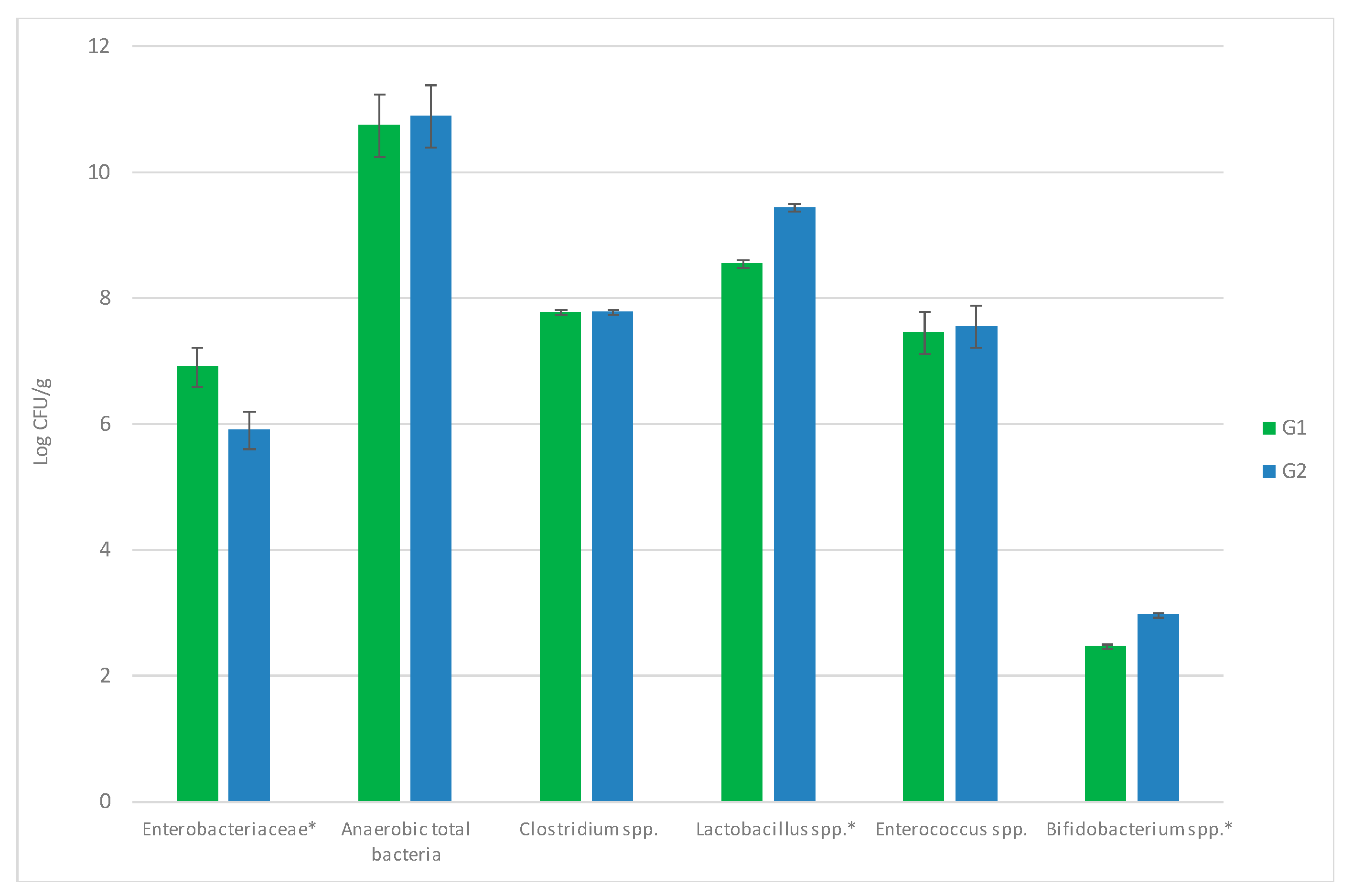
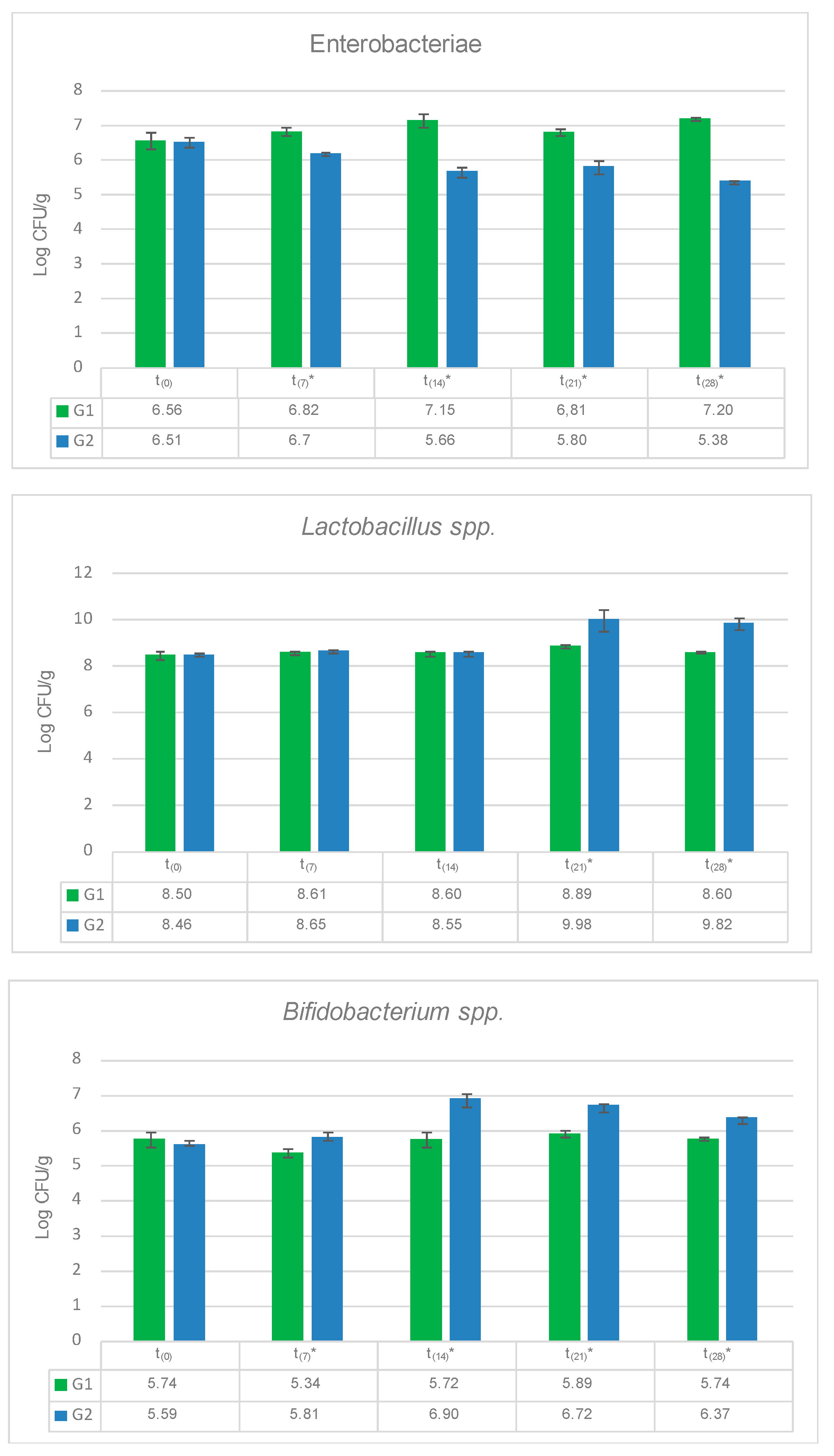
3.3. Influence of Allium Extract on Bacterial Populations in Gut
3.4. Influence of Allium Extract in Egg Biochemical Composition: Analysis of PTSO Residues in Egg Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commision. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, L268, 29–43. [Google Scholar]
- Sojoudi, M.R.; Dadashbeiki, M.; Bouyeh, M. Effects of different levels of symbiotic Technomos on broilers performance. Res. Opin. Anim. Vet. Sci. 2012, 2, 243–248. [Google Scholar]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.-D.; Niu, K.-M.; Kim, S.-K. The Genus Allium as Poultry Feed Additive: A Review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef]
- Cervantes, H.M. Antibiotic-free poultry production: Is it sustainable? J. Appl. Poult. Res. 2015, 24, 91–97. [Google Scholar] [CrossRef]
- Puvača, N.; Stanaćev, V.; Glamočić, D.; Lević, J.; Perić, L.; Milić, D. Beneficial effects of phytoadditives in broiler nutrition. World’s Poult. Sci. J. 2013, 69, 27–34. [Google Scholar] [CrossRef]
- Greathead, H. Plants and plant extracts for improving animal productivity. Proc. Nutr. Soc. 2003, 62, 279–290. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Cardozo, P.W.; Ferret, A.; Bach, A. Changes in rumen microbial fermentation are due to a combined effect of type of diet and pH. J. Anim. Sci. 2008, 86, 702–711. [Google Scholar] [CrossRef]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or yucca schidigera saponin extracts. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef]
- Hernández, F.; Madrid, J.; Garcia, V.; Orengo, J.; Megias, M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004, 83, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Brit. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lillehoj, H.S.; Hong, Y.H.; Jang, S.I.; Lillehoj, E.P.; Ionescue, C.; Mazuranok, L.; Bravo, D. In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Brit. Poult. Sci. 2010, 51, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Chemistry in a Salad Bowl: Allium Chemistry and Biochemistry. In Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry (RSC): Cambridge, UK, 2010; pp. 100–223. [Google Scholar]
- Augusti, K.T.; Mathew, P.T. Lipid lowering effect of allicin (diallyldisulphide-oxide) on long term feeding to normal rats. Experientia 1974, 30, 468–470. [Google Scholar] [CrossRef]
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef]
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.; Huo, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Kovačevića, D.B. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Use of Dialkyl-Thiosulfinate and/or Thiosulfonate to Improve the Resistance of an Animal Infected by a Pathogen. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013124441 (accessed on 21 December 2020).
- Ruiz, R.; García, M.; Lara, A.; Rubio, L.A. Garlic derivatives (PTS and PTS-O) differently affect the ecology of swine faecal microbiota in vitro. Vet. Microbiol. 2010, 29, 110–117. [Google Scholar] [CrossRef]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Rubio, L.A. Garlic derivative PTS-O is effective against broiler pathogens in vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef]
- Peinado, M.J.; Ruiz, R.; Echavarri, A.; Aranda-Olmedo, I.; Rubio, L.A. Garlic derivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed Sci. Tech. 2013, 181, 87–92. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; García-Campaña, A.M. A rapid and simple UHPLC-ESI-MS/MS method for the screening of propyl propane thiosulfonate, a new additive for animal feed. Anal. Methods 2016, 8, 3730–3739. [Google Scholar] [CrossRef]
- ROYAL DECREE 348/2000, of March 10, of the Ministry of Agriculture, Fisheries and Food, Which Incorporates Directive 98/58/CE, on the Protection of Animals on Livestock Farms. Available online: https://www.boe.es/buscar/pdf/2000/BOE-A-2000-4698-consolidado.pdf (accessed on 16 November 2020).
- Beerens, H. An elective and selective isolation medium for Bifidobacterium spp. Lett. Appl. Microbiol. 1990, 11, 155–157. [Google Scholar] [CrossRef]
- Hartemink, R.; Domenech, V.R.; Rombouts, F.M. LAMVAB—A new selective medium for the isolation of lactobacilli from feces. J. Microbiol. Methods 1997, 27, 77–84. [Google Scholar] [CrossRef]
- Abad, P.; Lara, F.J.; Arroyo-Manzanares, N.; Baños, A.; Guillamón, E.; García-Campaña, A.M. High-Performance liquid chromatography method for the monitoring of the Allium derivative propyl propane thiosulfonate used as natural additive in animal feed. Food Anal. Methods 2015, 8, 916–921. [Google Scholar] [CrossRef]
- Manju, G.U.; Reddy, B.S.V.; Gloridoss, G.; Prabhu, T.M.; Giridhar, K.S.; Suma, N. Effect of supplementation of lysine producing microbes vis-a-vis source and level of dietary protein on performance and egg quality characteristics of post-peak layers. Vet. World 2015, 8, 453–460. [Google Scholar] [CrossRef][Green Version]
- Yamane, H.; Kurauchi, I.; Denbow, D.M.; Furuse, M. Central functions of amino acids for the stress response in chicks. Asian-Austr. J. Anim. Sci. 2009, 22, 296–304. [Google Scholar] [CrossRef]
- Keshavarz, K.; Nakajima, S. The effect of dietary manipulations of energy, protein and fat during the growing and laying periods on early egg weight and egg components. Poult. Sci. 1995, 74, 50–61. [Google Scholar] [CrossRef]
- Zimmerman, R.A. Management of egg size through precise nutrient delivery. J. Applied Poult. Res. 1997, 6, 478–482. [Google Scholar] [CrossRef]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Baños, A.; Guillamón, E.; García, F.; Rodríguez-Nogales, A.; Gálvez, J. The Immunomodulatory properties of propyl-propane thiosulfonate contribute to its intestinal anti-inflammatory effect in experimental colitis. Mol. Nutr. Food. 2019, 63, 5. [Google Scholar] [CrossRef]
- Clark, C.E.F.; Akter, Y.; Hungerford, A.; Thomson, P.; Islam, M.R.; Groves, P.J.; O’She, C.J. The intake pattern and feed preference of layer hens selected for high or low feed conversion ratio. PLoS ONE 2019, 14, e0222304. [Google Scholar] [CrossRef] [PubMed]
- Official Methods of Analysis of AOAC International. 2015. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1755 (accessed on 3 September 2020).
- Bradley, R.L. Moisture and total solids analysis. In Compositional Analysis of Food; Nielsen, S.S., Ed.; Purdue University: West Lafayette, IN, USA, 2010; pp. 87–104. [Google Scholar]
- Marshall, M.R. Ash analysis. In Compositional Analysis of Food; Nielsen, S.S., Ed.; Purdue University: West Lafayette, IN, USA, 2010; pp. 107–114. [Google Scholar]
- Owusu-Apenten, R.K. Kjeldahl method, quantitative amino acids and combustion analysis. In Food Protein Analysis: Quantitative Effects On Processing; Pennsylvania University: Pennsylvania, PA, USA, 2002; Volume 18, pp. 1–45. [Google Scholar]
- Chang, S.K. Protein analysis. In Compositional Analysis of Food; Nielsen, S.S., Ed.; Purdue University: West Lafayette, IN, USA, 2010; pp. 135–146. [Google Scholar]
- BeMiller, J. Carbohydrate analysis. In Compositional Analysis of Food; Nielsen, S.S., Ed.; Purdue University: West Lafayette, IN, USA, 2010; pp. 149–178. [Google Scholar]
- Merril, A.L.; Watt, B.K. Energy value of food. In Agriculture Handbook Nº 74; Agricultural Research Service, United States Department of Agriculture: Washington, DC, USA, 1973. [Google Scholar]
- Waters Corporation. Analysing Feed Hydrolysate Samples Using the AccQ•Tag™ Method. Available online: http://www.waters.com/webassets/cms/library/docs/4acqtag.pdf (accessed on 3 September 2020).
- Abad, P.; Arroyo-Manzanares, N.; García-Campaña, A.M. Use of onion extract as a dairy cattle feed supplement: Monitoring propyl propane thiosulfonate as a marker of its effect on milk attributes. J. Agric. Food Chem. 2017, 65, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Food and Environment (Spain). 2017. Available online: http://www.huevo.org.es/huevo_salud_composicion_aminoacidos.asp (accessed on 3 September 2020).
- Gabriel, I.; Mallet, S.; Sibille, P. La microflore digestive des volailles: Facteurs de variation et conséquences pour l’animal. INRA. Prod. Anim. 2005, 18, 309–322. [Google Scholar]
- Adil, S.; Magray, S.M. Impact and manipulation of gut microflora in poultry: A Review. J. Anim. Vet. Adv. 2012, 11, 873–877. [Google Scholar] [CrossRef]
- Chambers, J.J.; Gong, J. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 2011, 44, 3149–3159. [Google Scholar] [CrossRef]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 4, 894–907. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Sánchez, C.J.; Martínez-Miró, S.; Ariza, J.J.; Madrid, J.; Orengo, J.; Aguinaga, M.A.; Baños, A.; Hernández, F. Effect of Alliaceae extract supplementation on performance and intestinal microbiota of growing-finishing pig. Animals 2020, 10, 1557. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, 13. [Google Scholar] [CrossRef]
| Ingredients (in Descending Order): Corn, Roasted Soybean Flour, Wheat, Barley, Fatty Acids, Sunflower Seed Flour, Dicalcium Phosphate, Sodium Chloride, Sodium Bicarbonate. | ||
|---|---|---|
| Additives | G1 | G2 |
| PTSO (mg kg−1) | -- | 30 |
| Trace element/trace element compounds (mg kg−1) | ||
| Manganese (manganese oxide) | 65.0 | 64.4 |
| Zinc (zinc oxide) | 37.0 | 36.6 |
| Copper (cupric sulfate pentahydrate) | 4.0 | 3.9 |
| Iron (ferrous carbonate) | 18.0 | 17.8 |
| Iodine (potassium iodide) | 1.9 | 1.9 |
| Cobalt (basic cobalt carbonate monohydrate) | 0.2 | 0.2 |
| Selenium (sodium selenite) | 0.1 | 0.1 |
| Vitamins (IU kg−1) | ||
| Vitamin A, E-672 | 7.5 | 7.4 |
| Vitamin D3, E-671 | 1.50 | 1.48 |
| Antioxidants (mg kg−1) | ||
| Butyldidroxytoluene (BHT), E-321 | 0.253 | 0.250 |
| Ethoxyquin, E-324 | 0.0369 | 0.0365 |
| Butyldidroxyanisole (BHA), E-320 | 0.023 | 0.023 |
| Digestives | ||
| Beta-glucanase endo-1,3 (4) EC 3.2.1.6 (U/kg) | 100 | 99 |
| Beta-xylanase endo-1,4 EC 3.2.1.8 (U/kg) | 70 | 69 |
| 4a1640 phytase EC 3.1.3.26 (FTU/kg) | 300 | 297 |
| Colorants (U/kg) | ||
| Canthaxanthin, E-161 | 3.0 | 3.0 |
| Beta apocarotenoic acid ethyl ester, E160f | 1.6 | 1.6 |
| Anti-caking agent (mg kg−1) | ||
| Sepiolite, E562 | 600 | 1560 |
| Amino acids, their salts and analogs (mg kg−1) | ||
| Methionine (DL-methionine) | 1.837 | 1.819 |
| Nutrients (%) | ||
| Crude protein | 16.0 | 16.0 |
| Crude fiber | 3.9 | 3.8 |
| Oils and crude oils and fats | 3.5 | 3.5 |
| Crude ash | 13.0 | 12.9 |
| Calcium | 3.9 | 3.9 |
| Phosphorous | 0.60 | 0.59 |
| Sodium | 0.20 | 0.20 |
| Methionine | 0.46 | 0.46 |
| Digestive Lysine | 0.97 | 0.96 |
| Carbohydrates | 57.6 | 57.0 |
| Growth Media | Bacterial Group | T (°C) | Culture Conditions |
|---|---|---|---|
| MacConkey agar | Enterobacteriaceae | 35 ± 1 °C | 18–24 h aerobic atmosphere |
| Wilkins-Chalgren | Total anaerobic bacteria | 35 ± 1 °C | 24–48 h anaerobic atmosphere |
| Sulfite-Polymyxin-Sulfadiazine agar | Clostridium spp. | 35 ± 1 °C | 24 h anaerobic atmosphere |
| LAMVAB | Lactobacillus spp. | 35 ± 1 °C | 72–120 h aerobic atmosphere |
| Slanetz Bartley agar | Enterococcus spp. | 37 ± 1 °C | 24–48 h aerobic atmosphere |
| Modified columbia agar | Total bifidobacteria | 37 ± 1 °C | 48–72 h anaerobic atmosphere |
| Compound | Retention Time (Min) | Precursor Ion (m/z) | Molecular Ion | DP 1 | EP 1 | CEP 1 | Product Ions 2 | CE | CXP 1 |
|---|---|---|---|---|---|---|---|---|---|
| CSSP | 1.7 | 196.0 | [M + H]+ | 36.0 | 6.0 | 12.0 | 107.0 (Q) | 11.0 | 4.0 |
| 179.0 (I) | 17.0 | ||||||||
| GSSP | 1.9 | 382.0 | [M + H]+ | 31.0 | 9.5 | 38.0 | 130.0 (Q) | 24.0 | 4.0 |
| 150.0 (I) | 27.0 | ||||||||
| PTSO | 4.0 | 183.0 | [M + H]+ | 21.0 | 10.5 | 10.0 | 141.1 (Q) | 13.0 | 4.0 |
| 99.0 (I) | 19.0 |
| Parameter | G1 | G2 | p |
|---|---|---|---|
| Feed intake (g per day) | 154 | 150 | >0.05 |
| Hens laying percentage (%) | 91 | 97.3 | >0.05 |
| Egg mass (g per day) | 62.79 | 69.29 | <0.05 |
| Feed conversion ratio | 2.45 | 2.16 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abad, P.; Arroyo-Manzanares, N.; Ariza, J.J.; Baños, A.; García-Campaña, A.M. Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens. Animals 2021, 11, 41. https://doi.org/10.3390/ani11010041
Abad P, Arroyo-Manzanares N, Ariza JJ, Baños A, García-Campaña AM. Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens. Animals. 2021; 11(1):41. https://doi.org/10.3390/ani11010041
Chicago/Turabian StyleAbad, Paloma, Natalia Arroyo-Manzanares, Juan J. Ariza, Alberto Baños, and Ana M. García-Campaña. 2021. "Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens" Animals 11, no. 1: 41. https://doi.org/10.3390/ani11010041
APA StyleAbad, P., Arroyo-Manzanares, N., Ariza, J. J., Baños, A., & García-Campaña, A. M. (2021). Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens. Animals, 11(1), 41. https://doi.org/10.3390/ani11010041





