Zinc Supplementation Forms Influenced Zinc Absorption and Accumulation in Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Experimental Design
2.2. Housing and Sample Collection
2.3. Chemical Composition of Feed Analysis
2.4. Mineral Concentration Analysis
2.5. Apparent Digestibility of Zn
2.6. Activity of Zinc Containing Enzymes
2.7. Real-Time Quantitative PCR Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of Zinc Supplementation Forms on Growth Performance and Apparent Digestibility of Zn
3.2. Effect of Zinc Supplementation Forms on Tissue Mineral Concentrations
3.3. Effect of Zn Supplementation Forms on Zn-Containing Enzymes in Duodenum, Jejunum, Ileum and Liver
3.4. Effect of Zn Supplementation Forms on MT mRNA and Protein Expressions in the Duodenum, Jejunum, Ileum and Liver
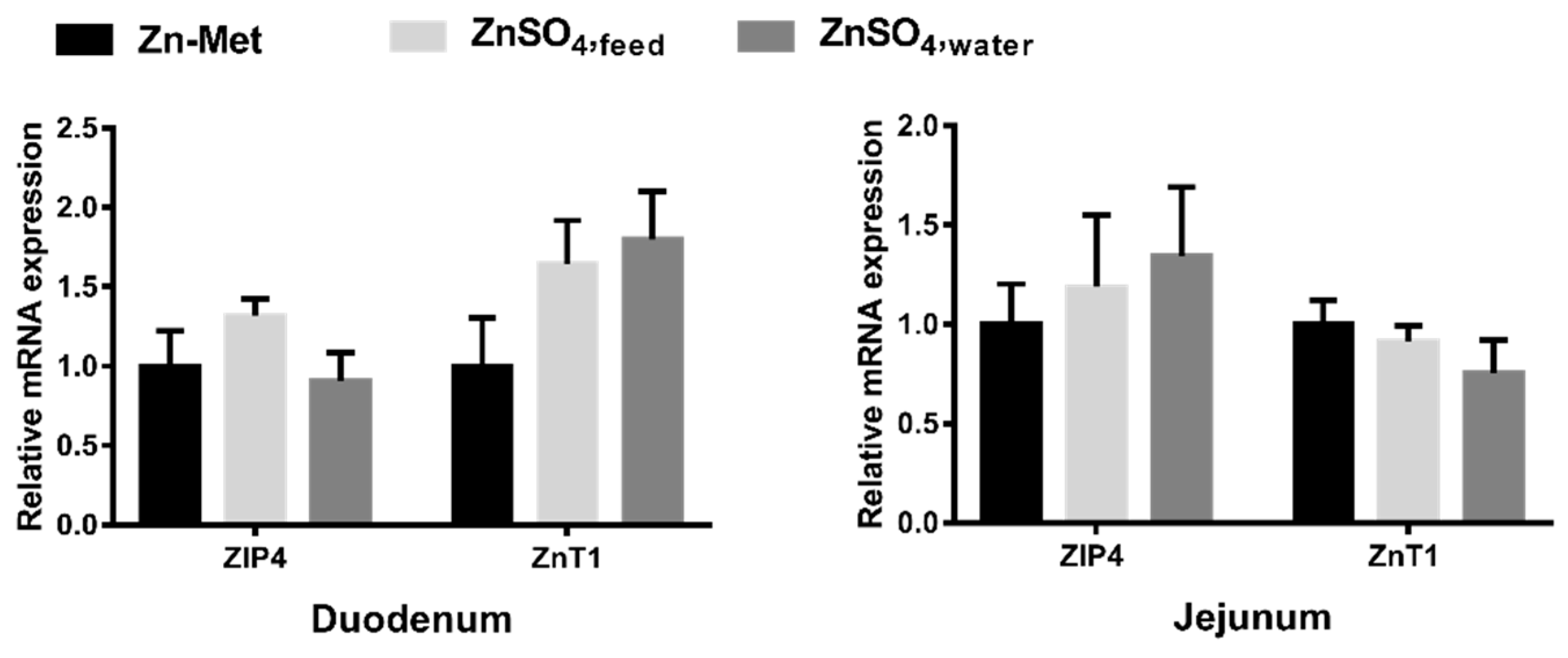
3.5. Effect of Zn Supplementation Forms on ZIP4 and ZnT1 mRNA Expressions in Duodenum and Jejunum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef]
- Brugger, D.; Windisch, W.M. Short-Term Subclinical Zinc Deficiency in Weaned Piglets Affects Cardiac Redox Metabolism and Zinc Concentration. J. Nutr. 2017, 147, 521–527. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Romeo, J.; Malavolta, M.; Costarelli, L.; Giacconi, R.; Diaz, L.E.; Marcos, A. Zinc: Dietary intake and impact of supplementation on immune function in elderly. Age (Dordr.) 2013, 35, 839–860. [Google Scholar] [CrossRef]
- Brugger, D.; Windisch, W.M. Subclinical zinc deficiency impairs pancreatic digestive enzyme activity and digestive capacity of weaned piglets. Br. J. Nutr. 2016, 116, 425–433. [Google Scholar] [CrossRef]
- Pieper, R.; Martin, L.; Schunter, N.; Villodre Tudela, C.; Weise, C.; Klopfleisch, R.; Zentek, J.; Einspanier, R.; Bondzio, A. Impact of high dietary zinc on zinc accumulation, enzyme activity and proteomic profiles in the pancreas of piglets. J. Trace Elem. Med. Biol. 2015, 30, 30–36. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, J.; Fanning, S.; Wang, L.; Li, M.; Maheshwari, N.; Sun, J.; Li, F. Effects of metal and metalloid pollutants on the microbiota composition of feces obtained from twelve commercial pig farms across China. Sci. Total Environ. 2019, 647, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ward, T.L.; Ji, F.; Peng, C.; Zhu, L.; Gong, L.; Dong, B. Effects of zinc sources and levels of zinc amino acid complex on growth performance, hematological and biochemical parameters in weanling pigs. Asian Australas. J. Anim. Sci. 2018, 31, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Olukosi, O.A.; van Kuijk, S.; Han, Y. Copper and zinc sources and levels of zinc inclusion influence growth performance, tissue trace mineral content, and carcass yield of broiler chickens. Poult. Sci. 2018, 97, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, Q.; Wang, L.; Wang, Y.; Cheng, Z.; Yang, Z.; Yang, W. The Effects of Partially or Completely Substituted Dietary Zinc Sulfate by Lower Levels of Zinc Methionine on Growth Performance, Apparent Total Tract Digestibility, Immune Function, and Visceral Indices in Weaned Piglets. Animals (Basel) 2019, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Zhou, W.; Feng, J.; Zou, X. Effects of zinc methionine supplementation on laying performance, zinc status, intestinal morphology, and expressions of zinc transporters’ mRNA in laying hens. J. Sci. Food Agric. 2019, 99, 6582–6588. [Google Scholar] [CrossRef]
- Kaewtapee, C.; Krutthai, N.; Poosuwan, K.; Poeikhampha, T.; Koonawootrittriron, S.; Bunchasak, C. Effects of adding liquid DL-methionine hydroxy analogue-free acid to drinking water on growth performance and small intestinal morphology of nursery pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2010, 94, 395–404. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bai, M.; Liu, H.; Xu, K.; Yu, R.; Oso, A.O.; Deng, J.; Yin, Y. Effects of coated cysteamine hydrochloride on muscle fiber characteristics and amino acid composition of finishing pigs. Asian Australas. J. Anim. Sci. 2019, 32, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Chen, Y.P.; Li, X.H.; Yang, W.L.; Wen, C.; Zhou, Y.M. Effects of Palygorskite Inclusion on the Growth Performance, Meat Quality, Antioxidant Ability, and Mineral Element Content of Broilers. Biol. Trace Elem. Res. 2016, 173, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shin, S.; Kuehn, I.; Bedford, M.; Rodehutscord, M.; Adeola, O.; Ajuwon, K.M. Effect of phytase on nutrient digestibility and expression of intestinal tight junction and nutrient transporter genes in pigs. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Falcone, E.L.; Abusleme, L.; Swamydas, M.; Lionakis, M.S.; Ding, L.; Hsu, A.P.; Zelazny, A.M.; Moutsopoulos, N.M.; Kuhns, D.B.; Deming, C.; et al. Colitis susceptibility in p47(phox-/-) mice is mediated by the microbiome. Microbiome 2016, 4, 13. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Zhu, X.; Han, H.; Ren, W.; Chen, S.; Bin, P.; Liu, G.; Huang, X.; Fang, R.; et al. Effects of Long-Term Protein Restriction on Meat Quality, Muscle Amino Acids, and Amino Acid Transporters in Pigs. J. Agric. food Chem. 2017, 65, 9297–9304. [Google Scholar] [CrossRef]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.; Smith, D.P.; Nelson, A.; Abdulkadir, B.; Skeath, T.; Petrosino, J.F.; Perry, J.D.; Berrington, J.E.; et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 2016, 4, 67. [Google Scholar] [CrossRef]
- Mallaki, M.; Norouzian, M.A.; Khadem, A.A. Effect of organic zinc supplementation on growth, nutrient utilization, and plasma zinc status in lambs. Turk. J. Vet. Anim. Sci. 2015, 39, 75–80. [Google Scholar] [CrossRef]
- Schlegel, P.; Sauvant, D.; Jondreville, C. Bioavailability of zinc sources and their interaction with phytates in broilers and piglets. Animal 2013, 7, 47–59. [Google Scholar] [CrossRef]
- Behjatian Esfahani, M.; Moravej, H.; Ghaffarzadeh, M.; Nehzati Paghaleh, G.A. Comparison the Zn-Threonine, Zn-Methionine, and Zn Oxide on Performance, Egg Quality, Zn Bioavailability, and Zn Content in Egg and Excreta of Laying Hens. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef]
- Ren, P.; Chen, J.; Wedekind, K.; Hancock, D.; Vázquez-Añón, M. Interactive effects of zinc and copper sources and phytase on growth performance, mineral digestibility, bone mineral concentrations, oxidative status, and gut morphology in nursery pigs. Transl. Anim. Sci. 2020, 4, txaa083. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Lu, L.; Zhang, L.; Zhang, X.; Li, H.; Lu, Y.; Luo, X. Relative bioavailability of zinc-methionine chelate for broilers fed a conventional corn-soybean meal diet. Biol. Trace Elem. Res. 2015, 165, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Windisch, W. Bioavailability of zinc glycinate in comparison with zinc sulphate in the presence of dietary phytate in an animal model with Zn labelled rats. J. Anim. Physiol. Anim. Nutr. (Berl.) 2006, 90, 216–222. [Google Scholar] [CrossRef]
- Grungreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef]
- Liu, B.; Xiong, P.; Chen, N.; He, J.; Lin, G.; Xue, Y.; Li, W.; Yu, D. Effects of Replacing of Inorganic Trace Minerals by Organically Bound Trace Minerals on Growth Performance, Tissue Mineral Status, and Fecal Mineral Excretion in Commercial Grower-Finisher Pigs. Biol. Trace Elem. Res. 2016, 173, 316–324. [Google Scholar] [CrossRef]
- Min, Y.N.; Liu, F.X.; Qi, X.; Ji, S.; Ma, S.X.; Liu, X.; Wang, Z.P.; Gao, Y.P. Effects of methionine hydroxyl analog chelated zinc on laying performance, eggshell quality, eggshell mineral deposition, and activities of Zn-containing enzymes in aged laying hens. Poult. Sci. 2018, 97, 3587–3593. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Cheng, Y.; Wen, C.; Zhou, Y. Effects of zinc bearing palygorskite supplementation on the growth performance, hepatic mineral content, and antioxidant status of broilers at early age. Asian Australas. J. Anim. Sci. 2017, 30, 1006–1012. [Google Scholar] [CrossRef][Green Version]
- Li, L.L.; Gong, Y.J.; Zhan, H.Q.; Zheng, Y.X.; Zou, X.T. Effects of dietary Zn-methionine supplementation on the laying performance, egg quality, antioxidant capacity, and serum parameters of laying hens. Poult. Sci. 2019, 98, 923–931. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Pal, D.; Mondal, S.; Selvaraju, S. Effect of Supplementation of Nano Zinc Oxide on Nutrient Retention, Organ and Serum Minerals Profile, and Hepatic Metallothionein Gene Expression in Wister Albino Rats. Biol. Trace Elem. Res. 2019, 190, 76–86. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhuo, Z.; Fang, S.; Yue, M.; Feng, J. Different Zinc Sources Have Diverse Impacts on Gene Expression of Zinc Absorption Related Transporters in Intestinal Porcine Epithelial Cells. Biol. Trace Elem. Res. 2016, 173, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Hu, Q.; Fang, S.; Feng, J. Dosage Effect of Zinc Glycine Chelate on Zinc Metabolism and Gene Expression of Zinc Transporter in Intestinal Segments on Rat. Biol. Trace Elem. Res. 2016, 171, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zetzsche, A.; Schunter, N.; Zentek, J.; Pieper, R. Accumulation of copper in the kidney of pigs fed high dietary zinc is due to metallothionein expression with minor effects on genes involved in copper metabolism. J. Trace Elem. Med. Biol. 2016, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Karweina, D.; Kreuzer-Redmer, S.; Muller, U.; Franken, T.; Pieper, R.; Baron, U.; Olek, S.; Zentek, J.; Brockmann, G.A. The Zinc Concentration in the Diet and the Length of the Feeding Period Affect the Methylation Status of the ZIP4 Zinc Transporter Gene in Piglets. PLoS ONE 2015, 10, e0143098. [Google Scholar] [CrossRef]
- Brugger, D.; Windisch, W.M. Zn metabolism of monogastric species and consequences for the definition of feeding requirements and the estimation of feed Zn bioavailability. J. Zhejiang Univ. Sci. B 2019, 20, 617–627. [Google Scholar] [CrossRef]
- VanValin, K.R.; Genther-Schroeder, O.N.; Carmichael, R.N.; Blank, C.P.; Deters, E.L.; Hartman, S.J.; Niedermayer, E.K.; Laudert, S.B.; Hansen, S.L. Influence of dietary zinc concentration and supplemental zinc source on nutrient digestibility, zinc absorption, and retention in sheep. J. Anim. Sci. 2018, 96, 5336–5344. [Google Scholar] [CrossRef]


| Ingredient | Content (%) | Analyzed Value | |
|---|---|---|---|
| Corn | 63.80 | Digestible energy (MJ.kg−1) | 14.05 |
| Soybean meal | 19.80 | Crude Protein (%) | 18.27 |
| Whey powder | 4.30 | Crude fat (%) | 4.20 |
| Fish meal | 9.00 | Crude fiber (%) | 2.55 |
| Soybean oil | 0.80 | Lysine (%) | 1.37 |
| Lysine | 0.38 | Methionine + Cysteine (%) | 0.76 |
| Threonine | 0.09 | Threonine (%) | 0.88 |
| Tryptophan | 0.01 | Calcium (%) | 0.80 |
| Limestone | 0.52 | Phosphorus (%) | 0.64 |
| Salt | 0.30 | Zinc (mg/kg) | 70.46 |
| 1% premix 1 | 1.00 | Copper(mg/kg) | 47.47 |
| Total | 100.00 | Iron(mg/kg) | 195.09 |
| Gene | Accession No. | 5′-3′ Primer Sequence |
|---|---|---|
| β-actin | XM_003357928 | F: CGTTGGCTGGTTGAGAATC |
| R: CGGCAAGACAGAAATGACAA | ||
| ZIP4 | XM_021090449 | F: TGCTGAACTTGGCATCTGGG |
| R: CGCCACGTAGAGAAAGAGGC | ||
| ZnT1 | NM_001139470 | F: CCAGGGGAGCAGGGAACCGA |
| R: TCAGCCCGTTGGAGTTGCTGC | ||
| MT | NM_001001266.2 | F: CTGTGCCTGAAGTCTGGGGAA |
| R: CACAGAAAAAGGGATGTAGCATG |
| Items | Zn-Met | ZnSO4,feed | ZnSO4,water | p-Value |
|---|---|---|---|---|
| Average daily gain (kg) | 0.443 ± 0.0102 | 0.471 ± 0.0531 | 0.435 ± 0.0462 | 0.209 |
| Average daily feed intake (kg) | 0.730 | 0.730 | 0.730 | 1.000 |
| Final body weight (kg) | 23.28 ± 0.263 | 23.90 ± 0.432 | 23.15 ± 0.312 | 0.274 |
| Feed: gain ratio | 1.67 ± 0.0403 | 1.56 ± 0.0732 | 1.69 ± 0.0714 | 0.265 |
| Apparent digestibility of Zn (%) | 40.29 ± 5.15 a | 23.43 ± 4.14 b | 46.21 ± 2.62 a | 0.004 |
| Tissue | Items | Zn-Met | ZnSO4,feed | ZnSO4,water | p-Value |
|---|---|---|---|---|---|
| Liver | Zn (mg/kg) | 248.00 ± 44.7 a | 162.50 ± 40.00 b | 233.50 ± 20.4 a | 0.002 |
| Cu (mg/kg) | 16.72 ± 2.64 | 14.54 ± 2.19 | 13.48 ± 3.08 | 0.148 | |
| Fe (mg/kg) | 111.10 ± 20.8 | 82.30 ± 46.20 | 87.90 ± 21.0 | 0.279 | |
| Jejunum | Zn (mg/kg) | 19.19 ± 0.49 a | 16.95 ± 0.43 b | 19.76 ± 0.75 a | 0.008 |
| Cu (mg/kg) | 1.86 ± 0.21 | 1.64 ± 0.13 | 1.74 ± 0.08 | 0.571 | |
| Fe (mg/kg) | 55.06 ± 5.52 | 56.15 ± 6.41 | 38.78 ± 2.81 | 0.991 | |
| Ileum | Zn (mg/kg) | 15.07 ± 0.54 | 17.03 ± 0.57 | 16.87 ± 0.73 | 0.075 |
| Cu (mg/kg) | 1.33 ± 0.26 | 1.32 ± 0.20 | 0.90 ± 0.23 | 0.336 | |
| Fe (mg/kg) | 8.40 ± 0.70 | 8.84 ± 0.95 | 7.79 ± 0.39 | 0.595 |
| Tissue | Item | Zn-Met | ZnSO4,feed | ZnSO4,water | p-Value |
|---|---|---|---|---|---|
| Duodenum | T-SOD | 52.16 ± 4.74 | 58.00 ± 7.77 | 55.37 ± 4.60 | 0.785 |
| AKP | 15.22 ± 1.92 | 15.63 ± 2.31 | 19.49 ± 1.66 | 0.273 | |
| 5′-NT | 10.85 ± 1.19 | 8.19 ± 0.75 | 9.47 ± 1.06 | 0.216 | |
| Jejunum | T-SOD | 72.67 ± 4.81 | 64.89 ± 3.80 | 82.82 ± 7.96 | 0.124 |
| AKP | 39.17 ± 2.64 a | 29.85 ± 1.60 b | 36.35 ± 30.06 ab | 0.031 | |
| 5′-NT | 15.81 ± 1.38 | 12.24 ± 1.03 | 17.71 ± 2.51 | 0.116 | |
| Ileum | T-SOD | 68.73 ± 4.1 a | 54.26 ± 2.38 b | 66.11 ± 4.62 a | 0.039 |
| AKP | 34.85 ± 1.70 | 31.02 ± 1.89 | 36.13 ± 3.95 | 0.406 | |
| 5′-NT | 15.95 ± 1.40 | 16.36 ± 1.67 | 15.11 ± 1.08 | 0.815 | |
| liver | T-SOD | 176.34 ± 72.06 | 232.20 ± 32.12 | 195.37 ± 32.34 | 0.478 |
| AKP | 32.20 ± 12.00 | 37.64 ± 10.78 | 16.48 ± 5.78 | 0.317 | |
| 5′-NT | 34.49 ± 7.95 | 45.97 ± 5.57 | 45.68 ± 8.79 | 0.503 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-F.; Azad, M.A.K.; Li, Z.-H.; Li, J.; Mo, K.-B.; Ni, H.-J. Zinc Supplementation Forms Influenced Zinc Absorption and Accumulation in Piglets. Animals 2021, 11, 36. https://doi.org/10.3390/ani11010036
Liu F-F, Azad MAK, Li Z-H, Li J, Mo K-B, Ni H-J. Zinc Supplementation Forms Influenced Zinc Absorption and Accumulation in Piglets. Animals. 2021; 11(1):36. https://doi.org/10.3390/ani11010036
Chicago/Turabian StyleLiu, Fen-Fen, Md. Abul Kalam Azad, Zhi-He Li, Jing Li, Kai-Bin Mo, and Heng-Jia Ni. 2021. "Zinc Supplementation Forms Influenced Zinc Absorption and Accumulation in Piglets" Animals 11, no. 1: 36. https://doi.org/10.3390/ani11010036
APA StyleLiu, F.-F., Azad, M. A. K., Li, Z.-H., Li, J., Mo, K.-B., & Ni, H.-J. (2021). Zinc Supplementation Forms Influenced Zinc Absorption and Accumulation in Piglets. Animals, 11(1), 36. https://doi.org/10.3390/ani11010036







