Automatic Assessment of Keel Bone Damage in Laying Hens at the Slaughter Line
Abstract
Simple Summary
Abstract
1. Introduction
2. Animals, Materials and Methods
2.1. Slaughterhouse and Animals
2.2. KBD Assessment by Human Assessors
2.3. Camera Vision System
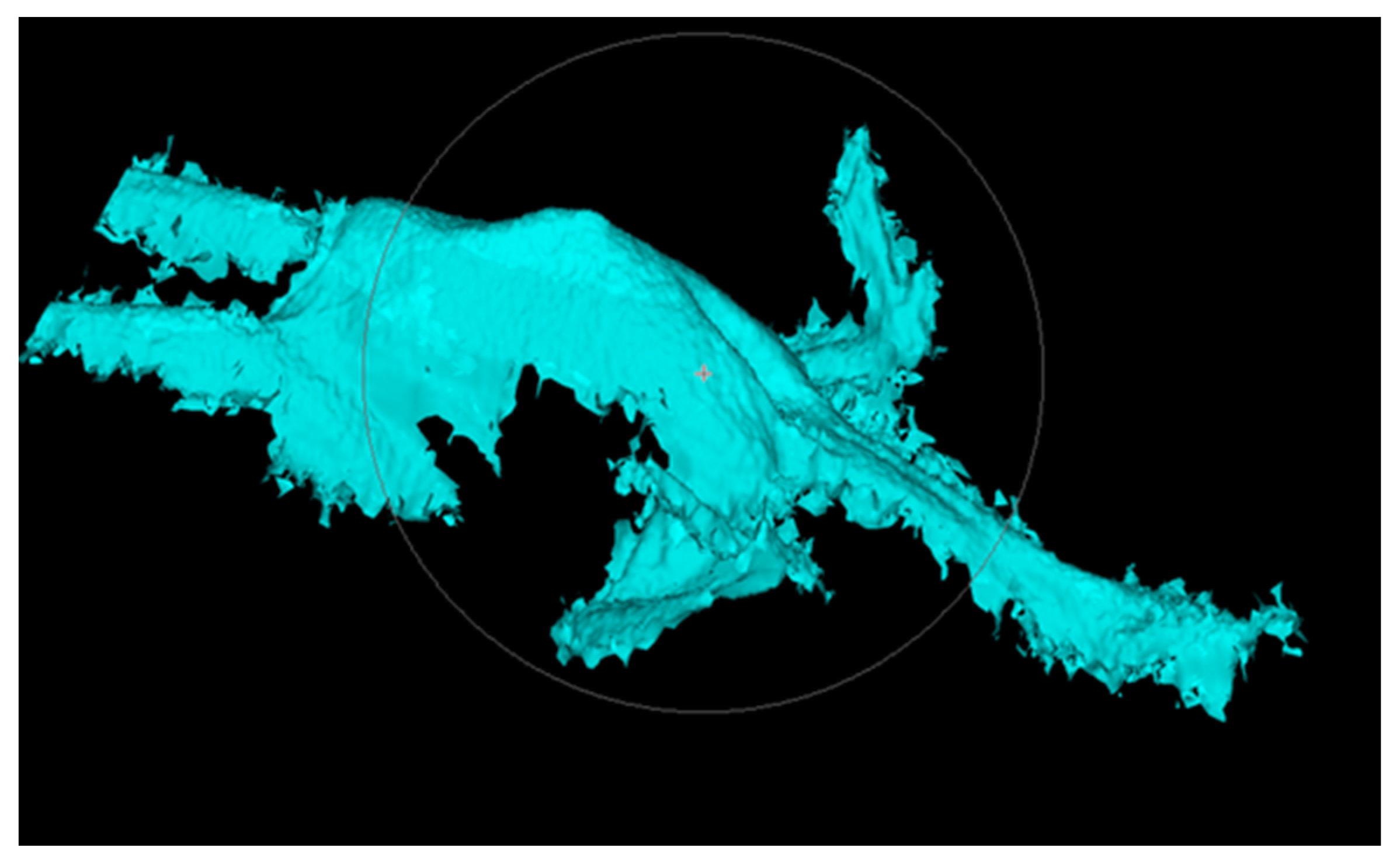
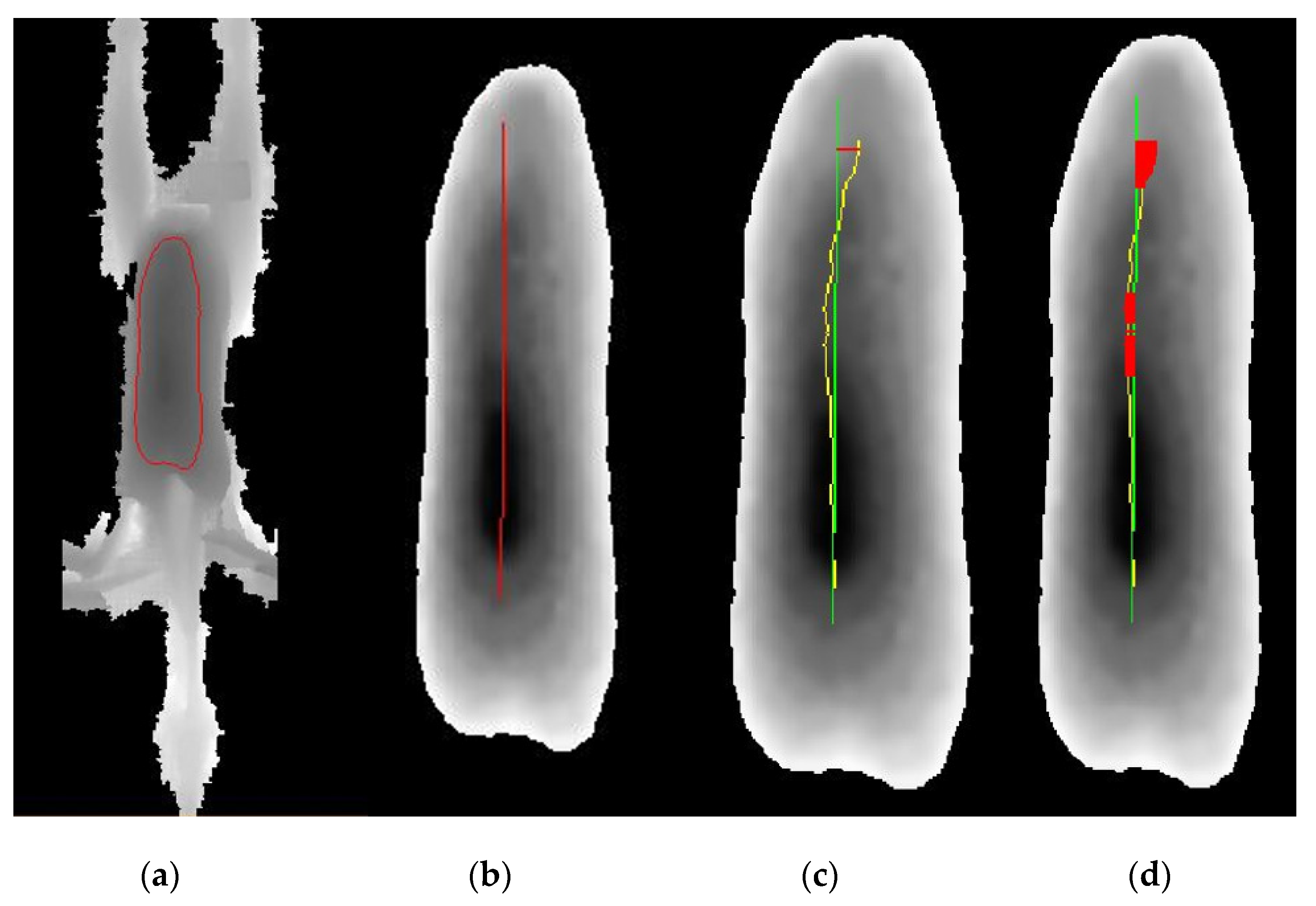
2.4. Development and Validation of the Automated System
2.5. Statistics
3. Results
3.1. KBD Assessment by Human Assessors
3.2. Validation of the System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards, G.J.; Wilkins, L.J.; Knowles, T.G.; Booth, F.; Toscano, M.J.; Nicol, C.J. Pop hole use by hens with different keel fracture status monitored throughout the laying period. Vet. Rec. 2012, 170, 494. [Google Scholar] [CrossRef] [PubMed]
- Heerkens, J.L.T.; Delezie, E.; Rodenburg, T.B.; Kempen, I.; Zoons, J.; Ampe, B.; Tuyttens, F.A.M. Risk factors associated with keel bone and foot pad disorders in laying hens housed in aviary systems. Poult. Sci. 2016, 95, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Rufener, C.; Baur, S.; Stratmann, A.; Toscano, M.J. Keel bone fractures affect egg laying performance but not egg quality in laying hens housed in a commercial aviary system. Poult. Sci. 2018, 98, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Jung, L.; Niebuhr, K.; Hinrichsen, L.K.; Gunnarsson, S.; Brenninkmeyer, C.; Bestman, M.; Heerkens, J.; Ferrari, P.; Knierim, U. Possible risk factors for keel bone damage in organic laying hens. Animal 2019, 13, 2356–2364. [Google Scholar] [CrossRef]
- Scholz, B.; Rönchen, S.; Hamann, H.; Hewicker-Trautwein, M.; Distl, O. Keel bone condition in laying hens: A histological evaluation of macroscopically assessed keel bones. Berl. Münch. Tierärzt. Wochenschr. 2008, 121, 89–94. [Google Scholar]
- Fleming, R.H.; McCormack, H.A.; McTeir, L.; Whitehead, C.C. Incidence, pathology and prevention of keel bone deformations in the laying hen. Br. Poult. Sci. 2004, 45, 320–330. [Google Scholar] [CrossRef]
- Saraiva, S.; Esteves, A.; Stilwell, G. Influence of different housing systems on prevalence of keel bone lesions in laying hens. Avian Pathol. 2019, 48, 454–459. [Google Scholar] [CrossRef]
- Rufener, C.; Makagon, M.M. Keel bone fractures in laying hens: A systematic review of prevalence across age, housing systems, and strains. Anim. Sci. J. 2020, 98, S36–S51. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Nicol, C.J.; Murrell, J.C. Do Laying Hens with Keel Bone Fractures Experience Pain? PLoS ONE 2012, 7, e42420. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Brownea, W.J.; Caplena, G.; Hothersalla, B.; Murrell, J.C.; Nicol, C.J. Positive affective state induced by opioid analgesia in laying hens with bone fractures. App. Anim. Behav. Sci. 2013, 147, 127–131. [Google Scholar] [CrossRef]
- Nasr, M.A.; Murrell, J.; Nicol, C.J. The effect of keel fractures on egg production, feed and water consumption in individual laying hens. Br. Poult. Sci. 2013, 54, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Casey-Trott, T.M.; Widowski, T.M. Behavioral Differences of Laying Hens with Fractured Keel Bones within Furnished Cages. Front. Vet. Sci. 2016, 3, 42. [Google Scholar] [CrossRef]
- Knierim, U.; Winckler, C. On-farm assessment in cattle: Validity, reliability and feasibility issues and future perspectives with special regard to the Welfare Quality® approach. Anim. Welf. 2009, 18, 451–458. [Google Scholar]
- Mullan, S.; Edwards, S.; Butterworth, A.; Whay, H.; Main, D. Interdependence of welfare outcome measures and potential confounding factors on finishing pig farms. Appl. Anim. Behav. Sci. 2009, 121, 25–31. [Google Scholar] [CrossRef]
- Tierschutzgesetz. Tierschutzgesetz as of 18 May 2006 (BGBl. I S. 1206, 1313), last amended by Article 101 at 20 November 2019 (BGBl. I S. 1626). Available online: https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 12 January 2021).
- Petrik, M.T.; Guerin, M.T.; Widowski, T.M. Keel fracture assessment of laying hens by palpation: Inter-observer reliability and accuracy. Vet. Rec. 2013, 173. [Google Scholar] [CrossRef] [PubMed]
- Casey-Trott, T.; Heerkens, J.L.T.; Petrik, M.; Regmi, P.; Schrader, L.; Toscano, M.J.; Widowski, T. Methods for assessment of keel bone damage in poultry. Poult. Sci. 2015, 94, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Nasirahmadi, A.; Edwards, S.A.; Sturm, B. Implementation of machine vision for detecting behaviour of cattle and pigs. Livest. Sci. 2017, 202, 25–38. [Google Scholar] [CrossRef]
- Ben Sassi, N.; Averós, X.; Estevez, I. Technology and Poultry Welfare. Animals 2016, 6, 62. [Google Scholar] [CrossRef]
- Fernandes, A.F.A.; Dórea, J.R.R.; Rosa, G.J.M. Image Analysis and Computer Vision Applications in Animal Sciences: An Overview. Front. Vet. Sci. 2020, 7, 551269. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Patterson, J.; Cheng, H. Use of thermography to screen for subclinical bumblefoot in poultry. Poult. Sci. 2009, 88, 1176–1180. [Google Scholar] [CrossRef]
- Vanderhasselt, R.F.; Sprenger, M.; Duchateau, L.; Tuyttens, F. Automated assessment of footpad dermatitis in broiler chickens at the slaughter-line: Evaluation and correspondence with human expert scores. Poult. Sci. 2013, 92, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Toppel, K.; Spindler, B.; Kaufmann, F.; Gauly, M.; Kemper, N.; Andersson, R. Foot Pad Health as Part of On-Farm-Monitoring in Turkey Flocks. Front. Vet. Sci. 2019, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.M.; Temple, S.M.; Bennett, D.C.; Sprayberry, K.A.; Makagon, M.M.; Blatchford, R.A. The Reliability and Accuracy of Palpation, Radiography, and Sonography for the Detection of Keel Bone Damage. Animals 2019, 9, 894. [Google Scholar] [CrossRef]
- Berckmans, D. General introduction to precision livestock farming. Anim. Front. 2017, 7, 6–11. [Google Scholar] [CrossRef]
| Year | N of Hens | PABAK | Experience 1 | Pair No. |
|---|---|---|---|---|
| 2017 | ||||
| A | 110 | 0.78 | yes | 1 |
| B | 110 | 0.78 | yes | 2 |
| C | 100 | 0.90 | yes | 3 |
| D | 157 | 0.81 | yes | 3 |
| 2018 | ||||
| E | 76 | 0.82 | no | 4 |
| F | 90 | 0.73 | yes | 3 |
| G | 90 | 0.76 | no | 5 |
| H | 150 | 0.75 | yes | 6 |
| I | 400 | 0.65 | yes | 3 |
| J | 400 | 0.78 | yes | 3 |
| K | 400 | 0.67 | yes | 7 |
| 2019 | ||||
| L | 76 | 0.82 | no | 8 |
| M | 68 | 0.62 | no | 9 |
| N | 46 | 0.57 | no | 10 |
| Assessments | True Positive | False Positive | True Negative | False Negative | N Total |
|---|---|---|---|---|---|
| Opt1–1 | 32 | 26 | 50 | 81 | 189 |
| Opt1–2 | 9 | 3 | 28 | 20 | 60 |
| Opt2–3 | 15 | 8 | 41 | 29 | 93 |
| Opt2–4 | 23 | 5 | 34 | 17 | 79 |
| Opt2–5 1 | 38 | 9 | 30 | 2 | 79 |
| Assessments | Sensitivity | Specificity | Accuracy | Precision | Prevalence 1 | True Prevalence 2 | PABAK | N |
|---|---|---|---|---|---|---|---|---|
| Opt1–1 | 0.28 | 0.66 | 0.43 | 0.55 | 30.7 | 59.8 | −0.13 | 189 |
| Opt1–2 | 0.31 | 0.90 | 0.62 | 0.75 | 20.0 | 48.3 | 0.23 | 60 |
| Opt2–3 | 0.34 | 0.84 | 0.60 | 0.65 | 24,7 | 47,3 | 0.20 | 93 |
| Opt2–4 | 0.58 | 0.87 | 0.72 | 0.82 | 35.4 | 50.6 | 0.44 | 79 |
| Opt2–5 3 | 0.95 | 0.77 | 0.86 | 0.81 | 38.3 | 50.6 | 0.72 | 79 |
| Assessments | Batch Size | Assessor | Camera | ||||
|---|---|---|---|---|---|---|---|
| N | Prevalence % | N 1 | Prevalence % | Difference (% Points) | |||
| Learning phase | 1 | 15,815 | 481 | 39.1 | 13,315 | 21.5 | 17.6 |
| 2 | 1044 | 393 | 38.3 | 824 | 41.7 | 3.4 | |
| 3 | 1780 | 538 | 46.7 | 1426 | 34.5 | 12.2 | |
| 4 | 9235 | 210 | 55.2 | 8436 | 22.7 | 32.5 | |
| 5 | 2732 | 548 | 40.0 | 1747 | 21.2 | 19.0 | |
| Opitmization step 1 | 6 | 906 | 665 | 39.2 | 762 | 31.6 | 7.6 |
| 7 | 10,510 | 314 | 29.3 | 8985 | 25.9 | 3.4 | |
| 8 | 1724 | 1247 | 54.1 | 1411 | 32.5 | 21.6 | |
| 9 | 7105 | 2344 | 29.0 | 6241 | 19.0 | 10.0 | |
| 10a | 15,249 | 1307 | 68.7 | 12,845 | 45.7 | 23.0 | |
| 10b 2 | 15,249 | 1187 | 68.9 | 12,845 | 45.7 | 23.2 | |
| 11 | 3091 | 392 | 43.6 | 2663 | 46.8 | 3.2 | |
| Optimization step 2 | 12 | 275 | 253 | 32.4 | 251 | 24.3 | 8.1 |
| 13 | 745 | 241 | 35.3 | 665 | 21.8 | 13.5 | |
| 14 | 4255 | 369 | 23.0 | 3784 | 19.5 | 3.5 | |
| 15a | 4516 | 556 | 30.2 | 4103 | 17.3 | 12.9 | |
| 15b | 4516 | 414 | 18.8 3 | 4103 | 17.3 | 1.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, L.; Nasirahmadi, A.; Schulte-Landwehr, J.; Knierim, U. Automatic Assessment of Keel Bone Damage in Laying Hens at the Slaughter Line. Animals 2021, 11, 163. https://doi.org/10.3390/ani11010163
Jung L, Nasirahmadi A, Schulte-Landwehr J, Knierim U. Automatic Assessment of Keel Bone Damage in Laying Hens at the Slaughter Line. Animals. 2021; 11(1):163. https://doi.org/10.3390/ani11010163
Chicago/Turabian StyleJung, Lisa, Abozar Nasirahmadi, Jan Schulte-Landwehr, and Ute Knierim. 2021. "Automatic Assessment of Keel Bone Damage in Laying Hens at the Slaughter Line" Animals 11, no. 1: 163. https://doi.org/10.3390/ani11010163
APA StyleJung, L., Nasirahmadi, A., Schulte-Landwehr, J., & Knierim, U. (2021). Automatic Assessment of Keel Bone Damage in Laying Hens at the Slaughter Line. Animals, 11(1), 163. https://doi.org/10.3390/ani11010163





