Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
- (1)
- How and when to pet the dog,
- (2)
- Luring technique (to capture the dogs’ attention),
- (3)
- Turn to the inmate’s right or left,
- (4)
- The command “sit”,
- (5)
- Nose-working activity,
- (6)
- The command “stay”,
- (7)
- Management of the leash,
- (8)
- Recall,
- (9)
- The command “give the paw”.
2.3. Sample Collection and Cortisol Measurement in Dogs
2.4. Data Analysis
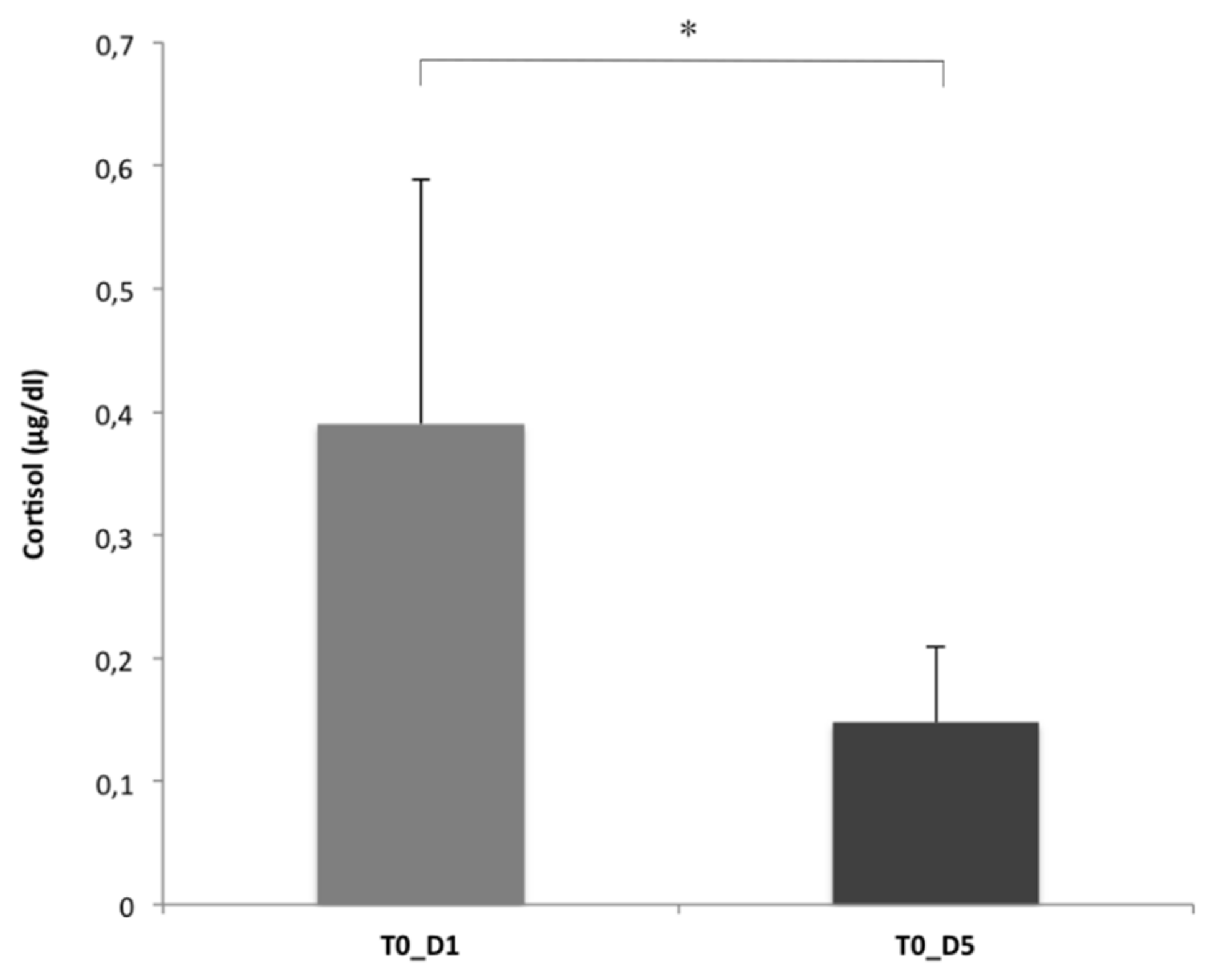
3. Results
Dog Salivary Cortisol Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dotson, M.J.; Hyatt, E.M. Understanding dog–human companionship. J. Bus. Res. 2008, 61, 457–466. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Stipo, C.; Quaranta, A. Like Owner, Like Dog: Correlation between the Owner’s Attachment Profile and the Owner-Dog Bond. PLoS ONE 2013, 8, e78455. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.H.; Beck, A.M. Understanding our kinship with animals: Input for health care professionals interested in the human/animal bond. In Handbook on Animal-Assisted Therapy: Theoretical Foundations and Guidelines for Practice; Fine, A.H., Ed.; Academic Press: London, UK, 2015; pp. 3–10. [Google Scholar]
- Fine, A.H.; Weaver, S.J. The human-animal bond and animal-assisted intervention. In Oxford Textbook of Nature and Public Health: The Role of Nature in Improving the Health of a Population; Frumkin, H., Ed.; Oxford University Press: Oxford, UK, 2018; pp. 132–138. [Google Scholar]
- Braun, C.; Stangler, T.; Narveson, J.; Pettingell, S. Animal-assisted therapy as a pain relief intervention for children. Complement. Ther. Clin. Pract. 2009, 15, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Veggiotti, P.; Palestrini, C.; De Giorgis, V.; Raschetti, R.; Tumminelli, M.; Mencherini, S.; Papotti, F.; Klersy, C.; Albertini, R.; et al. Post-operative benefits of animal-assisted therapy in pediatric surgery: A randomised study. PLoS ONE 2015, 10, e0125813. [Google Scholar] [CrossRef]
- Binfet, J.T.; Passmore, H.A. Hounds and homesickness: The effects of an animal-assisted therapeutic intervention for first-year university students. Anthrozoӧs 2016, 29, 441–454. [Google Scholar] [CrossRef]
- Fiocco, A.J.; Hunse, A.M. The buffer effect of therapy dog exposure on stress reactivity in undergraduate students. Int. J. Environ. Res. Public Health 2017, 14, 707. [Google Scholar] [CrossRef]
- Ward-Griffin, E.; Klaiber, P.; Collins, H.K.; Owens, R.L.; Coren, S.; Chen, F.S. Petting away pre-exam stress: The effect of therapy dog sessions on student well-being. Stress Health 2018, 34, 468–473. [Google Scholar] [CrossRef]
- Cole, K.M.; Gawlinski, A.; Steers, N.; Kotlerman, J. Animal-assisted therapy in patients hospitalized with heart failure. Am. J. Crit. Care 2007, 16, 575–585. [Google Scholar] [CrossRef]
- Hoffmann, A.O.; Lee, A.H.; Wertenauer, F.; Ricken, R.; Jansen, J.J.; Gallinat, J.; Lang, U.E. Dog-assisted intervention significantly reduces anxiety in hospitalized patients with major depression. Eur. J. Integr. Med. 2009, 1, 145–148. [Google Scholar] [CrossRef]
- Klemm, P.; Waddington, C.; Bradley, E.; Bucher, L.; Collins, M.; Lyons, D.L.; Seckel, M.A.; Urban, M. Unleashing animal-assisted therapy. Nursing 2010, 40, 12–13. [Google Scholar] [CrossRef]
- O’Haire, M.E.; McKenzie, S.J.; McCune, S.; Slaughter, V. Effects of classroom animal-assisted activities on social functioning in children with autism spectrum disorder. J. Altern. Complement. Med. 2014, 20, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.; Correia, R.; Lima, M.; Magalhães, A.; de Sousa, L. Can dogs prime autistic children for therapy? Evidence from a single case study. J. Altern. Complement. Med. 2011, 17, 655–659. [Google Scholar] [PubMed]
- Le Roux, M.C.; Swartz, L.; Swart, E. The effect of an animal-assisted reading program on the reading rate, accuracy and comprehension of grade 3 students: A randomized control study. Child Youth Care Forum 2014, 43, 655–673. [Google Scholar] [CrossRef]
- Nordgren, L.; Engstrӧm, G. Animal-assisted intervention in dementia: Effects on quality of life. Clin. Nurs. Res. 2014, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Contalbrigo, L.; De Santis, M.; Toson, M.; Montanaro, M.; Farina, L.; Costa, A.; Nava, F.A. The Efficacy of Dog Assisted Therapy in Detained Drug Users: A Pilot Study in an Italian Attenuated Custody Institute. Int. J. Environ. Res. Public Health 2017, 14, 683. [Google Scholar] [CrossRef] [PubMed]
- Baybutt, M.; Chemlal, K. Health-promoting prison: Theory to practice. Glob. Health Promot. 2016, 23, 66–74. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Minunno, M.; Quaranta, A. Communication in dogs. Animals 2018, 8, 131. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Quaranta, A. Orienting asymmetries and physiological reactivity in dogs’ response to human emotional faces. Learn. Behav. 2018, 46, 574–585. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Fornelli, S.; Quaranta, A. Lateralized behavior and cardiac activity of dogs in response to human emotional vocalizations. Sci. Rep. 2018, 8, 77. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Quaranta, A. The dog nose “KNOWS” fear: Asymmetric nostril use during sniffing at canine and human emotional stimuli. Behav. Brain Res. 2016, 304, 34–41. [Google Scholar] [CrossRef]
- Quaranta, A.; d’Ingeo, S.; Amoruso, R.; Siniscalchi, M. Emotion Recognition in Cats. Animals 2020, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; Scott, S.K.; McComb, K. Domestic horses (Equus caballus) discriminate between negative and positive human nonverbal vocalisations. Sci. Rep. 2018, 8, 13052. [Google Scholar] [CrossRef] [PubMed]
- d’Ingeo, S.; Quaranta, A.; Siniscalchi, M.; Stomp, M.; Coste, C.; Bagnard, C.; Hausberger, M.; Cousillas, H. Horses perception of human voices is modulated by the valence of previous horse-human interactions: A behavioural and electrophysiological study. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fureix, C.; Jego, P.; Sankey, C.; Hausberger, M. How horses (Equus caballus) see the world: Humans as significant “objects”. Anim. Cogn. 2009, 12, 643–654. [Google Scholar] [CrossRef]
- Lefebvre, S.L.; Reid-Smith, R.J.; Waltner-Toews, D.; Weese, J.S. Incidence of acquisition of methicillin-resistant Staphylococcus aureus, Clostridium difficile, and other health-care-associated pathogens by dogs that participate in animal-assisted interventions. J. Am. Vet. Med. Assoc. 2009, 234, 1404–1417. [Google Scholar]
- Lefebvre, S.L.; Peregrine, A.S.; Golab, G.C.; Gumley, N.R.; Waltner-Toews, D.; Weese, J.S. A veterinary perspective on the recently published guidelines for animal-assisted interventions in health-care facilities. J. Am. Vet. Med. Assoc. 2008, 233, 394–402. [Google Scholar] [CrossRef]
- Lefebvre, S.L.; Arroyo, L.G.; Weese, J.S. Epidemic Clostridium difficile strain in hospital visitation dog. Emerg. Infect. Dis. 2006, 12, 1036. [Google Scholar] [CrossRef]
- Lefebvre, S.L.; Waltner-Toews, D.; Peregrine, A.S.; Reid-Smith, R.; Hodge, L.; Arroyo, L.G.; Weese, J.S. Prevalence of zoonotic agents in dogs visiting hospitalized people in Ontario: Implications for infection control. J. Hosp. Infect. 2006, 62, 458–466. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Wolfhagen, M.J.; Box, A.T.; Heck, M.E.; Wannet, W.J.; Fluit, A.C. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2004, 10, 2235. [Google Scholar] [CrossRef]
- Iannuzzi, D.; Rowan, A.N. Ethical issues in animal-assisted therapy programs. Anthrozoӧs 1991, 4, 154–163. [Google Scholar]
- Marinelli, L.; Normando, S.; Siliprandi, C.; Salvadoretti, M.; Mongillo, P. Dog assisted interventions in a specialized centre and potential concerns for animal welfare. Vet. Res. Commun. 2009, 33, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Glenk, L.M. Current perspectives on therapy dog welfare in animal-assisted interventions. Animals 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, D.; Ciani, F.; Zaccherini, A.; Tafuri, S.; Avallone, L.; d’Ingeo, S.; Quaranta, A. Human-Animal Relationship Dysfunction: A Case Study of Animal Hoarding in Italy. Animals 2020, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Reisner, I.R.; Shofer, F.S. Effects of gender and parental status on knowledge and attitudes of dog owners regarding dog aggression toward children. J. Am. Vet. Med. Assoc. 2008, 233, 1412–1419. [Google Scholar] [CrossRef]
- Bloom, T.; Friedman, H. Classifying dogs’ (Canis familiaris) facial expressions from photographs. Behav. Process. 2013, 96, 1–10. [Google Scholar] [CrossRef]
- Meints, K.; Racca, A.; Hickey, N. How to prevent dog bite injuries? Children misinterpret dogs’ facial expressions. Inj. Prev. 2010, 16, 1136. [Google Scholar] [CrossRef]
- Lakestani, N.N.; Donaldson, M.L.; Waran, N. Interpretation of dog behavior by children and young adults. Anthrozoös 2014, 27, 65–80. [Google Scholar] [CrossRef]
- Tami, G.; Gallagher, A. Description of the behaviour of domestic dog (Canis familiaris) by experienced and inexperienced people. Appl. Anim. Behav. Sci. 2009, 120, 159–169. [Google Scholar] [CrossRef]
- Wan, M.; Bolger, N.; Champagne, F.A. Human perception of fear in dogs varies according to experience with dogs. PLoS ONE 2012, 7, e51775. [Google Scholar]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Salivary cortisol and behavior in therapy dogs during animal-assisted interventions: A pilot study. J. Vet. Behav. 2014, 9, 98–106. [Google Scholar] [CrossRef]
- Palestrini, C.; Calcaterra, V.; Cannas, S.; Talamonti, Z.; Papotti, F.; Buttram, D.; Pelizzo, G. Stress level evaluation in a dog during animal-assisted therapy in pediatric surgery. J. Vet. Behav. 2017, 17, 44–49. [Google Scholar] [CrossRef]
- Esposito, L.; Auletta, L.; Ciani, F.; Pelagalli, A.; Pasolini, M.P.; Lamagna, B.; Piscopo, N.; Amici, A. Hair cortisol levels in captive brown hare (Lepus europaeus): Potential effect of sex, age, and breeding technology. Eur. J. Wildl. Res. 2017, 63, 62. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; Bernardina, W.; Van Hoff, J.A.; de Vries, H.W.; Mol, J.A. Chronic stress in dogs subjected to social and spatial restrictions. II. Hormonal and immunological responses. Physiol. Behav. 1999, 66, 243–254. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; Janssen, N.S.; Mol, J.A. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 1996, 30, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Wilding, C. The genetic basis of size in pet dogs: The study of quantitative genetic variation in an undergraduate laboratory practical. Biochem. Mol. Biol. Educ. 2018, 46, 623–629. [Google Scholar] [CrossRef]
- Pageat, P. Patologia Comportamentale del Cane; Verga, M., Carenzi, C., Eds.; Zecchini, M., Translator; Le Point Veterinaire Italie: Milano, Italy, 1999. [Google Scholar]
- Rep. Atti n. 60/CSR del 25 Marzo 2015. Accordo Tra il Governo, le Regioni e le Province Autonome di Trento e di Bolzano sul Documento Recante. “Linee Guida Nazionali per gli Interventi Assistiti con gli Animali (IAA)”, Italia. 2017. Available online: http://www.salute.gov.it/imgs/C_17_opuscoliPoster_276_allegato.pdf (accessed on 15 December 2020).
- Siniscalchi, M.; Marzulli, M.; Celi, P.; Quaranta, A. Cortisolo salivare in cani adibiti a Pet Therapy. Obiettivi Doc. Vet. 2007, 4, 25–30. [Google Scholar]
- Oyama, D.; Hyodo, M.; Doi, H.; Kurachi, T.; Takata, M.; Koyama, S.; Satoh, T.; Watanabe, G. Saliva collection by using filter paper for measuring cortisol levels in dogs. Domest. Anim. Endocrinol. 2013, 46, 20–25. [Google Scholar] [CrossRef]
- Siniscalchi, M.; McFarlane, J.R.; Kauter, K.G.; Quaranta, A.; Rogers, L.J. Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Res. Vet. Sci. 2013, 94, 49–54. [Google Scholar]
- Shiverdecker, M.; Schiml, P.A.; Hennessy, M.B. Human interaction moderates plasma cortisol and behavioral responses of dogs to shelter housing. Physiol. Behav. 2013, 109, 75–79. [Google Scholar] [CrossRef]
- Gunter, L.M.; Feuerbacher, E.N.; Gilchrist, R.J.; Wynne, C.D.L. Evaluating the effects of a temporary fostering program on shelter dog welfare. PeerJ 2019, 7, e6620. [Google Scholar] [CrossRef]
- Tuber, D.S.; Hennessy, M.B.; Sanders, S.; Miller, J.A. Behavioral and glucocorticoid responses of adult domestic dogs (Canis familiaris) to companionship and social separation. J. Comp. Psychol. 1996, 110, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.B.; Williams, M.T.; Miller, D.D.; Douglas, C.W.; Voith, V.L. Influence of male and female petters on plasma cortisol and behaviour: Can human interaction reduce the stress of dogs in a public animal shelter? Appl. Anim. Behav. Sci. 1998, 61, 63–77. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Davis, H.N.; Williams, M.T.; Mellott, C.; Douglas, C.W. Plasma cortisol levels of dogs at a county animal shelter. Physiol. Behav. 1997, 62, 485–490. [Google Scholar] [CrossRef]
- Horváth, Z.; Dóka, A.; Miklósi, Á. Affiliative and disciplinary behavior of human handlers during play with their dog affects cortisol concentrations in opposite directions. Horm. Behav. 2008, 54, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.B.; Willen, R.M.; Schiml, P.A. Psychological Stress, Its Reduction, and Long-Term Consequences: What Studies with Laboratory Animals Might Teach Us about Life in the Dog Shelter. Animals 2020, 10, 2061. [Google Scholar] [CrossRef]
- Ng, Z.Y.; Pierce, B.J.; Otto, C.M.; Buechner-Maxwell, V.A.; Siracusa, C.; Werre, S.R. The effect of dog-human interaction on cortisol and behavior in registered animal-assisted activity dogs. Appl. Anim. Behav. Sci. 2014, 159, 69–81. [Google Scholar] [CrossRef]
- Protopopova, A. Effects of sheltering on physiology, immune function, behavior, and the welfare of dogs. Physiol. Behav. 2016, 159, 95–103. [Google Scholar] [CrossRef]
- Stephen, J.M.; Ledger, R.A. A longitudinal evaluation of urinary cortisol in kennelled dogs, Canis familiaris. Physiol. Behav. 2006, 87, 911–916. [Google Scholar] [CrossRef]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Therapy dogs’ salivary cortisol levels vary during animal-assisted interventions. Anim. Welf. 2013, 22, 369–378. [Google Scholar] [CrossRef]
- Sandri, A.; Colussi, A.; Perrotta, M.G.; Stefanon, B. Salivary cortisol concentration in healthy dogs is affected by size, sex, and housing context. J. Vet. Behav. 2015, 10, 302–306. [Google Scholar] [CrossRef]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release and heart rate variability in horses during road transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef]
- Chulayo, A.Y.; Bradley, G.; Muchenje, V. Effects of transport distance, lairage time and stunning efficiency on cortisol, glucose, HSPA1A and how they relate with meat quality in cattle. Meat Sci. 2016, 117, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Palme, R.; Robia, C.; Baumgartner, W.; Möstl, E. Transport stress in caftle as reflected by an increase in faecal cortisol metabolite concentrations. Vet. Rec. 2000, 146, 108. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.H.; Parrott, R.F.; Goode, J.A.; Lloyd, D.M.; Rodway, R.G.; Broom, D.M. Behavioural and hormonal responses of pigs during transport: Effect of mixing and duration of journey. Anim. Sci. 1996, 62, 547–554. [Google Scholar] [CrossRef]
- Radisavljević, K.; Vučinić, M.; Becskei, Z.; Stanojković, A.; Ostović, M. Comparison of stress level indicators in blood of free-roaming dogs after transportation and housing in the new environment. J. Appl. Anim. Res. 2017, 45, 52–55. [Google Scholar] [CrossRef]
- Tuli, J.S.; Smith, J.A.; Morton, D.B. Stress measurements in mice after transportation. Lab. Anim. 1995, 29, 132–138. [Google Scholar] [CrossRef]
- Schmidt, A.; Hödl, S.; Möstl, E.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release, heart rate, and heart rate variability in transport-naive horses during repeated road transport. Domest. Anim. Endocrinol. 2010, 39, 205–213. [Google Scholar] [CrossRef]
- Zorawski, M.; Killcross, S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol. Learn. Mem. 2002, 78, 458–464. [Google Scholar] [CrossRef]
- Zorawski, M.; Killcross, S. Glucocorticoid receptor agonist enhances Pavlovian appetitive conditioning but disrupts outcome-specific associations. Behav. Neurosci. 2003, 117, 1453–1457. [Google Scholar]
- Trevisi, E.; Bertoni, G. Some physiological and biochemical methods for acute and chronic stress evaluationin dairy cows. Ital. J. Anim. Sci. 2009, 8, 265–286. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Oh, Y.I.; Song, K.H.; Seo, K.W. Evaluation of salivary vasopressin as an acute stress biomarker in healthy dogs with stress due to noise and environmental challenges. BMC Vet. Res. 2020, 16, 331. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, A.; Uchida, Y.; Nakade, T.; Taguchi, K. Salivary secretory IgA concentrations in beagle dogs. J. Vet. Med. Sci. 2003, 65, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Part, C.E.; Kiddie, J.L.; Hayes, W.A.; Mills, D.S.; Neville, R.F.; Morton, D.B.; Collins, L.M. Physiological, physical and behavioural changes in dogs (Canis familiaris) when kennelled: Testing the validity of stress parameters. Physiol. Behav. 2014, 133, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Previde, E.P.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]

| Subjects | Sex | Age (Years) | Size | Time Spent in the Kennel (Months) |
|---|---|---|---|---|
| Gigio | Male | 3 | Small | 20 |
| Ketty | Female | 2 | Medium | 16 |
| Sara | Female | 2 | Large | 18 |
| Lupetto | Male | 2 | Medium | 16 |
| Lean | Female | 3 | Large | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Angelo, D.; d’Ingeo, S.; Ciani, F.; Visone, M.; Sacchettino, L.; Avallone, L.; Quaranta, A. Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study. Animals 2021, 11, 345. https://doi.org/10.3390/ani11020345
d’Angelo D, d’Ingeo S, Ciani F, Visone M, Sacchettino L, Avallone L, Quaranta A. Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study. Animals. 2021; 11(2):345. https://doi.org/10.3390/ani11020345
Chicago/Turabian Styled’Angelo, Danila, Serenella d’Ingeo, Francesca Ciani, Michele Visone, Luigi Sacchettino, Luigi Avallone, and Angelo Quaranta. 2021. "Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study" Animals 11, no. 2: 345. https://doi.org/10.3390/ani11020345
APA Styled’Angelo, D., d’Ingeo, S., Ciani, F., Visone, M., Sacchettino, L., Avallone, L., & Quaranta, A. (2021). Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study. Animals, 11(2), 345. https://doi.org/10.3390/ani11020345






