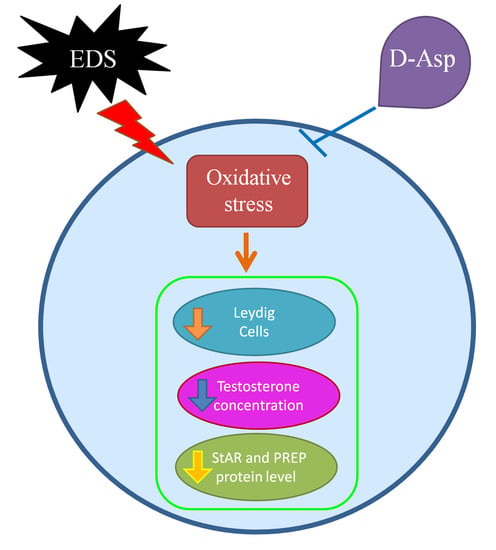
Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Antibodies
2.2. Animals and Experiments
2.3. Histology
2.4. Serum Testosterone Evaluation
2.5. TUNEL Assay
2.6. Thiobarbiturc Acid-Reactive Species (TBARS) Levels Assessment
2.7. Protein Extraction and Western Blotting Analysis
2.8. Immunofluorescence Analysis
2.9. Statistical Analysis
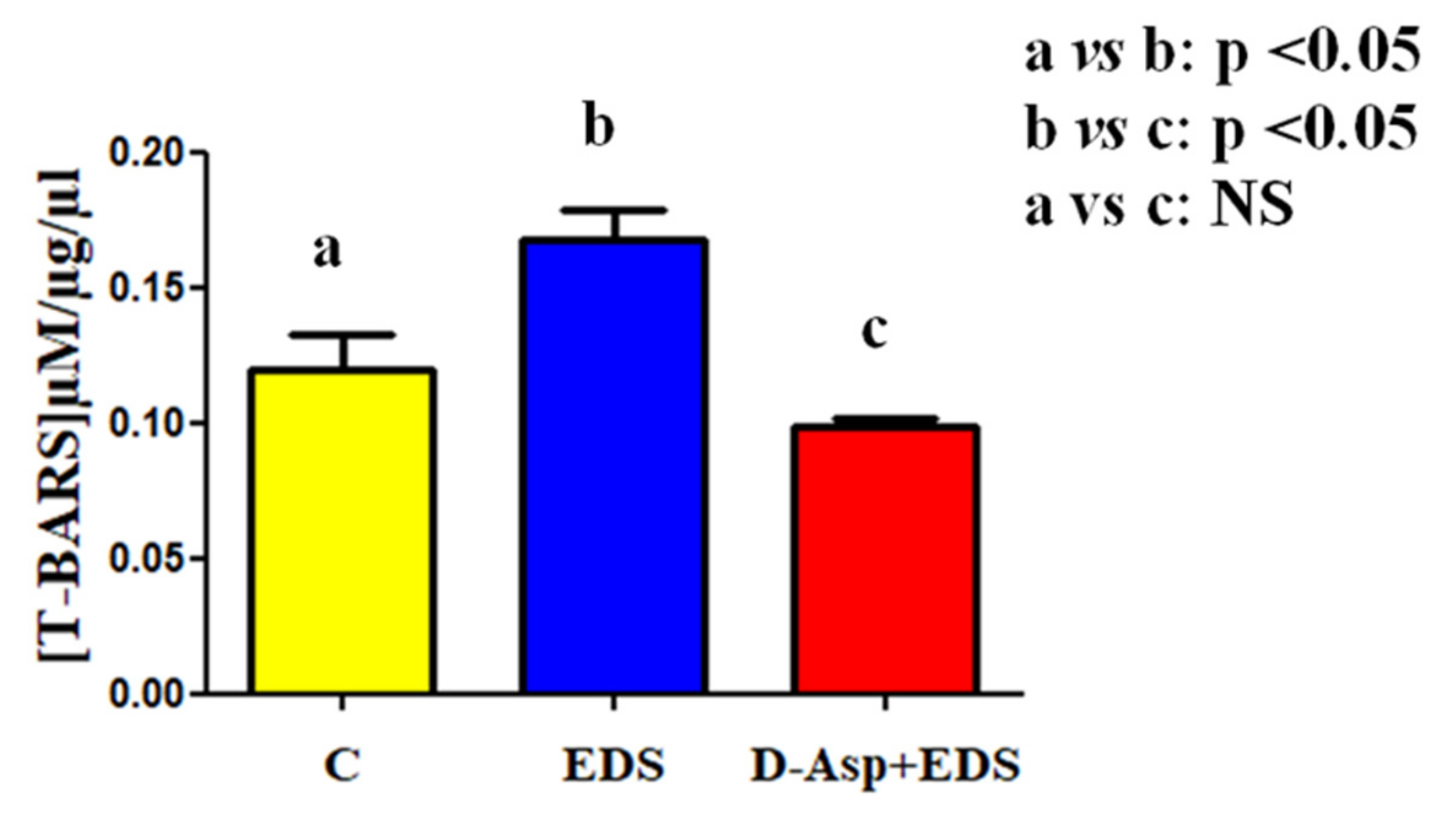
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, G.Q.; Garbers, D.L. Male germ cell specification and differentiation. Dev. Cell 2002, 2, 537–547. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jian, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shen, J.; Xia, Z.; Zhang, R.; Zhang, P.; Zhao, C.; Xing, J.; Chen, L.; Chen, W.; Lin, M.; et al. Proteomic Analysis of Proteins Involved in Spermiogenesis in Mouse. J. Proteome Res. 2010, 9, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Ergoli, M.; Venditti, M.; Piccillo, E.; Minucci, S.; Politano, L. Study of Expression of Genes Potentially Responsible for Reduced Fitness in Patients with Myotonic Dystrophy Type 1 and Identification of New Biomarkers of Testicular Function. Mol. Reprod. Dev. 2020, 87, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Grive, K.J.; Hu, Y.; Shu, E.; Grimson, A.; Elemento, O.; Grenier, J.K.; Cohen, P.E. Dynamic transcriptome profiles within spermatogonial and spermatocyte populations during postnatal testis maturation revealed by single-cell sequencing. PLoS Genetics 2019, 15, 1007810. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: Background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech. 2010, 73, 241–278. [Google Scholar] [CrossRef]
- Huhtaniemi, I. Mechanisms in Endocrinology: Hormonal regulation of spermatogenesis: Mutant mice challenging old paradigms. Eur. J. Endocrinol. 2018, 179, 143–150. [Google Scholar] [CrossRef]
- Endo, T.; Mikedis, M.M.; Nicholls, P.K.; Page, D.C.; de Rooij, D.G. Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules 2019, 9, 775. [Google Scholar] [CrossRef]
- Flück, C.E.; Pandey, A.V. Steroidogenesis of the testis—New genes and pathways. Ann. Endocrinol. Paris. 2014, 75, 40–47. [Google Scholar] [CrossRef]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef]
- Tremblay, J.J. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 2015, 103, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Dolci, S. Paracrine mechanisms involved in the control of early stages of Mammalian spermatogenesis. Front. Endocrinol. 2013, 4, 181. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Furuchi, T.; Homma, H. Free D-aspartate in mammals. Biol. Pharm. Bull. 2005, 28, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, A.; Di Fiore, M.M.; Fisher, G.H.; Milone, A.; Seleni, A.; D’Aniello, S.; Perna, A.F.; Ingrosso, D. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J. 2000, 14, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Homma, H.; Lee, J.A.; Imai, K. D-Aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett. 1999, 444, 160–164. [Google Scholar] [CrossRef]
- Nagata, Y.; Homma, H.; Matsumoto, M.; Imai, K. Stimulation of steroidogenic acute regulatory protein (StAR) gene expression by D-aspartate in rat Leydig cells. FEBS Lett. 1999, 454, 317–320. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Chieffi, P.; Di Fiore, M.M.; Senese, R.; Chieffi Baccari, G. D-Aspartate Induces Proliferative Pathways in Spermatogonial GC-1 Cells. J. Cell. Physiol. 2016, 231, 490–495. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Di Fiore, M.M.; Di Giacomo Russo, F.; Chieffi, P.; Usiello, A.; Pinelli, C.; Baccari, G.C. AMPA receptor expression in mouse testis and spermatogonial GC-1 cells: A study on its regulation by excitatory amino acids. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Santillo, A.; Falvo, S.; Longobardi, S.; Chieffi Baccari, G. Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia. Int. J. Mol. Sci. 2016, 17, 11727. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, Y.; Chen, H.; Jesus, T.; Tang, E.; Li, N.; Lian, Q.; Ge, R.-S.; Cheng, C.Y. Cell polarity, cell adhesion, and spermatogenesis: Role of cytoskeletons. F1000Res. 2017, 6, 1565. [Google Scholar] [CrossRef]
- Santillo, A.; Venditti, M.; Minucci, S.; Chieffi Baccari, G.; Falvo, S.; Rosati, L.; Di Fiore, M.M. D-Asp upregulates PREP and GluA2/3 expressions and induces p-ERK1/2 and p-Akt in rat testis. Reproduction 2019, 158, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Santillo, A.; Falvo, S.; Fiore, M.M.D.; Baccari, G.C.; Minucci, S. D-Aspartate Upregulates DAAM1 Protein Levels in the Rat Testis and Induces Its Localization in Spermatogonia Nucleus. Biomolecules 2020, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Giacone, F.; Condorelli, R.A.; Mongioì, L.M.; Bullara, V.; La Vignera, S.; Calogero, A.E. In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine 2017, 56, 408–415. [Google Scholar] [CrossRef]
- D’Aniello, G.; Ronsini, S.; Guida, F.; Spinelli, P.; D’Aniello, A. Occurrence of D-aspartic acid in human seminal plasma and spermatozoa: Possible role in reproduction. Fertil. Steril. 2005, 84, 1444–1449. [Google Scholar] [CrossRef]
- Tirabassi, G.; Vignini, A.; Tiano, L.; Buldreghini, E.; Brugè, F.; Silvestri, S.; Orlando, P.; D’Aniello, A.; Mazzanti, L.; Lenzi, A.; et al. Protective effects of coenzyme Q10 and aspartic acid on oxidative stress and DNA damage in subjects affected by idiopathic asthenozoospermia. Endocrine 2015, 49, 549–552. [Google Scholar] [CrossRef]
- Minucci, S.; Chieffi Baccari, G.; Di Matteo, L.; Fasano, S.; D’Antonio, M.; Pierantoni, R.; Chieffi, G. Resumption of testicular activity in Gobius paganellus after administration of ethane 1,2- dimethane sulfonate (EDS). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 102, 319–323. [Google Scholar] [CrossRef]
- Minucci, S.; Fasano, S.; Di Matteo, L.; Chieffi Baccari, G.; Pierantoni, R. Morphological and hormonal changes in the frog, Rana esculenta, testis after administration of ethane dimethane sulfonate. Gen. Comp. Endocrinol. 1990, 79, 335–345. [Google Scholar] [CrossRef]
- Minucci, S.; Di Matteo, L.; Fasano, S.; Chieffi Baccari, G.; Pierantoni, R. Regeneration of the testicular interstitial compartment after ethane dimethane sulfonate treatment in the hypophysectomized frog Rana esculenta: Independence of pituitary control. Gen. Comp. Endocrinol. 1994, 95, 84–91. [Google Scholar] [CrossRef]
- Minucci, S.; Fasano, S.; Marmorino, C.; Chieffi, P.; Pierantoni, R. Ethane 1,2-dimethane sulfonate effects on the testis of the lizard, Podarcis s. sicula Raf: Morphological and hormonal changes. Gen. Comp. Endocrinol. 1995, 97, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Minucci, S.; Vitiello, I.I.; Marmorino, C.; Di Matteo, L.; Baccari, G.C. Mast cell-Leydig cell relationships in the testis of the lizard Podarcis s. sicula Raf: Thermal manipulation, ethane 1,2- dimethane sulphonate (EDS) and sex hormone treatment. Zygote 1995, 3, 259–264. [Google Scholar] [CrossRef]
- Minucci, S.; De Rienzo, G.; Di Sena, R.; Cobellis, G.; Meccariello, R.; Pierantoni, R.; Fasano, S. Effects of multiple injections of ethane 1,2-dimethane sulphonate (EDS) on the frog, Rana esculenta, testicular activity. J. Exp. Zool. 2000, 287, 384–393. [Google Scholar] [CrossRef]
- Palmiero, C.; Ferrara, D.; De Rienzo, G.; d’Istria, M.; Minucci, S. Ethane 1,2-dimethane sulphonate is a useful tool for studying cell-to-cell interactions in the testis of the frog, Rana esculenta. Gen. Comp. Endocrinol. 2003, 131, 38–47. [Google Scholar] [CrossRef]
- Ferrara, D.; Palmiero, C.; Branno, M.; Pierantoni, R.; Minucci, S. Testicular activity of Mos in the frog, Rana esculenta: A new role in spermatogonial proliferation. Biol. Reprod. 2004, 70, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Izzo, G.; d’Istria, M.; Ferrara, D.; Serino, I.; Aniello, F.; Minucci, S. Connexin 43 expression in the testis of the frog Rana esculenta. Zygote. 2006, 14, 349–357. [Google Scholar] [CrossRef]
- Kerr, J.B.; Knell, C.M.; Abbott, M.; Donachie, K. Ultrastructural analysis of the effect of ethane dimethanesulphonate on the testis of the rat, guinea pig, hamster and mouse. Cell. Tissue Res. 1987, 249, 451–457. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Boni, R.; Santillo, A.; Falvo, S.; Gallo, A.; Esposito, S.; Baccari, G.C. D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs. Biomolecules 2019, 9, 445. [Google Scholar] [CrossRef]
- Lama, S.; Vanacore, D.; Diano, N.; Nicolucci, C.; Errico, S.; Dallio, M.; Federico, A.; Loguercio, C.; Stiuso, P. Ameliorative effect of Silybin on bisphenol A induced oxidative stress, cell proliferation and steroid hormones oxidation in HepG2 cell cultures. Sci. Rep. 2019, 9, 3228. [Google Scholar] [CrossRef]
- Kechiche, S.; Venditti, M.; Knani, L.; Jabłońska, K.; Dzięgiel, P.; Messaoudi, I.; Reiter, R.J.; Minucci, S. First evidence of the protective role of melatonin in counteracting cadmium toxicity in the rat ovary via the mTOR pathway. Environ. Pollut 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Chemek, M.; Minucci, S.; Messaoudi, I. Cadmium-induced toxicity increases prolyl endopeptidase (PREP) expression in the rat testis. Mol. Reprod. Dev. 2020, 87, 565–573. [Google Scholar] [CrossRef]
- Chemek, M.; Venditti, M.; Boughamoura, S.; Mimouna, S.B.; Messaoudi, I.; Minucci, S. Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J. Cell. Physiol. 2018, 233, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Sabir, S.; Rehman, K. Bisphenol A-induced metabolic disorders: From exposure to mechanism of action. Environ. Toxicol. Pharmacol. 2020, 77, 103373. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Baskaran, S.; Agarwal, A.; Henkel, R. Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 2020, e13646. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mollier, J.; Brocklesby, R.W.K.; Caves, C.; Jayasena, C.N.; Minhas, S. Endocrine-disrupting chemicals and male reproductive health. Reprod. Med. Biol. 2020, 19, 243–253. [Google Scholar] [CrossRef]
- Zini, A.; Schlegel, P.N. Effect of hormonal manipulation on mRNA expression of antioxidant enzymes in the rat testis. J. Urol. 2003, 169, 767–771. [Google Scholar] [CrossRef]
- Barbato, V.; Talevi, R.; Braun, S.; Merolla, A.; Sudhakaran, S.; Longobardi, S.; Gualtieri, R. Supplementation of sperm media with zinc, D-aspartate and co-enzyme Q10 protects bull sperm against exogenous oxidative stress and improves their ability to support embryo development. Zygote 2017, 25, 168–175. [Google Scholar] [CrossRef]
- Gualtieri, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Rizos, D.; Longobardi, S.; Talevi, R. Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology 2014, 82, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef] [PubMed]
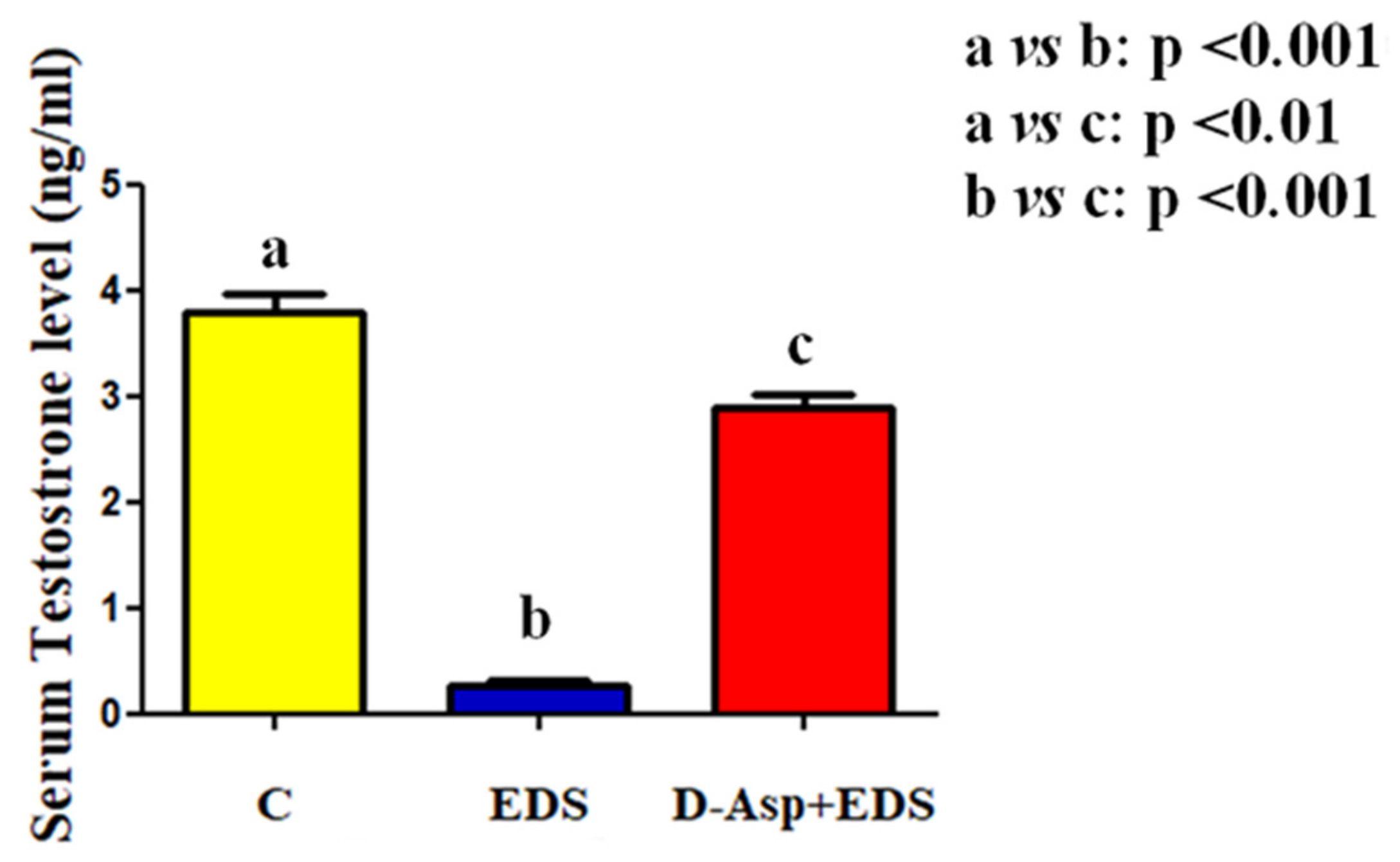

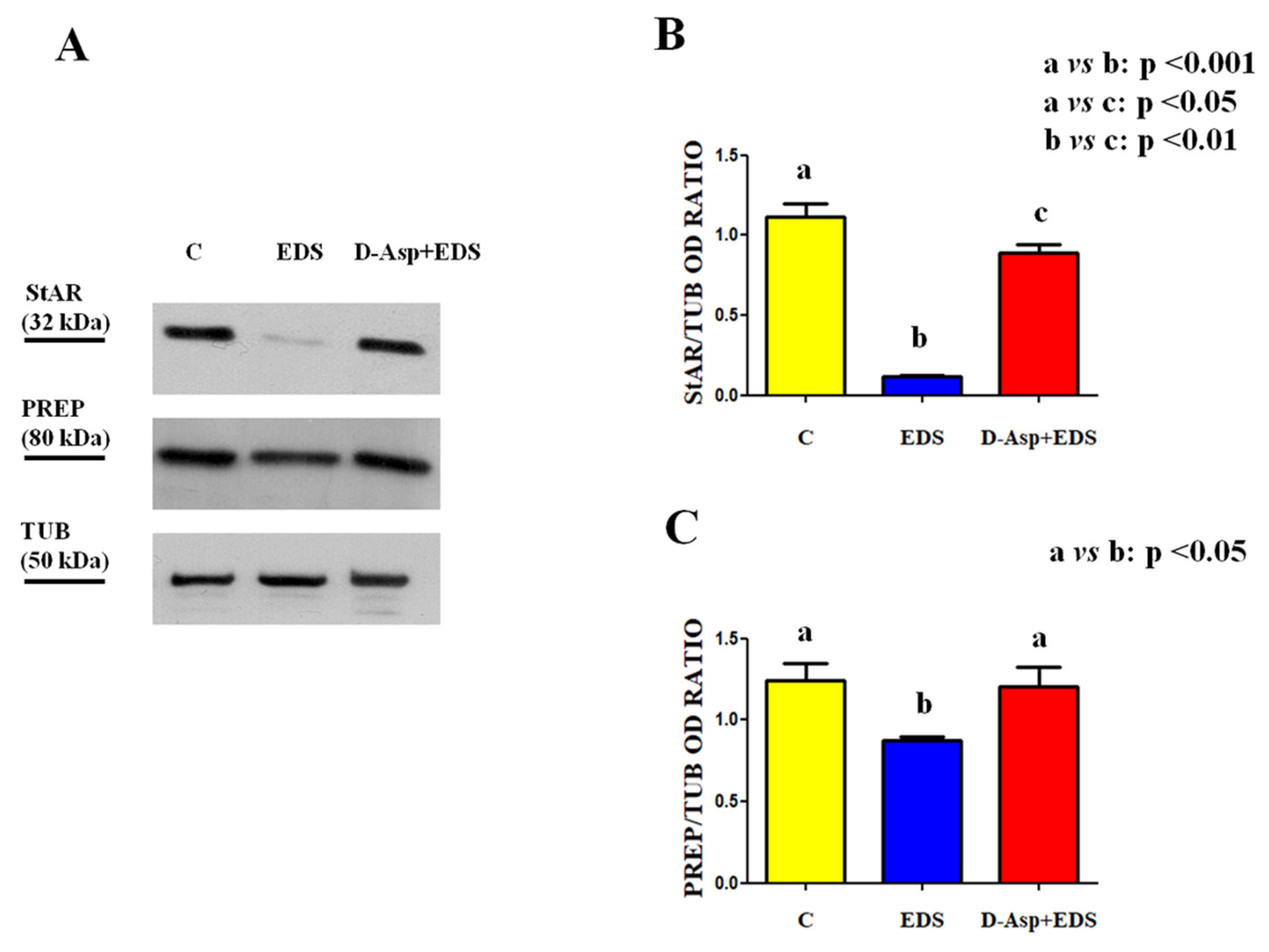
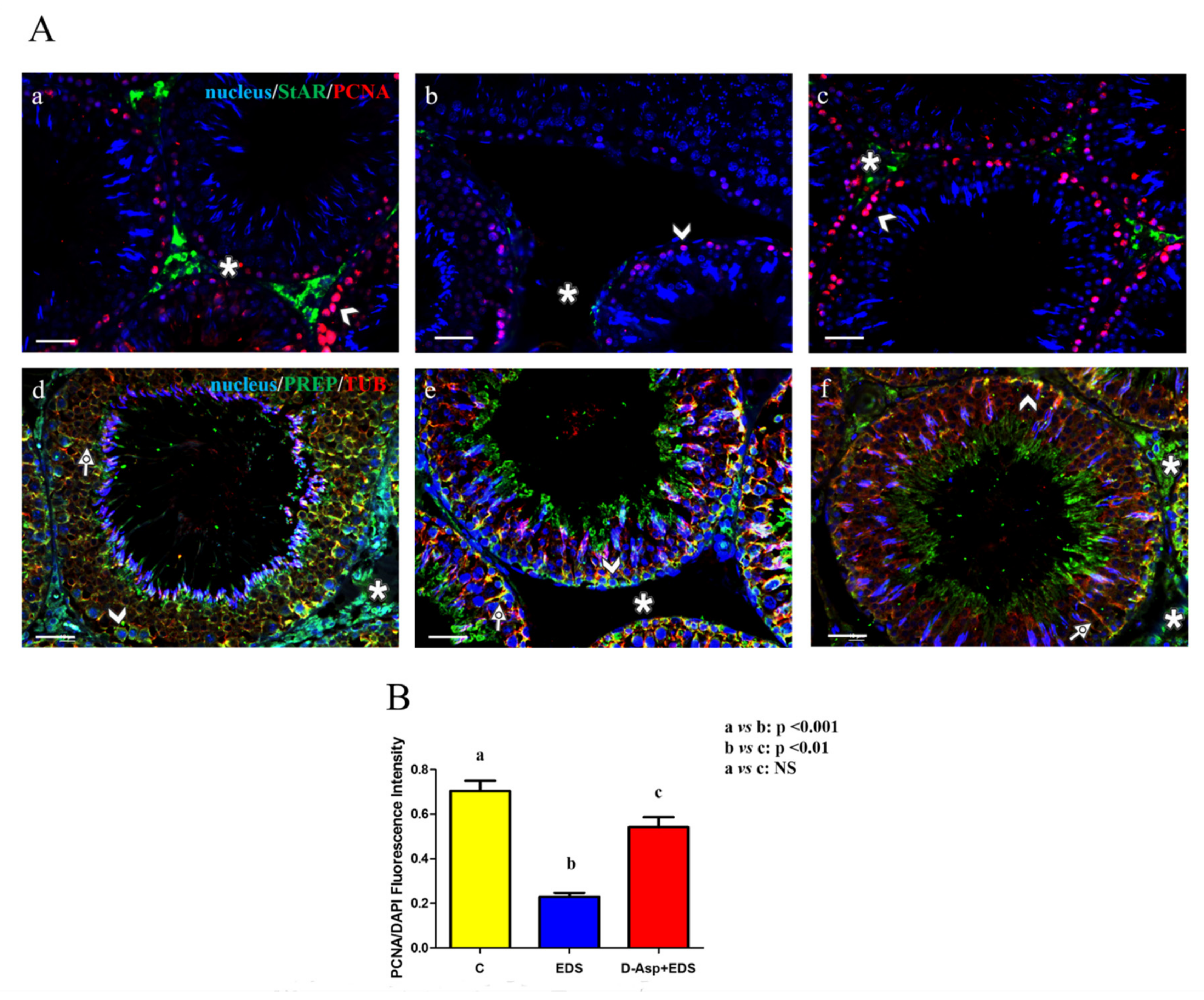
| Groups | Epithelium Thickness (µm) | Epithelium Diameter (µm) Empty Lumen (%) |
|---|---|---|
| C | 80.94 ± 3.37a | 237.61 ± 2.11a 27 ± 8a |
| EDS | 51.16 ± 2.39b | 179.81 ± 4.58c 69 ± 7d |
| D-Asp + EDS | 69.91 ± 5.21c | 221.04 ± 4.02a 33 ± 12a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venditti, M.; Romano, M.Z.; Aniello, F.; Minucci, S. Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis. Animals 2021, 11, 133. https://doi.org/10.3390/ani11010133
Venditti M, Romano MZ, Aniello F, Minucci S. Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis. Animals. 2021; 11(1):133. https://doi.org/10.3390/ani11010133
Chicago/Turabian StyleVenditti, Massimo, Maria Zelinda Romano, Francesco Aniello, and Sergio Minucci. 2021. "Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis" Animals 11, no. 1: 133. https://doi.org/10.3390/ani11010133
APA StyleVenditti, M., Romano, M. Z., Aniello, F., & Minucci, S. (2021). Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis. Animals, 11(1), 133. https://doi.org/10.3390/ani11010133