Transdermal Fentanyl Uptake at Two Different Patch Locations in Swiss White Alpine Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
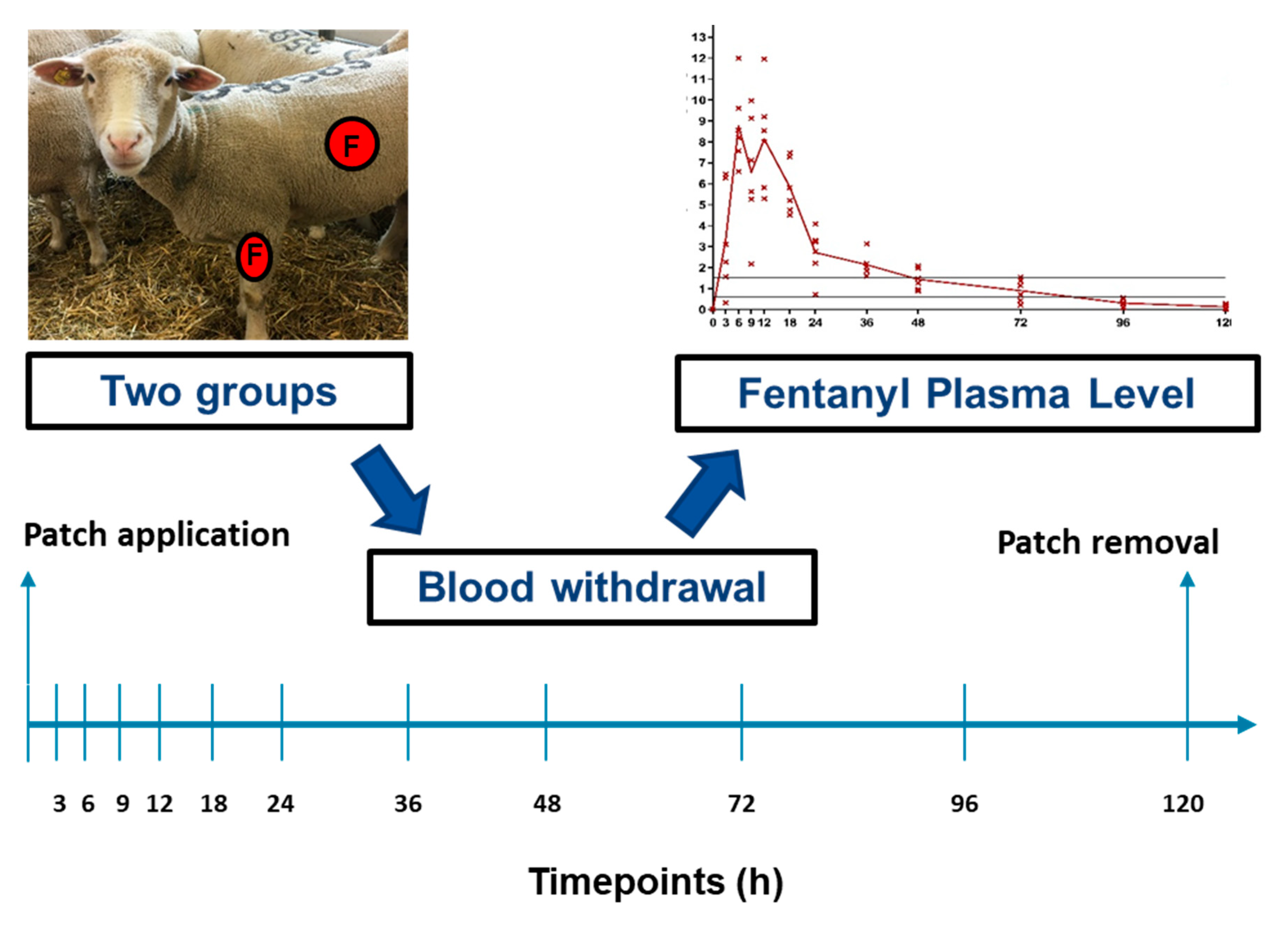
2.1. Study Design
2.2. Animals
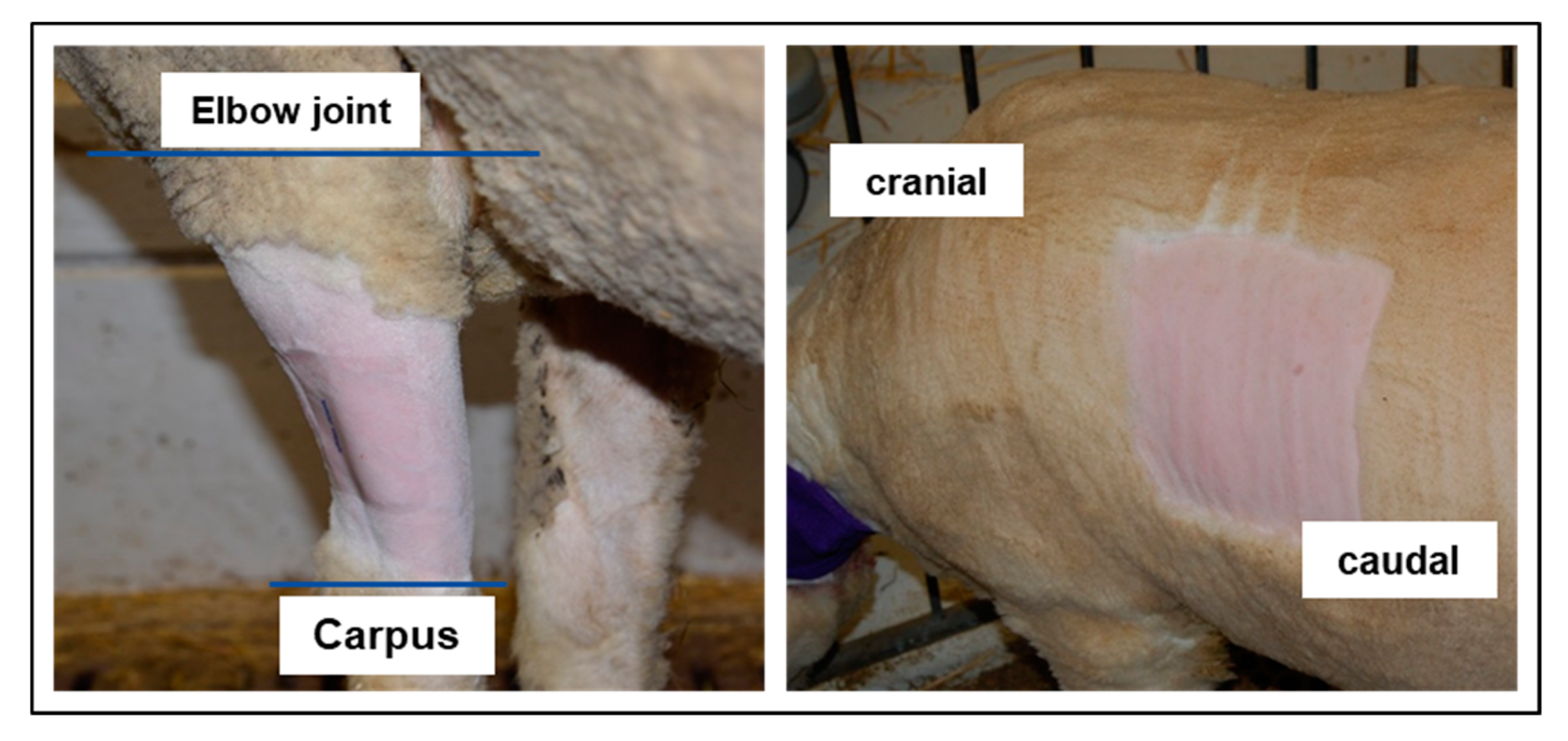
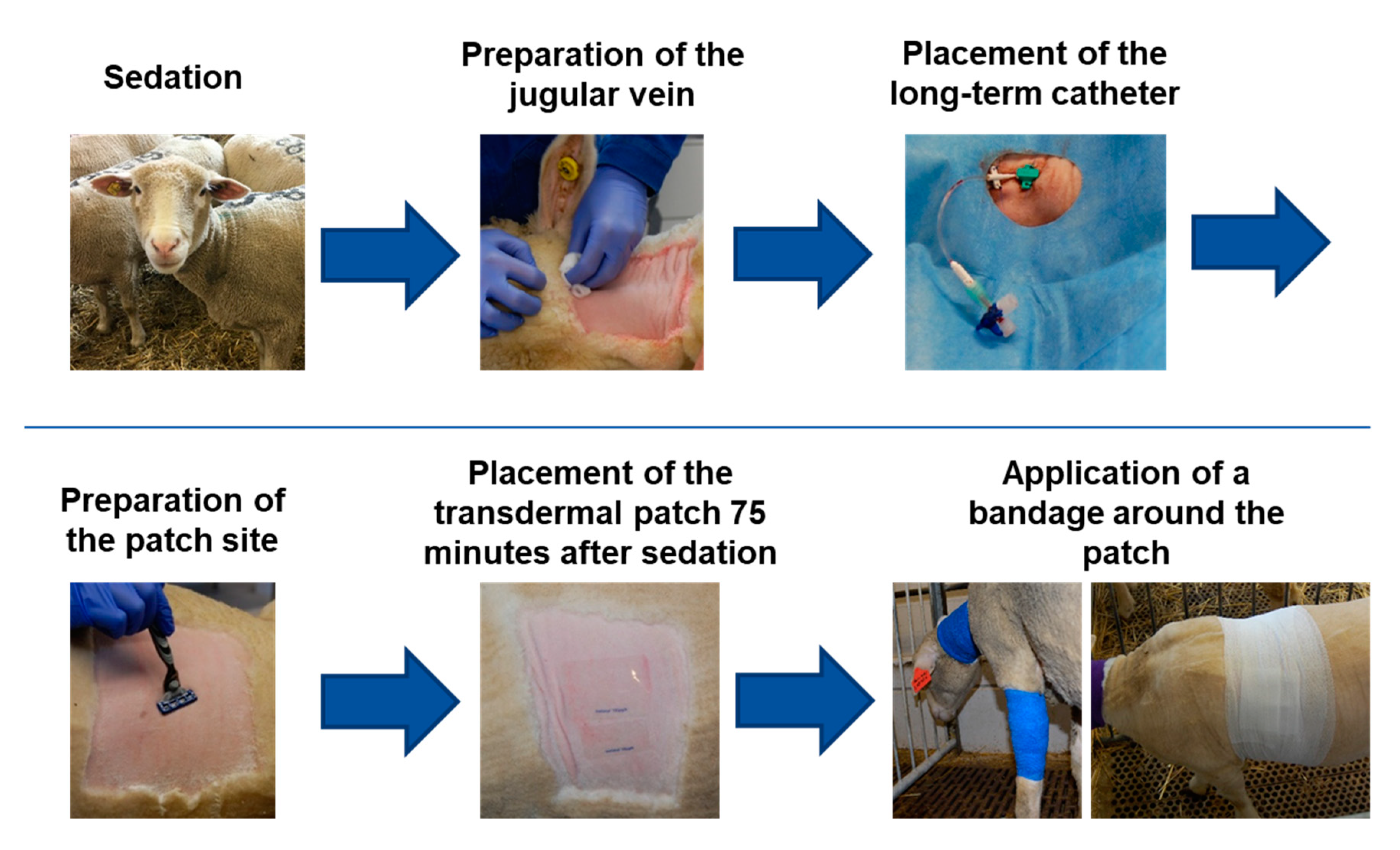
2.3. Transdermal Fentanyl Patch Application and Long-Term Catheter
2.4. Blood Withdrawal
2.5. Blood Analysis
2.6. Study Outcomes
2.7. Statistics
3. Results
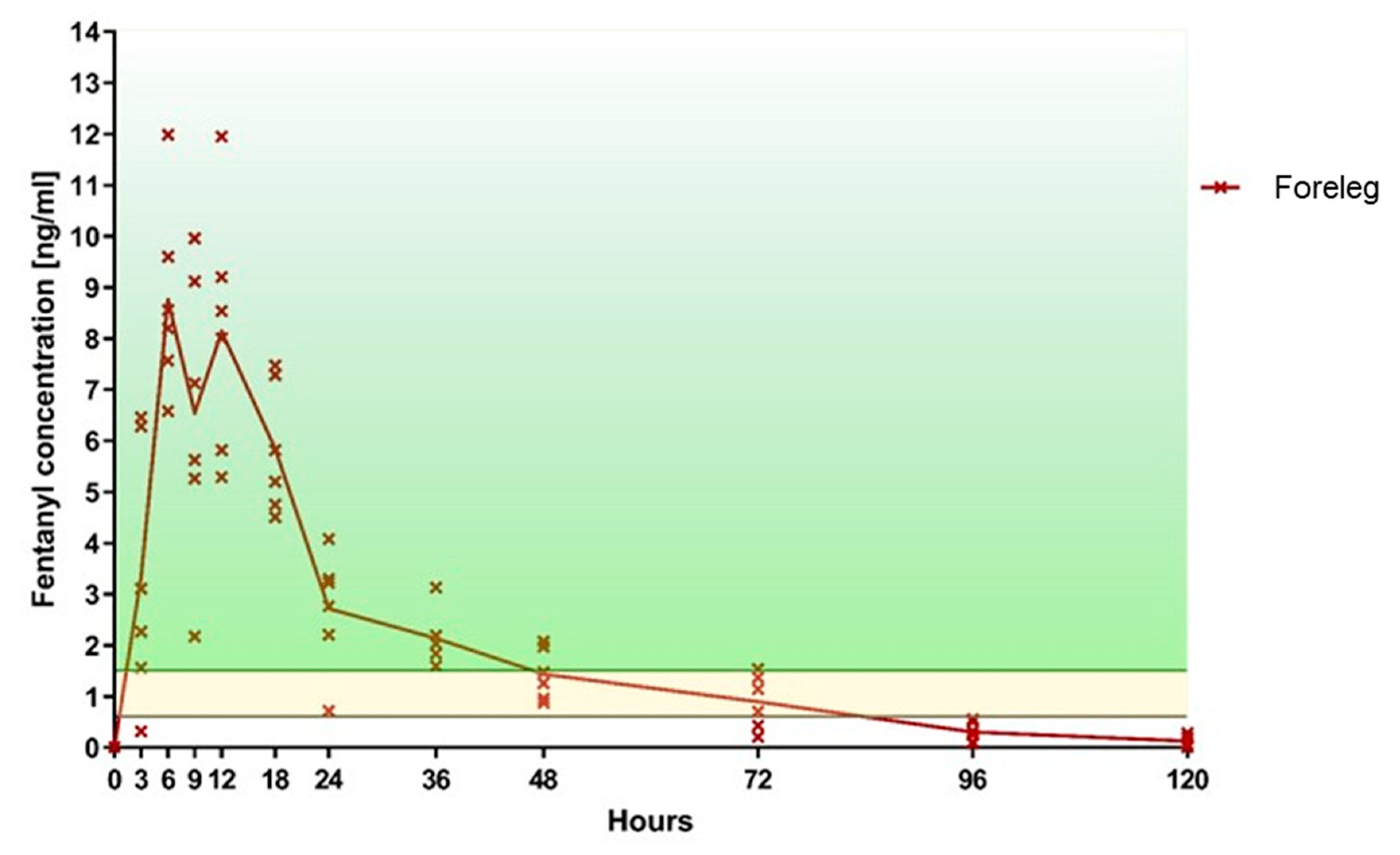
3.1. Primary Outcome: Fentanyl Plasma Level
3.2. Secondary Outcomes
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martini, L.; Fini, M.; Giavaresi, G.; Giardino, R. Sheep model in orthopedic research: A literature review. Comp. Med. 2001, 51, 292–299. [Google Scholar] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Pearce, A.; Richards, R.; Milz, S.; Schneider, E.; Pearce, S. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeiter, S.; Montavon, P.; Schneider, E.; Ito, K. Plate Stabilization With Bone Rivets: An Alternative Method for Internal Fixation of Fractures. J. Orthop. Trauma 2004, 18, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.L.K.; Ritzman, T.F.; Schneider, E.; Knothe, U.R. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum for the repair of long-bone defects. JBJS 2007, 89, 307–316. [Google Scholar] [CrossRef]
- Yamamuro, T.; Shikata, J.; Okumura, H.; Kitsugi, T.; Kakutani, Y.; Matsui, T.; Kokubo, T. Replacement of the lumbar vertebrae of sheep with ceramic prostheses. J. Bone Jt. Surg. Br. 1990, 72, 889–893. [Google Scholar] [CrossRef]
- den Boer, F.C.; Patka, P.; Bakker, F.C.; Wippermann, B.W.; van Lingen, A.; Vink, G.Q.; Boshuizen, K.; Haarman, H.J.T.M. New segmental long bone defect model in sheep: Quantitative analysis of healing with dual energy X-ray absorptiometry. J. Orthop. Res. 1999, 17, 654–660. [Google Scholar] [CrossRef]
- Boot, W.; D’Este, M.; Schmid, T.; Zeiter, S.; Richards, R.G.; Eglin Moriarty, T.F.D. Local antibiotic delivery from a thermoresponsive hyaluronan hydrogel successfully treats a chronic implant-related infection in a single stage revision in sheep. In Orthopaedic Proceedings Volume 99-B, Issue Supp 22, Proceedings of the European Bone and Joint Infection Society (EBJIS), Nantes, France, 7–9 September 2017; The Bone & Joint Journal: Nantes, France, 2017; p. 99. [Google Scholar]
- Pobloth, A.M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.; Roth, C.P.; Schaser, K.D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, 423. [Google Scholar] [CrossRef]
- Newman, E.; Turner, A.; Wark, J. The potential of sheep for the study of osteopenia: Current status and comparison with other animal models. Bone 1995, 16, S277–S284. [Google Scholar] [CrossRef]
- Carbone, L.; Austin, J. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef]
- Flecknell, P. Analgesia from a veterinary perspective. Br. J. Anaesth. 2008, 101, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Huxley, J.; Whay, H. Current attitudes of cattle practitioners to pain and the use of analgesics in cattle. Vet. Rec. 2006, 159, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.; Christie, M.; Nicholson, A. 14—Opioid Analgesics, in Small Animal Clinical Pharmacology, 2nd ed.; Maddison, J.E., Page, S.W., Church, D.B., Eds.; W.B. Saunders: Edinburgh, UK, 2008; pp. 309–329. [Google Scholar]
- Janssen, P.A. A review of the chemical features associated with strong morphine-like activity. BJA Br. J. Anaesth. 1962, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Dyson, D.H. Perioperative pain management in veterinary patients. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1309–1327. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Allen, H.W.; Olsson, G.L. Patient-controlled Epidural Analgesia with Bupivacaine and Fentanyl on Hospital Wards Prospective Experience with 1030 Surgical Patients. Anesthesiol. J. Am. Soc. Anesthesiol. 1998, 88, 688–695. [Google Scholar]
- Twycross, R.G. Palliative Care Formulary; Radcliffe Publishing: Oxford, UK, 2002. [Google Scholar]
- Vadivelu, N.; Mitra, S.; Narayan, D. Recent advances in postoperative pain management. Yale J. Biol. Med. 2010, 83, 11–25. [Google Scholar]
- Christou, C.; Oliver, R.A.; Rawlinson, J.; Walsh, W.R. Transdermal fentanyl and its use in ovine surgery. Res. Vet. Sci. 2015, 100, 252–256. [Google Scholar] [CrossRef]
- Holley, F.; Van Steennis, C. Postoperative analgesia with fentanyl: Pharmacokinetics and pharmacodynamics of constant-rate iv and transdermal delivery. BJA Br. J. Anaesth. 1988, 60, 608–613. [Google Scholar] [CrossRef]
- Ahern, B.J.; Soma, L.R.; Boston, R.C.; Schaer, T.P. Comparison of the analgesic properties of transdermally administered fentanyl and intramuscularly administered buprenorphine during and following experimental orthopedic surgery in sheep. Am. J. Vet. Res. 2009, 70, 418–422. [Google Scholar] [CrossRef]
- Robinson, T.M.; Kruse-Elliott, K.T.; Markel, M.D.; Pluhar, G.E.; Massa, K.; Bjorling, D.E. A comparison of transdermal fentanyl versus epidural morphine for analgesia in dogs undergoing major orthopedic surgery. J. Am. Anim. Hosp. Assoc. 1999, 35, 95–100. [Google Scholar] [CrossRef]
- Mills, P.C.; Cross, S.E. Regional differences in transdermal penetration of fentanyl through equine skin. Res. Vet. Sci. 2007, 82, 252–256. [Google Scholar] [CrossRef]
- Orsini, J.A.; Moate, P.J.; Kuersten, K.; Soma, L.R.; Boston, R.C. Pharmacokinetics of fentanyl delivered transdermally in healthy adult horses—Variability among horses and its clinical implications. J. Vet. Pharm. 2006, 29, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.G.; Baird, N. Sheep & Goat Medicine; Elsevier Health Sciences: Philadelphia, PA, USA, 2012. [Google Scholar]
- Ivar Seldinger, S. Catheter replacement of the needle in percutaneous arteriography: A new technique. Acta Radiol. 2008, 49 (Suppl. 434), 47–52. [Google Scholar] [CrossRef] [PubMed]
- Grape, S.; Schug, S.A.; Lauer, S.; Schug, B.S. Formulations of fentanyl for the management of pain. Drugs 2010, 70, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.B.; Motzel, S.L.; Das, S.R. Postoperative pain management using fentanyl patches in dogs. Contemp. Top. Lab. Anim. Sci. 2003, 42, 21–26. [Google Scholar]
- Pettifer, G.R.; Hosgood, G. The effect of rectal temperature on perianesthetic serum concentrations of transdermally administered fentanyl in cats anesthetized with isoflurane. Am. J. Vet. Res. 2003, 64, 1557–1561. [Google Scholar] [CrossRef]
- Riviere, J.E.; Papich, M.G. Potential and problems of developing transdermal patches for veterinary applications. Adv. Drug Deliv. Rev. 2001, 50, 175–203. [Google Scholar] [CrossRef]
- Lyne, A.G.; Hollis, D.E. The skin of the sheep: A comparison of body regions. Aust. J. Biol. Sci. 1968, 21, 499–527. [Google Scholar] [CrossRef]
- Bouclier, M.; Cavey, D.; Kail, N.; Hensby, C. Experimental models in skin pharmacology. Pharmacol. Rev. 1990, 42, 127–154. [Google Scholar]
- Bartek, M.J.; LaBudde, J.A.; Maibach, H.I. Skin permeability in vivo: Comparison in rat, rabbit, pig and man. J. Investig. Dermatol. 1972, 58, 114–123. [Google Scholar] [CrossRef]
- Jen, K.Y.; Dyson, M.C.; Lester, P.A.; Nemzek, J.A. Pharmacokinetics of a Transdermal Fentanyl Solution in Suffolk Sheep (Ovis aries). J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 550–557. [Google Scholar]
- Musk, G.C.; Catanchin, C.S.M.; Usuda, H.; Woodward, E.; Kemp, M.W. The uptake of transdermal fentanyl in a pregnant sheep model. Vet. Anaesth. Analg. 2017, 44, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. The transdermal delivery of fentanyl. Eur. J. Pharm. Biopharm. 2013, 84, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Thiede, A.J.; Garcia, K.D.; Stolarik, D.F.; Ma, J.; Jenkins, G.J.; Nunamaker, E.A. Pharmacokinetics of sustained-release and transdermal buprenorphine in Göttingen minipigs (Sus scrofa domestica). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 692–699. [Google Scholar] [PubMed]
- Marchionatti, E.; Lardé, H.; Steagall, P.V. Opioid-induced adverse effects in a Holstein calf. Vet. Anaesth. Analg. 2015, 42, 229–230. [Google Scholar] [CrossRef]
- Grimm, K.A.; Lamont, L.A.; Tranquilli, W.J.; Greene, S.A.; Robertson, S.A. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Linton, D.; Wilson, M.; Newbound, G.; Freise, K.; Clark, T. The effectiveness of a long-acting transdermal fentanyl solution compared to buprenorphine for the control of postoperative pain in dogs in a randomized, multicentered clinical study. J. Vet. Pharmacol. Ther. 2012, 35, 53–64. [Google Scholar] [CrossRef]
- Otto, K.A.; Short, C.E. Pharmaceutical control of pain in large animals. Appl. Anim. Behav. Sci. 1998, 59, 157–169. [Google Scholar] [CrossRef]
- Varvel, J.R.; Shafer, S.L.; Hwang, S.S.; Coen, P.A.; Stanski, D.R. Absorption characteristics of transdermally administered fentanyl. Anesthesiol. J. Am. Soc. Anesthesiol. 1989, 70, 928–934. [Google Scholar] [CrossRef]
- Calis, K.A.; Kohler, D.R.; Corso, D.M. Transdermally administered fentanyl for pain management. Clin. Pharm. 1992, 11, 22–36. [Google Scholar]
- Gourlay, G.K.; Kowalski, S.R.; Plummer, J.L.; Cousins, M.J.; Armstrong, P.J. Fentanyl blood concentration-analgesic response relationship in the treatment of postoperative pain. Anesth. Analg. 1988, 67, 329–337. [Google Scholar] [CrossRef]
- Rohrbach, H.; Andersen, O.K.; Zeiter, S.; Wieling, R.; Spadavecchia, C. Repeated electrical stimulations as a tool to evoke temporal summation of nociceptive inputs in healthy, non-medicated experimental sheep. Physiol. Behav. 2015, 142, 85–89. [Google Scholar] [CrossRef]
- Berset, C.M.; Lanker, U.; Zeiter, S. Survey on Sheep Usage in Biomedical Research. Animals 2020, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Solassol, I.; Caumette, L.; Bressolle, F.; Garcia, F.; Thézenas, S.; Astre, C.; Culine, S.; Coulouma, R.; Pinguet, F. Inter- and intra-individual variability in transdermal fentanyl absorption in cancer pain patients. Oncol. Rep. 2005, 14, 1029–1036. [Google Scholar] [CrossRef]
- Suárez-Morales, M.; Mendoza-Popoca, C.Ú. Intravenous total anesthesia in neuroanesthesia: Influence of gender and gender/age on fentanyl consumption. Rev. Mex. Anestesiol. 2008, 31, 160–165. [Google Scholar]
- Kest, B.; Sarton, E.; Dahan, A. Gender Differences in Opioid-mediated AnalgesiaAnimal and Human Studies. Anesthesiol. J. Am. Soc. Anesthesiol. 2000, 93, 539–547. [Google Scholar]
- Badillo-Martinez, D.; Kirchgessner, A.L.; Butler, P.D.; Bodnar, R. Monosodium glutamate and analgesia induced by morphine: Test-specific effects. Neuropharmacology 1984, 23, 1141–1149. [Google Scholar] [CrossRef]
- Craft, R.; Stratmann, J.; Bartok, R.; Walpole, T.; King, S. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology 1999, 143, 1–7. [Google Scholar] [CrossRef]
- Kasson, B.G.; George, R. Endocrine influences on the actions of morphine: IV. Effects of sex and strain. Life Sci. 1984, 34, 1627–1634. [Google Scholar] [CrossRef]
- Bartok, R.E.; Craft, R.M. Sex differences in opioid antinociception. J. Pharmacol. Exp. Ther. 1997, 282, 769–778. [Google Scholar]
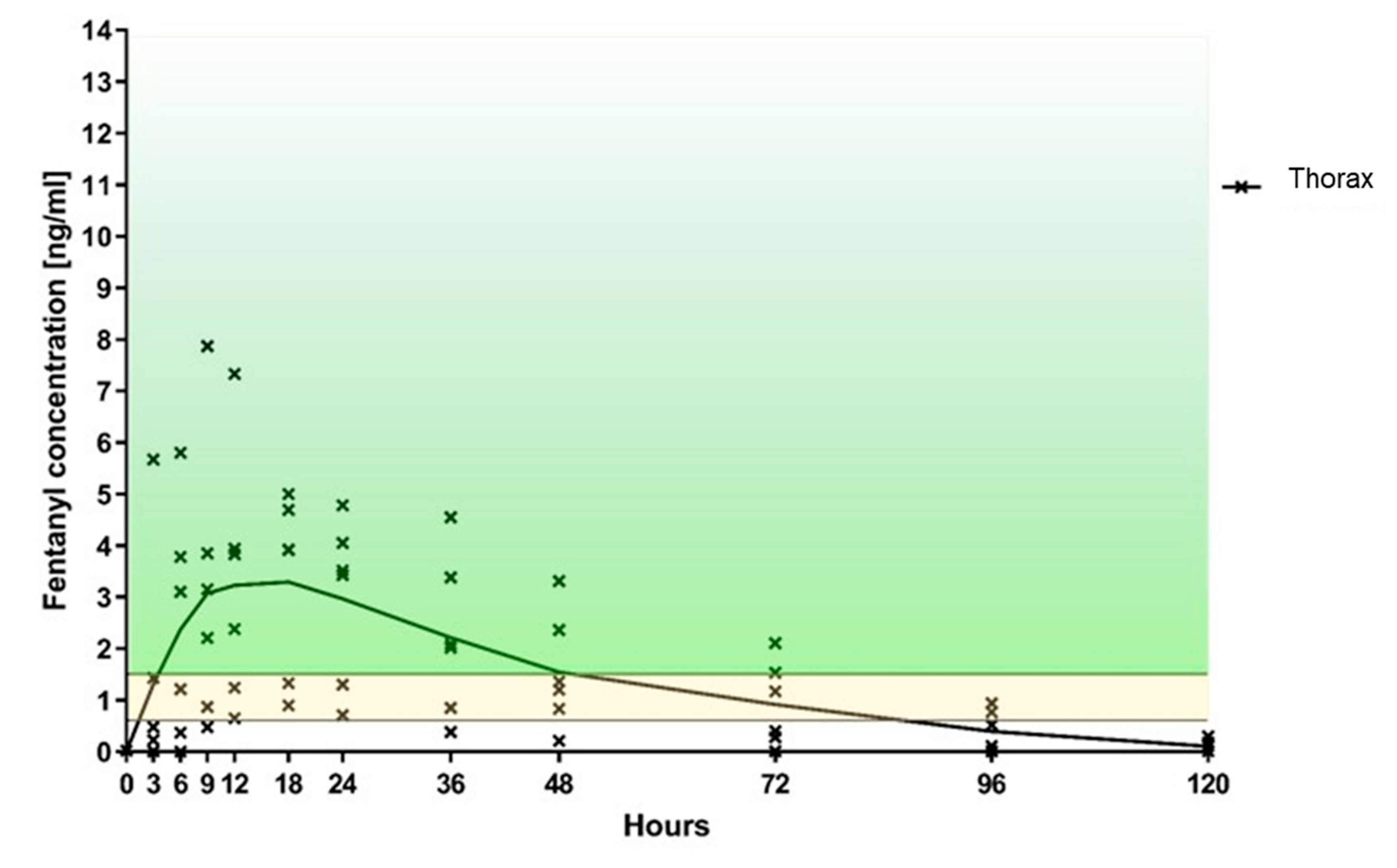
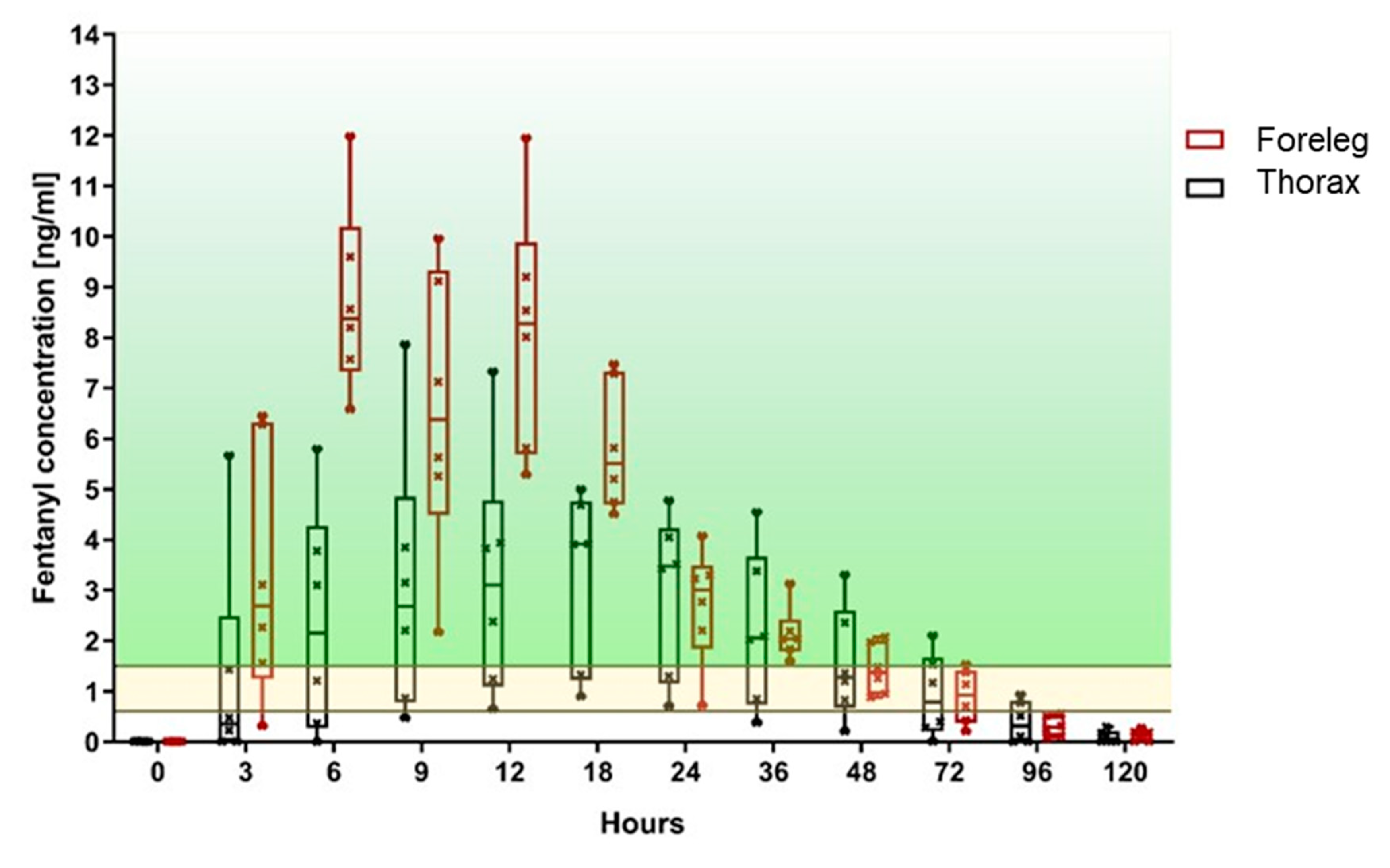
| Fentanyl Concentration ng/ml | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foreleg | Thorax | |||||||||||
| Timepoint | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 0 h | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 3 h | 3.11 | 6.46 | 1.56 | 0.32 | 2.27 | 6.28 | 5.67 | 0.01 | 1.43 | 0.01 | 0.48 | 0.23 |
| 6 h | 8.56 | 7.57 | 6.58 | 8.20 | 11.99 | 9.60 | 5.80 | 0.37 | 3.78 | 0.00 | 3.10 | 1.21 |
| 9 h | 9.12 | 9.96 | 5.63 | 7.13 | 5.26 | 2.17 | 7.87 | 0.48 | 3.85 | 0.87 | 3.15 | 2.21 |
| 12 h | 9.20 | 5.29 | 8.54 | 8.01 | 11.95 | 5.82 | 7.33 | 0.65 | 3.94 | 1.24 | 3.83 | 2.38 |
| 18 h | 7.29 | 4.51 | 4.75 | 5.20 | 7.48 | 5.82 | 5.00 | 0.90 | 3.91 | 1.33 | 3.92 | 4.69 |
| 24 h | 2.21 | 3.30 | 4.08 | 0.72 | 2.77 | 3.23 | 4.05 | 0.71 | 3.52 | 1.30 | 3.43 | 4.78 |
| 36 h | 2.03 | 1.84 | 3.13 | 1.60 | 2.19 | 2.04 | 2.09 | 0.38 | 3.38 | 0.85 | 2.02 | 4.55 |
| 48 h | 1.26 | 0.88 | 1.97 | 1.48 | 0.96 | 2.08 | 1.20 | 0.21 | 2.36 | 0.83 | 1.36 | 3.31 |
| 72 h | 1.37 | 0.71 | 1.14 | 0.43 | 0.21 | 1.54 | 1.17 | 0.01 | 1.53 | 0.40 | 0.28 | 2.11 |
| 96 h | 0.51 | 0.08 | 0.32 | 0.56 | 0.11 | 0.26 | 0.52 | 0.01 | 0.77 | 0.11 | 0.01 | 0.94 |
| 120 h | 0.19 | 0.01 | 0.20 | 0.07 | 0.01 | 0.28 | 0.30 | 0.01 | 0.18 | 0.01 | 0.01 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchholz, T.; Hildebrand, M.; Heider, A.; Stenger, V.; Arens, D.; Spadavecchia, C.; Zeiter, S. Transdermal Fentanyl Uptake at Two Different Patch Locations in Swiss White Alpine Sheep. Animals 2020, 10, 1675. https://doi.org/10.3390/ani10091675
Buchholz T, Hildebrand M, Heider A, Stenger V, Arens D, Spadavecchia C, Zeiter S. Transdermal Fentanyl Uptake at Two Different Patch Locations in Swiss White Alpine Sheep. Animals. 2020; 10(9):1675. https://doi.org/10.3390/ani10091675
Chicago/Turabian StyleBuchholz, Tim, Maria Hildebrand, Anja Heider, Valentina Stenger, Daniel Arens, Claudia Spadavecchia, and Stephan Zeiter. 2020. "Transdermal Fentanyl Uptake at Two Different Patch Locations in Swiss White Alpine Sheep" Animals 10, no. 9: 1675. https://doi.org/10.3390/ani10091675
APA StyleBuchholz, T., Hildebrand, M., Heider, A., Stenger, V., Arens, D., Spadavecchia, C., & Zeiter, S. (2020). Transdermal Fentanyl Uptake at Two Different Patch Locations in Swiss White Alpine Sheep. Animals, 10(9), 1675. https://doi.org/10.3390/ani10091675





