Pork Production with Entire Males: Directions for Control of Boar Taint
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Why Is Castration an Issue Now?
1.2. Raising Entire Males
2. Boar Taint
3. Boar Taint Metabolism
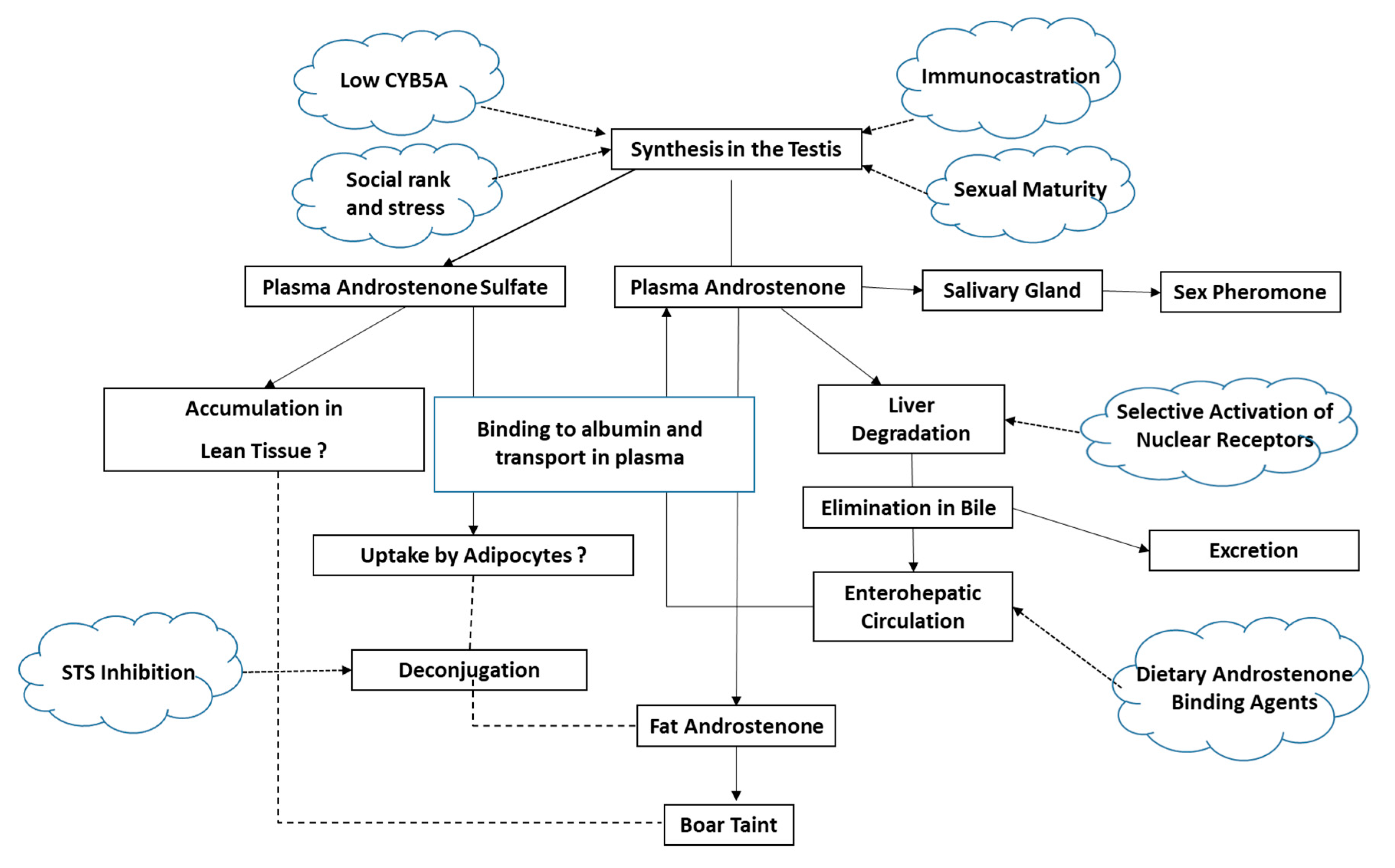
3.1. Androstenone Synthesis, Metabolism and Transport
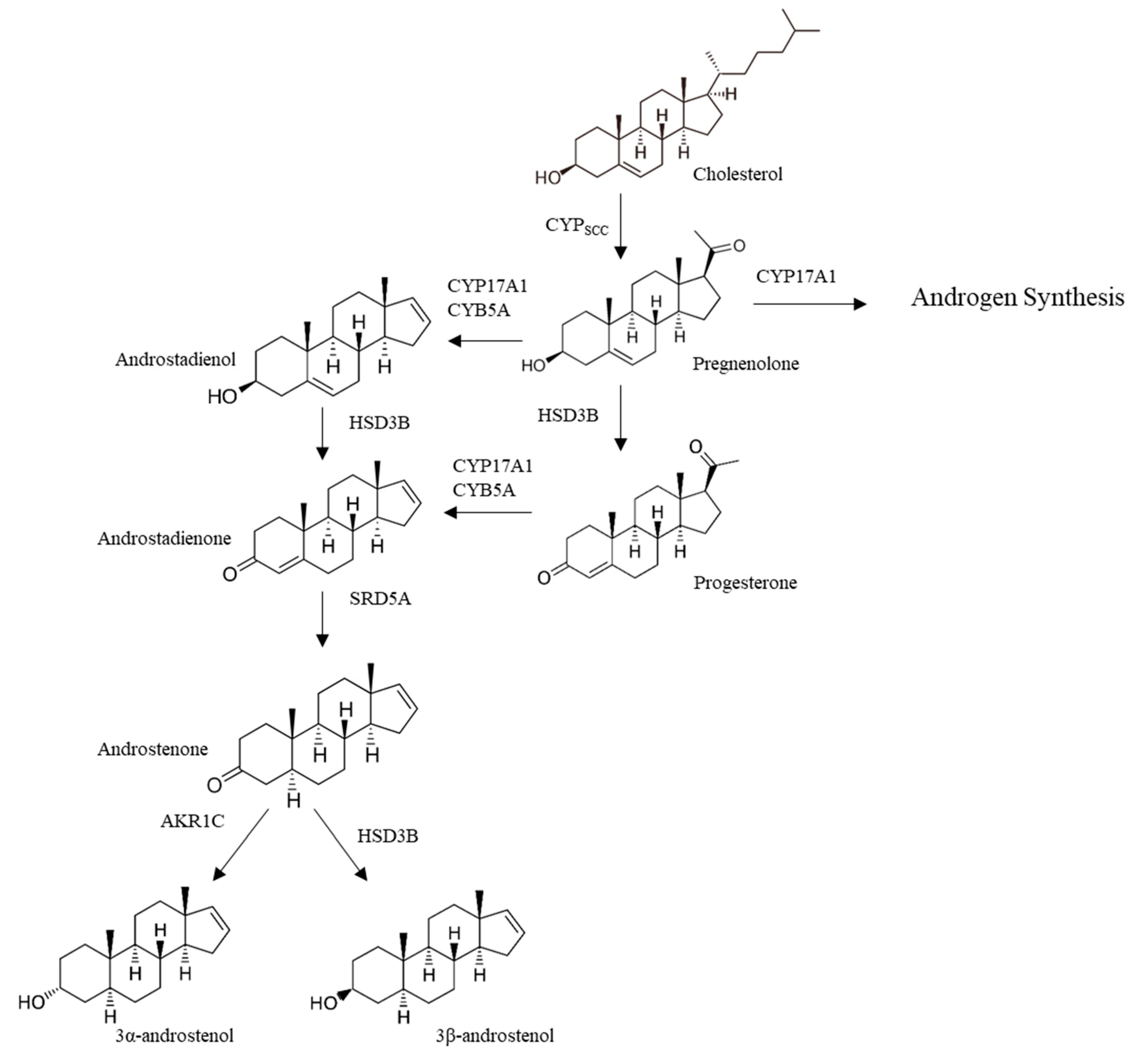
3.1.1. Androstenone Synthesis
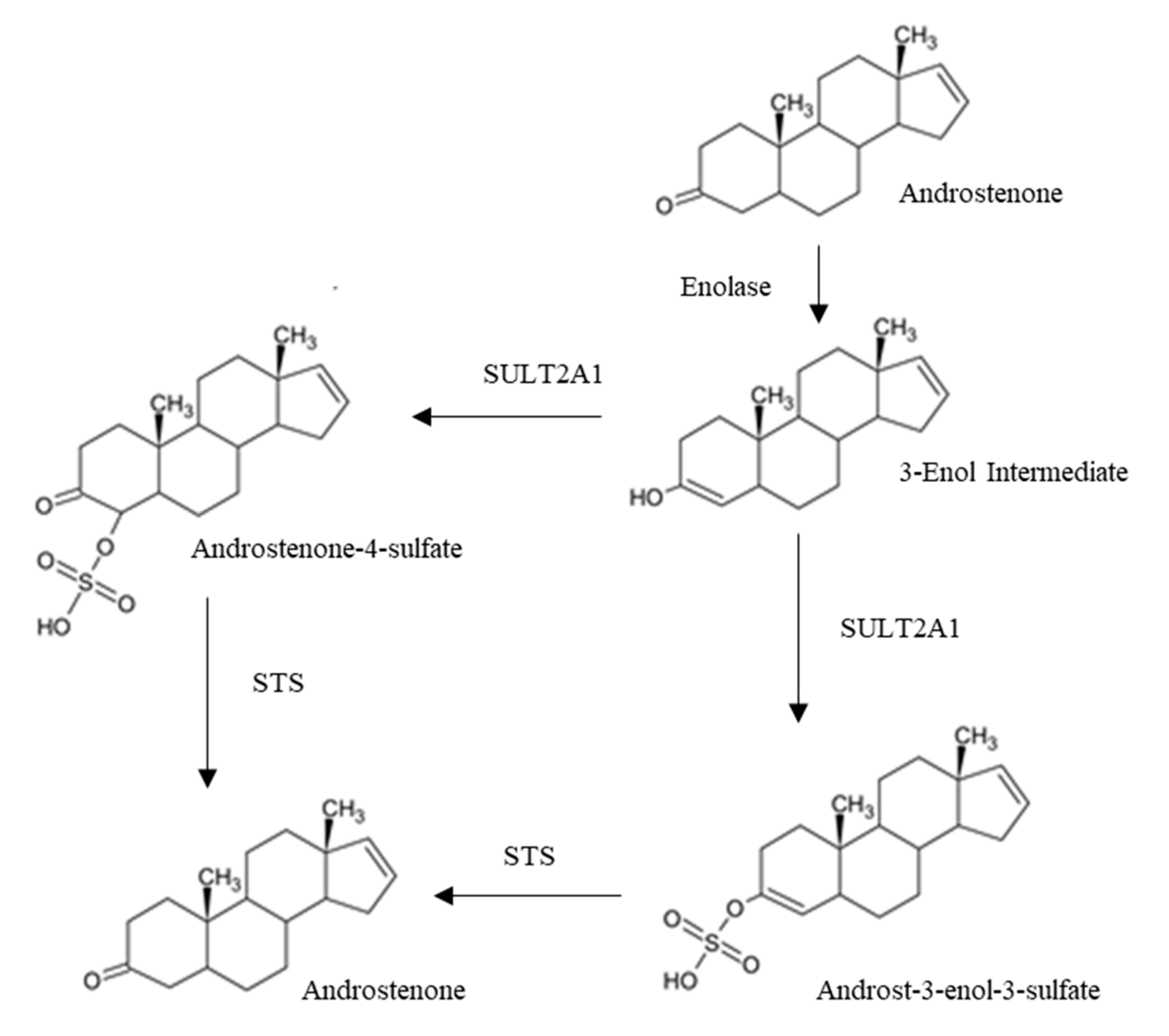
3.1.2. Androstenone Metabolism
3.1.3. Androstenone Transport
3.1.4. Aspects That Are Not Yet Well Understood
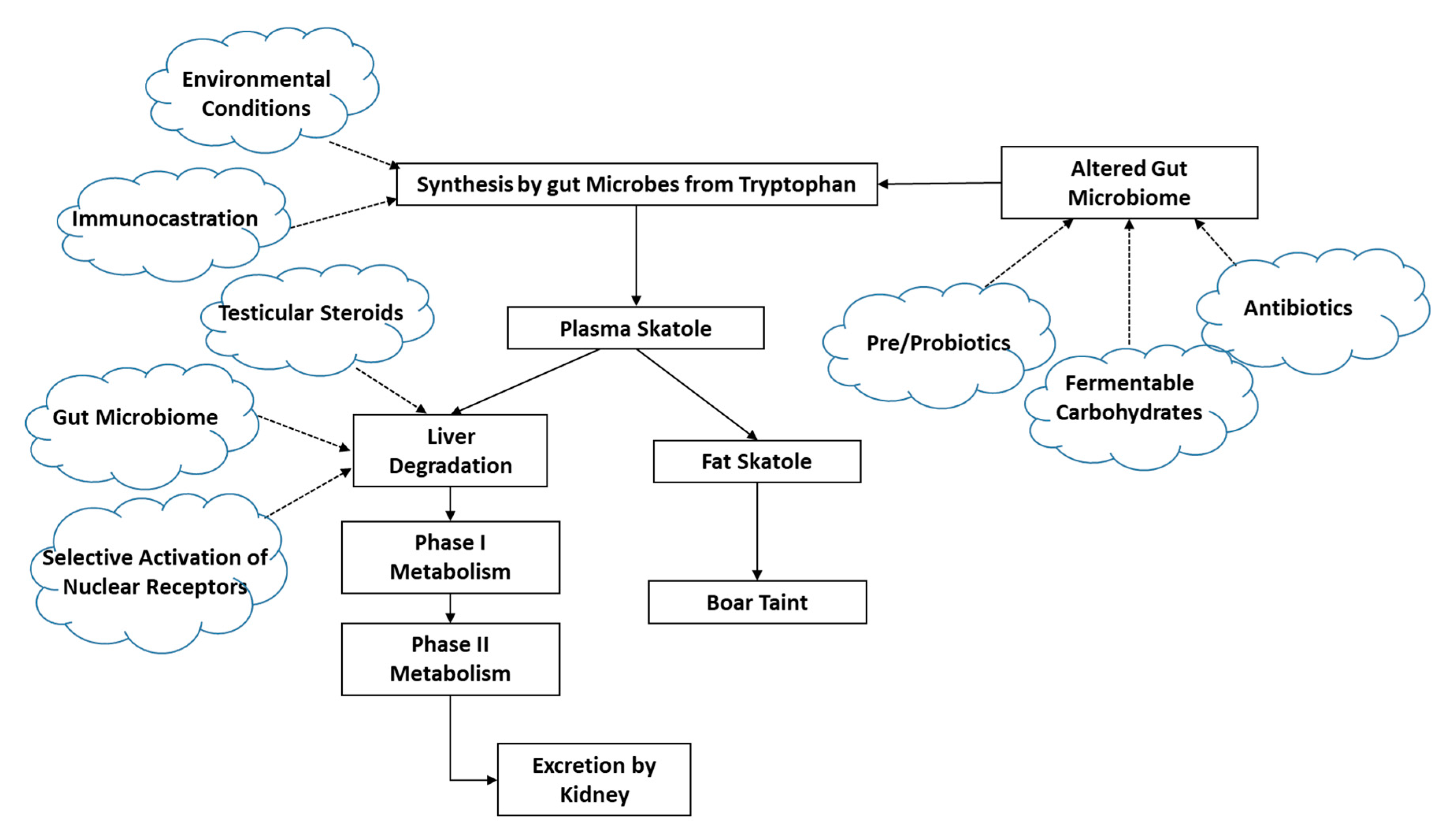
3.2. Skatole Synthesis and Metabolism
3.2.1. Skatole Production
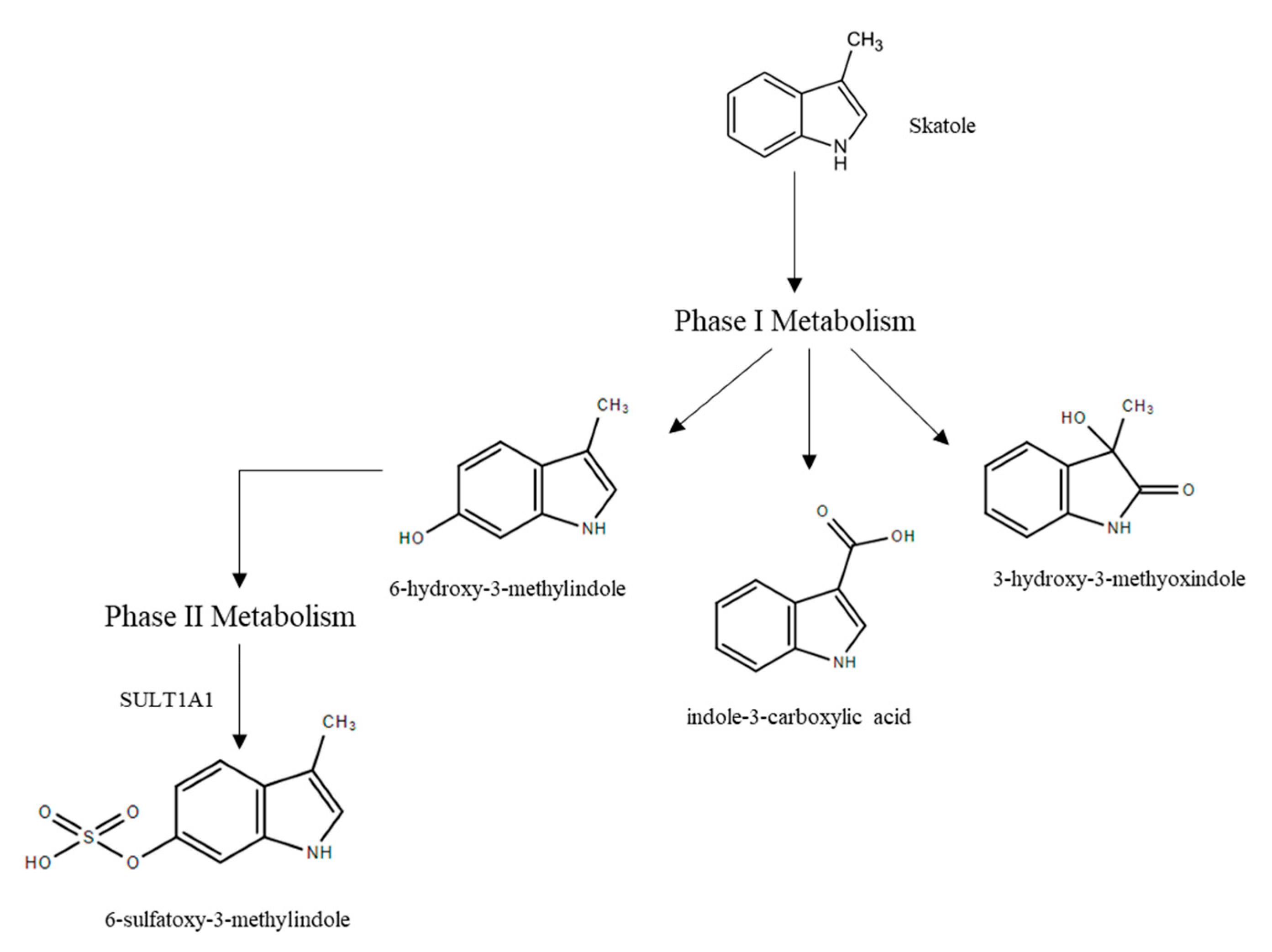
3.2.2. Skatole Metabolism and Clearance
3.2.3. Aspects That Are Not Yet Well Understood
4. Potential Solutions for the Boar Taint Problem
4.1. Assessment of Boar Taint and Carcass Sorting
4.2. Dietary Approaches
4.3. Environmental and Management Approaches
4.4. Effect of Sexual Maturity
4.5. Immunocastration
4.6. Role of Genetics
4.6.1. Selective Breeding
4.6.2. Gene Editing
5. Developing Custom Solutions for Boar Taint
5.1. Control of Androstenone
5.2. Control of Skatole
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gunn, M.G.; Allen, P.; Bonneau, M.; Byrne, D.V.; Cinotti, S.; Fredriksen, B.; Hansen, L.L.; Karlsson, A.H.; Linder, M.G.; Lundström, K.; et al. Welfare aspects of the castration of piglets. Scientific report on the scientific panel for animal health and welfare on a request from the commission related to welfare aspects of the castration of piglets. EFSA J. 2004, 91, 1–18. [Google Scholar]
- Bonneau, M.; Weiler, U. Pros and cons of alternatives to piglet castration: Welfare, boar taint, and other meat quality traits. Animals 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Tuyttens, F.A.M.; De Groot, J.; Van Reenen, K.; De Bourdeaud’huy, A.; Struelens, E. Differences in aggressive and sexual behaviour in entire male pigs versus barrows. In Proceedings of the EAAP Working Group on Production and Utilisation of Meat from Entire Male Pigs, Monells, Spain, 26–27 March 2008; pp. 34–35. [Google Scholar]
- Boyle, L.A.; Björklund, L. Effects of fattening boars in mixed or single sex groups and split marketing on pig welfare. Anim. Welf. 2007, 16, 259–262. [Google Scholar]
- Rydhmer, L.; Zamaratskaia, G.; Andersson, H.K.; Algers, B.; Guillemet, R.; Lundström, K. Aggressive and sexual behaviour of growing and finishing pigs reared in groups, without castration. Acta Agric. Scand. Sect. A 2006, 56, 109–119. [Google Scholar] [CrossRef]
- Morrow-Tesch, J.L.; McGlone, J.J.; Salak-Johnson, J.L. Heat and social stress effects on pig immune measures. J. Anim. Sci. 1994, 72, 2599–2609. [Google Scholar] [CrossRef]
- Babol, J.; Squires, E.J. Quality of meat from entire male pigs. Food Res. Internat. 1995, 28, 201–212. [Google Scholar] [CrossRef]
- Stookey, J.M.; Gonyou, H.W. The effects of regrouping on behavioral and production parameters in finishing swine. J. Anim. Sci. 1994, 72, 2804–2811. [Google Scholar] [CrossRef]
- Fredriksen, B.; Lium, B.M.; Marka, C.H.; Mosveen, B.; Nafstad, O. Entire male pigs in farrow-to-finish pens—effects on animal welfare. Appl. Anim. Behav. Sci. 2008, 110, 258–268. [Google Scholar] [CrossRef]
- Fu, L.; Li, H.; Liang, T.; Zhou, B.; Chu, Q.; Schinckel, A.P.; Yang, X.; Zhao, R.; Li, P.; Huang, R. Stocking density affects welfare indicators of growing pigs of different group sizes after regrouping. Appl. Anim. Behav. Sci. 2016, 174, 42–50. [Google Scholar] [CrossRef]
- McGlone, J.J.; Curtis, S.E. Behavior and performance of weanling pigs in pens equipped with hide areas. J. Anim. Sci. 1985, 60, 20–24. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Squires, E.J. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 2009, 3, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.L.S. 5-alpha-androst-16-en-3-one: Compound responsible for taint in boar fat. J. Sci. Food Agric. 1968, 19, 31–38. [Google Scholar] [CrossRef]
- Gower, D.B. 16-unsaturated C19 steroids a review of their chemistry, biochemistry and possible physiological role. J. Steroid Biochem. 1972, 3, 45–103. [Google Scholar] [CrossRef]
- Fischer, J.; Wüst, M. Quantitative determination of the boar taint compounds androstenone, skatole, indole, 3α-androstenol and 3β-androstenol in wild boars (Sus scrofa) reveals extremely low levels of the tryptophan-related degradation products. Food Chem. 2012, 135, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
- Vold, E. Fleischproduktionseigenschaften bei Ebern und Kastraten. IV. Organoleptische und gaschromatographische Untersuchungen wasserdampfflüchtiger Stoffe des Rückenspecks von Ebern; Report No. 238; Institute of Animal Genetics and Breeding, NLH: Vollebekk, Norway, 1970. [Google Scholar]
- Walstra, P.; Maarse, G. Onderzoek gestachlengen van mannelijke mestvarkens I.V.O.; Rapport C-147 et Rapport No. 2; Researchgroep voor Vlees en Vleeswaren T. N. O.: Zeist, The Netherlands, 1970. [Google Scholar]
- Brooks, R.I.; Pearson, A.M. Steroid hormone pathways in the pig with special emphasis on boar odor: A review. J. Anim. Sci. 1986, 62, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Faraut, T.; Prunier, A. Pathways and genes involved in steroid hormone metabolism in male pigs: A review and update. J. Steroid Biochem. Mol. Biol. 2014, 140, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Feve, K.; Louveau, I.; Riquet, J.; Prunier, A. Exploration of steroidogenesis-related genes in testes, ovaries, adrenals, liver and adipose tissue in pigs. Anim. Sci. J. 2016, 87, 1041–1047. [Google Scholar] [CrossRef]
- Robic, A.; Feve, K.; Riquet, J.; Prunier, A. Transcript levels of genes implicated in steroidogenesis in the testes and fat tissue in relation to androstenone accumulation in fat of pubertal pigs. Domest. Anim. Endocrinol. 2016, 57, 1–9. [Google Scholar] [CrossRef]
- Babol, J.; Squires, E.J.; Bonneau, M. Factors regulating the concentrations of 16-androstene steroids in submaxillary salivary glands of pigs. J. Anim. Sci. 1996, 74, 413–419. [Google Scholar] [CrossRef]
- Yamazaki, T.; Ohno, T.; Sakaki, T.; Akiyoshi-Shibata, M.; Yabusaki, Y.; Imai, T.; Kominami, S. Kinetic analysis of successive reactions catalyzed by bovine cytochrome P45017α,lyase. Biochemistry 1998, 37, 2800–2806. [Google Scholar] [CrossRef]
- Billen, M.J.; Squires, E.J. The role of porcine cytochrome b5A and cytochrome b5B in the regulation of cytochrome P45017A1 activities. J. Steroid Biochem. Mol. Biol. 2009, 113, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J.; Gray, M.S.; Lou, Y. Effect of mutations in porcine CYB5A and CYP17A1 on the metabolism of pregnenolone. J. Steroid Biochem. Mol. Biol. 2019, 195, 105–469. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.A.; Squires, E.J. Testicular sulfoconjugation of the 16-androstene steroids by hydroxysteroid sulfotransferase: Its effect on the concentrations of 5α-androstenone in plasma and fat of the mature domestic boar. J. Anim. Sci. 2005, 83, 358–365. [Google Scholar] [CrossRef]
- Laderoute, H.; Bone, C.; Squires, E.J. The sulfoconjugation of androstenone and dehydroepiandrosterone by human and porcine sulfotransferase enzymes. Steroids 2018, 136, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, A.; Hum, D.W.; Beaulieu, M.; Lévesque, É.; Guillemette, C.; Tchernof, A.; Bélanger, G.; Turgeon, D.; Dubois, S. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J. Steroid Biochem. Mol. Biol. 1998, 65, 301–310. [Google Scholar] [CrossRef]
- Moe, M.; Grindflek, E.; Doran, O. Expression of 3beta-hydroxysteroid dehydrogenase, cytochrome P450c17 and sulfotransferase 2B1 proteins in liver and testis of pigs of two breeds: Relationship with adipose tissue androstenone concentration. J. Anim. Sci. 2007, 85, 2924–2931. [Google Scholar] [CrossRef]
- Sinclair, P.A.; Squires, E.J.; Raeside, J.I.; Renaud, R. Synthesis of free and sulphoconjugated 16-androstene steroids by the Leydig cells of the mature domestic boar. J. Steroid Biochem. Mol. Biol. 2005, 96, 217–228. [Google Scholar] [CrossRef]
- Laderoute, H.; Bone, C.; Brewer, D.; Squires, E.J. The synthesis of 16-androstene sulfoconjugates from primary porcine Leydig cell culture. Steroids 2019, 146, 14–20. [Google Scholar] [CrossRef]
- Reed, M.J.; Purohit, A.; Woo, L.W.L.; Newman, S.P.; Potter, B.V.L. Steroid sulfatase: Molecular biology, regulation and inhibition. Endocr. Rev. 2005, 26, 171–202. [Google Scholar] [CrossRef]
- Ampuero Kragten, S.; Chavarria-Drecourt, H.; Bee, G. Parallel analysis of boar taint compounds in meat and in back fat. Adv. Anim. Biosci. 2018, 9, s38. [Google Scholar] [CrossRef]
- Bonneau, M.; Le Denmat, M.; Vaudelet, J.C.; Veloso Nunes, J.R.; Mortensen, A.B.; Mortensen, H.P. Contributions of fat androstenone and skatole to boar taint: I. Sensory attributes of fat and pork meat. Livest. Prod. Sci. 1992, 32, 63–80. [Google Scholar] [CrossRef]
- Riches, Z.; Stanley, E.L.; Bloomer, J.C.; Coughtrie, M.W.H. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: The SULT “pie”. Drug Metab. Dispos. 2009, 37, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, W.L.; Dziuk, P.J. Absorption and metabolism of estrogens from the stomach and duodenum of pigs. Dom. Anim. Endocrinol. 1994, 11, 197–208. [Google Scholar] [CrossRef]
- Jen, K.; Squires, E.J. Efficacy of non-nutritive sorbent materials as intestinal-binding agents for the control of boar taint. Animal 2011, 5, 1814–1820. [Google Scholar] [CrossRef]
- Andreassen, T.K. The role of plasma-binding proteins in the cellular uptake of lipophilic vitamins and steroids. Horm. Metab. Res. 2006, 38, 279–290. [Google Scholar] [CrossRef]
- Cook, B.; Hunter, R.H.; Kelly, A.S. Steroid-binding proteins in follicular fluid and peripheral plasma from pigs, cows and sheep. J. Soc. Reprod. Fert. 1977, 51, 65–71. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Dahl, E.; Madej, A.; Squires, E.J.; Andresen, Ø. Studies on 5α-androst-16-en-3one binding to porcine serum plasma and testicular cytosolic fraction and to human serum. J. Steroid Biochem. Mol. Biol. 2008, 111, 24–28. [Google Scholar] [CrossRef]
- Bone, C.; Anderson, C.; Lou, Y.; Squires, E.J. The characterization of androstenone transport in boar plasma. J. Steroid Biochem. Mol. Biol. 2019, 185, 218–224. [Google Scholar] [CrossRef]
- Bone, C.; Cameron, J.; Squires, E.J. Inter-animal variability in androstenone transport and the potential effect on the development of boar taint. In Proceedings of the American Society of Animal Science, Madison, WI, USA, 19–23 July 2020. [Google Scholar]
- Hagenbuch, B.; Meier, P.J. The superfamily of organic anion transporting polypeptides. Biochem. Biophys. Acta 2003, 1609, 1–18. [Google Scholar] [CrossRef]
- Dalla Valle, L.; Toffolo, V.; Nardi, A.; Fiore, C.; Bernante, P.; Di Liddo, R.; Parnigotto, P.P.; Colombo, L. Tissue-specific transcriptional initiation and activity of steroid sulfatase complementing dehydroepiandrosterone sulfate uptake and intracrine steroid activations in human adipose tissue. J. Endocrinol. 2006, 190, 129–139. [Google Scholar] [CrossRef]
- Ugele, B.; St-Pierre, M.V.; Pihusch, M.; Bahn, A.; Hantschmann, P. Characterization and identification of steroid sulphate transporters of human placenta. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E390–E398. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, R.; Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Claus, R.; Dehnhard, M.; Herzog, A.; Bernal-Barragan, H.; Gimenez, T. Parallel measurements of indole and skatole (3-methylindole) in feces and blood plasma of pigs by HPLC. Livest. Prod. Sci. 1993, 34, 115–126. [Google Scholar] [CrossRef]
- Knarreborg, A.; Beck, J.; Jensen, M.T.; Laue, A. Effect of non-starch polysaccharides on production and absorption of indolic compounds in entire male pigs. Anim. Sci. 2002, 74, 445–453. [Google Scholar] [CrossRef]
- Lanthier, F.; Lou, Y.; Squires, E.J. Skatole metabolism in the intact prepubescent male pig: The relationship between hepatic enzyme activity and skatole concentrations in plasma and fat. Livest. Sci. 2007, 106, 145–153. [Google Scholar] [CrossRef]
- Li, X.; Jensen, B.B.; Canibe, N. The mode of action of chicory roots on skatole production in entire male pigs is neither via reducing the population of skatole-producing bacteria nor increased butyrate production in the hindgut. Appl. Environ. Microbiol. 2019, 85, e02327-18. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.Y.; Amenyogbe, N.; Brwon, E.M.; Findlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Zamaratskaia, G. Regulation of porcine hepatic cytochrome P450 – Implication for boar taint. Comp. Struct. Biotech. 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Hansen, L.L.; Larsen, A.E.; Jen, B.B.; Hansen-Møller, J.; Barton-Gade, P. Influence of stocking rate and faeces deposition in the pen at different temperatures on skatole concentration (boar taint) in subcutaneous fat. Anim. Sci. 1994, 59, 99–110. [Google Scholar] [CrossRef]
- Jensen, B.B.; Jensen, M.T. Microbial production of skatole in the digestive tract of entire male pigs. In Skatole and Boar Taint; Jensen, W.K., Ed.; Danish Meat Research Institute: Roskilde, Denmark, 1998; pp. 41–76. [Google Scholar]
- Han, X.; Zhou, M.; Cao, X.; Du, X.; Meng, F.; Bu, G.; Kong, F.; Huang, A.; Zeng, X. Mechanistic insight into the role of immunocastration on eliminating skatole in boars. Theriogenology 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Metz, C.; Claus, R. Active immunization of boars against GnRH does not affect growth hormone, but lowers IGF-1 in plasma. Livest. Prod. Sci. 2003, 81, 129–137. [Google Scholar] [CrossRef]
- Diaz, G.J.; Skordos, K.W.; Yost, G.S.; Squires, E.J. Identification of phase I metabolites of 3-methylindole produced by pig liver microsomes. Drug Metab. Disp. 1999, 27, 1150–1156. [Google Scholar]
- Diaz, G.J.; Squires, E.J. Phase II in vitro metabolism of 3-methylindole metabolites in porcine liver. Xenobiotica 2003, 33, 485–498. [Google Scholar] [CrossRef]
- Baek, C.; Hansen-Møller, J.; Friiis, C.; Cornett, C.; Hansen, S.H. Identification of selected metabolites of skatole in plasma and urine from pigs. J. Agric. Food Chem. 1997, 45, 2332–2340. [Google Scholar] [CrossRef]
- Brunius, C.; Vidanarachchi, J.K.; Tomankova, J.; Lundström, K.; Andersson, K.; Zamaratskaia, G. Skatole metabolites in urine as a biological marker of pigs with enhanced hepatic metabolism. Animal 2016, 10, 1734–1740. [Google Scholar] [CrossRef]
- Wiercinska, P.; Lou, Y.; Squires, E.J. The roles of different porcine cytochrome P450 enzymes and cytochrome b5A in skatole metabolism. Animal 2012, 6, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Doran, E.; Whittington, F.W.; Wood, J.D.; McGivan, J.D. Cytochrome P450IIE1 (CYP2E1) is induced by skatole and this induction is blocked by androstenone in isolated pig hepatocytes. Chem. Biol. Interact. 2002, 140, 81–92. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Zamaratskaia, G.; Ekstrand, B. In vitro cytochrome P450 2E1 and 2A activities in the presence of testicular steroids. Reprod. Domest. Anim. 2011, 46, 149–154. [Google Scholar] [CrossRef]
- Kiros, T.G.; Pinloche, E.; D’Inca, R.; Auclair, E.; Van Kessel, A. Model development: Establishing pigs with homogenous microbial profile in the hind gut. Can. J. Anim. Sci. 2018, 98, 498–507. [Google Scholar] [CrossRef]
- Gray, M.A.; Squires, E.J. Effects of nuclear receptor transactivation on steroid hormone synthesis and gene expression in porcine Leydig cells. J. Steroid Biochem. Mol. Biol. 2013, 113, 93–100. [Google Scholar] [CrossRef]
- Gray, M.A.; Squires, E.J. Effects of nuclear receptor transactivation on boar taint metabolism and gene expression in porcine hepatocytes. J. Steroid Biochem. Mol. Biol. 2013, 113, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M. Consumer studies on sensory acceptability of boar taint: A review. Meat Sci. 2012, 92, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Haugen, J.-E.; Brunius, C.; Zamaratskaia, G. Review of analytical methods to measure boar taint compounds in porcine adipose tissue: The need for harmonised methods. Meat Sci. 2012, 90, 9–19. [Google Scholar] [CrossRef]
- Mathur, P.K.; Ten Napel, J.; Bloemhof, S.; Heres, L.; Knol, E.F.; Mulder, H.A. A human nose scoring system for boar taint and its relationship with androstenone and skatole. Meat Sci. 2012, 91, 414–422. [Google Scholar] [CrossRef]
- EU Health and Food Safety Directorate. Establishing Best Practices on the Production, the Processing and the Marketing of Meat from Uncastrated Pigs or Pigs Vaccinated against Boar Taint (Immunocastrated). Final Report 14 March 2019. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/aw_prac_farm_pigs_cast-alt_establishing-best-practices.pdf (accessed on 15 September 2020).
- Sachar, M.; Ma, X. Nuclear receptors in herb-drug interactions. Drug Metab. Rev. 2013, 45, 73–78. [Google Scholar] [CrossRef]
- Prunier, A.; Brillouet, A.; Merlot, E.; Meurier-Salaun, M.C.; Tallet, C. Influence of housing and season on pubertal development, boar taint compounds and skin lesions of male pigs. Animal 2013, 7, 2035–2043. [Google Scholar] [CrossRef]
- Giersing, M.; Lundström, K.; Andersson, A. Social effects and boar taint: Significance for production of slaughter boars (Sus scrofa). J. Anim. Sci. 2000, 78, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, R.; Jungbluth, I.; Stefanski, V.; Weiler, U. Pre-slaughter conditions influence skatole and androstenone in adipose tissue of boars. Meat Sci. 2015, 99, 60–67. [Google Scholar] [CrossRef]
- Davidson, J.M.; Levine, S. Endocrine Regulation of Behavior. Ann. Rev. Physiol. 1972, 34, 375–408. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Rydhmer, L.; Chen, G.; Madej, A.; Andersson, H.K.; Lundström, K. Boar taint is related to endocrine and anatomical changes at puberty but not to aggressive behaviour in entire male pigs. Reprod. Domest. Anim. 2005, 40, 500–506. [Google Scholar] [CrossRef]
- Brown, S.N.; Knowles, T.G.; Edwards, J.E.; Warriss, P.D. Relationship between food deprivation before transport and aggression in pigs held in lairage before slaughter. Vet. Rec. 1999, 145, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.M.; Velarde, A.; Dalmau, A.; Saucier, L.; Faucitano, L. Can the monitoring of animal welfare parameters predict pork meat quality variation through the supply chain (from farm to slaughter)? J. Anim. Sci. 2016, 94, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Goumon, S.; Faucitano, L. Influence of loading handling and facilities on the subsequent response to pre-slaughter stress in pigs. Livest. Sci. 2017, 200, 6–13. [Google Scholar] [CrossRef]
- Bonneau, M.; Meusy-Dessolle, N.; Léglise, P.C.; Claus, R. Relationships between fat and plasma androstenone and plasma testosterone in fatty and lean young boars during growth and after hCG stimulation. Acta Endocrinol. 1982, 101, 119–128. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Babol, J.; Andersson, H.; Lundström, K. Plasma skatole and androstenone levels in entire male pigs and relationship between boar taint compounds, sex steroids and thyroxine at various ages. Livest. Prod. Sci. 2004, 87, 91–98. [Google Scholar] [CrossRef]
- Schwarzenberger, F.; Toole, G.S.; Christie, H.L.; Raeside, J.I. Plasma levels of several androgens and estrogens from birth to puberty in male domestic pigs. Acta Endocrinol. 1993, 128, 173–177. [Google Scholar] [CrossRef]
- Ford, J.J. Differentiation of sexual behaviour in pigs. J. Reprod. Fertil. Suppl. 1990, 40, 311–321. [Google Scholar]
- Sinclair, P.A.; Squires, E.J.; Raeside, J.I.; Britt, J.H.; Hegdpeth, V.G. The effect of early postnatal treatment with a gonadotropin-releasing hormone agonist on the developmental profiles of testicular hormones in the intact male pig. J. Anim. Sci. 2001, 79, 1003–1010. [Google Scholar] [CrossRef]
- Cameron, J.; Bergeron, R.; Squires, E.J. Early plasma androstenone concentrations may indicate extent of boar taint at slaughter. In Proceedings of the American Society of Animal Science, Madison, WI, USA, 19–23 July 2020. [Google Scholar]
- Batorek, N.; Candek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef]
- Aluwe, M.; Degezelle, I.; Depuydt, L.; Fremaut, D.; Van den Broek, A.; Millet, S. Immunocastrated male pigs: Effect of 4 v.6 weeks time post second injection on performance, carcass quality and meat quality. Animal 2016, 10, 1466–1473. [Google Scholar] [CrossRef]
- Cronin, G.M.; Dunshea, F.R.; Butler, K.L.; McCauley, I.; Barnett, J.L.; Hemsworth, P.H. The effects of immuno- and surgical-castration on the behaviour and consequently growth of group-housed, male finisher pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- Rydhmer, L.; Lundström, K.; Andersson, K. Immunocastration reduces aggressive and sexual behaviour in male pigs. Animal 2010, 4, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Brewster, V.; Nevel, A. Immunocastration with ImprovacTM reduces aggressive and sexual behaviours in male pigs. Appl. Anim. Behav. Sci. 2013, 145, 32–36. [Google Scholar] [CrossRef]
- Dalla Costa, O.A.; Tavernari, F.D.C.; Lopes, L.D.S.; Dalla Costa, F.A.; Feddern, V.; de Lima, G.J.M.M. Performance, carcass and meat quality of pigs submitted to immunocastration and different feeding programs. Res. Vet. Sci. 2020, 131, 137–145. [Google Scholar] [CrossRef]
- Baes, C.; Mattei, S.; Luther, H.; Ampuero, S.; Sidler, X.; Bee, G.; Spring, P.; Hofer, A. A performance test for boar taint compounds in live boars. Animal 2013, 7, 714–720. [Google Scholar] [CrossRef]
- Große-Brinkhaus, C.; Storck, L.C.; Frieden, L.; Neuhoff, C.; Schellander, K.; Looft, C.; Tholen, E. Genome-wide association analyses for boar taint components and testicular traits revealed regions having pleiotropic effects. BMC Genetics 2015, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Brinke, I.; Große-Brinkhaus, C.; Roth, K.; Proll-Cornelissen, M.J.; Henne, H.; Schellander, K.; Tholen, E. Genomic background and genetic relationships between boar taint and fertility traits in German Landrace and Large White. BMC Genet. 2020, 21, 61. [Google Scholar] [CrossRef]
- Zadinova, K.; Stupka, R.; Stratil, A.; Citek, J.; Vehovsky, K.; Urbanova, D. Boar taint - the effects of selected candidate genes associated with androstenone and skatole levels—A review. Anim. Sci. Pap. Rep. 2016, 34, 107–127. [Google Scholar]
- van Son, M.; Agarwal, R.; Kent, M.P.; Grove, H.; Grindflek, E.; Lien, S. Exploiting whole genome sequence data to fine map and characterize candidate genes within a quantitative trait loci region affecting androstenone on porcine chromosome 5. Anim. Genet. 2017, 48, 653–659. [Google Scholar] [CrossRef]
- Drag, M.H.; Kogelman, L.J.A.; Maribo, H.; Meinert, L.; Thomsen, P.D.; Kadarmideen, N. Characterization of eQTls associated with androstenone by RNA sequencing in porcine testis. Physiol. Genom. 2019, 51, 488–499. [Google Scholar] [CrossRef]
- Stephenson, M.; Darlington, G.S.; Schenkel, F.S.; Squires, E.J.; Ali, R.A. DSRIG: Incorporating graphical structure in the regularized modelling of SNP data. J. Bioinform. Comput. Biol. 2019, 17, 1950017. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J.; Schenkel, F.S. Managing Boar Taint: Focus on Genetic Markers. In Proceeding of the London Swine Conference, London, ON, Canada, 31 March–1 April 2010. [Google Scholar]
- Squires, J.; Jafarikia, M.; Schenkel, F.; Wyss, S.; Fortin, F.; Van Berkel, W.; de Wolde, R.; Sullivan, B. Potential applications of genomics to reduce boar taint levels in three Canadian swine breeds. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- Kurtz, S.; Petersen, B. Pre-determination of sex in pigs by application of CRISPR/Cas system for genome editing. Theriogenology 2019, 137, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lievens, A.; Petrillo, M.; Querci, M.; Patak, A. Genetically modified animals: Options and issues for traceability and enforcement. Trends Food Sci. Technol. 2015, 44, 159–176. [Google Scholar] [CrossRef]
- Petersen, B. Basics of genome editing technology and its application in livestock species. Reprod. Dom. Anim. 2017, 52 (Suppl. 3), 4–13. [Google Scholar] [CrossRef]
- Sato, M.; Miyoshi, K.; Kawaguchi, H.; Inada, E.; Saitoh, I.; Tanimoto, A. Recent Advance in Genome Editing-Based Gene Modification in Pigs. In Theriogenology; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gonen, S.; Jenko, J.; Gorjanc, G.; Mileham, A.J.; Whitelaw, C.B.A.; Hickey, J.M. Potential of gene drives with genome editing to increase genetic gain in livestock breeding programs. Genet. Sel. Evol. 2017, 49, 3. [Google Scholar] [CrossRef] [PubMed]
- Sonstegard, T.; Fahrenkrug, S.; Carlson, D. Precision animal breeding to make genetically castrated animals for improved animal welfare and alternative breeding applications. J. Anim. Sci. 2017, 95 (Suppl. 2), 149–150. [Google Scholar] [CrossRef]
- Yunes, M.C.; Teixeira, D.L.; von Keyserlingk, M.A.G.; Hotzel, M.J. Is gene editing an acceptable alternative to castration in pigs? PLoS ONE 2019, 14, e0218176. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squires, E.J.; Bone, C.; Cameron, J. Pork Production with Entire Males: Directions for Control of Boar Taint. Animals 2020, 10, 1665. https://doi.org/10.3390/ani10091665
Squires EJ, Bone C, Cameron J. Pork Production with Entire Males: Directions for Control of Boar Taint. Animals. 2020; 10(9):1665. https://doi.org/10.3390/ani10091665
Chicago/Turabian StyleSquires, E. James, Christine Bone, and Jocelyn Cameron. 2020. "Pork Production with Entire Males: Directions for Control of Boar Taint" Animals 10, no. 9: 1665. https://doi.org/10.3390/ani10091665
APA StyleSquires, E. J., Bone, C., & Cameron, J. (2020). Pork Production with Entire Males: Directions for Control of Boar Taint. Animals, 10(9), 1665. https://doi.org/10.3390/ani10091665




