2.2. Stock and Husbandry
The study included three purebred and three crossbred genotypes of domestic chicken (
Gallus gallus domesticus). The purebred genotypes were Vorwerkhuhn (VH), Bresse Gauloise (BG) and White Rock (WR). VH is a local chicken breed from Germany, which was originally bred for dual-purpose usage. The BG hens originate from the Bresse region in the south of France Burgundy County, where they are marketed as a delicacy with protected designation of origin (PDO) [
23]. They achieve an annual laying performance of around 250 eggs [
24]. WR is a commercial layer line from Lohmann Tierzucht GmbH (Cuxhaven, Germany), which originates, amongst others, from Plymouth Rock chicken. As high performing line, WR is a founder population of some modern laying hybrids with brown eggshell color. To build up grandparent stocks, birds of the VH and BG breed were provided by fancy breeders of a conservation flock for poultry species. Based on these animals, parent stocks of VH and BG purebreds were generated, which were then used to generate the test animals. The WR hens were provided as day-old chicks by Lohmann Tierzucht GmbH (Cuxhaven, Germany).
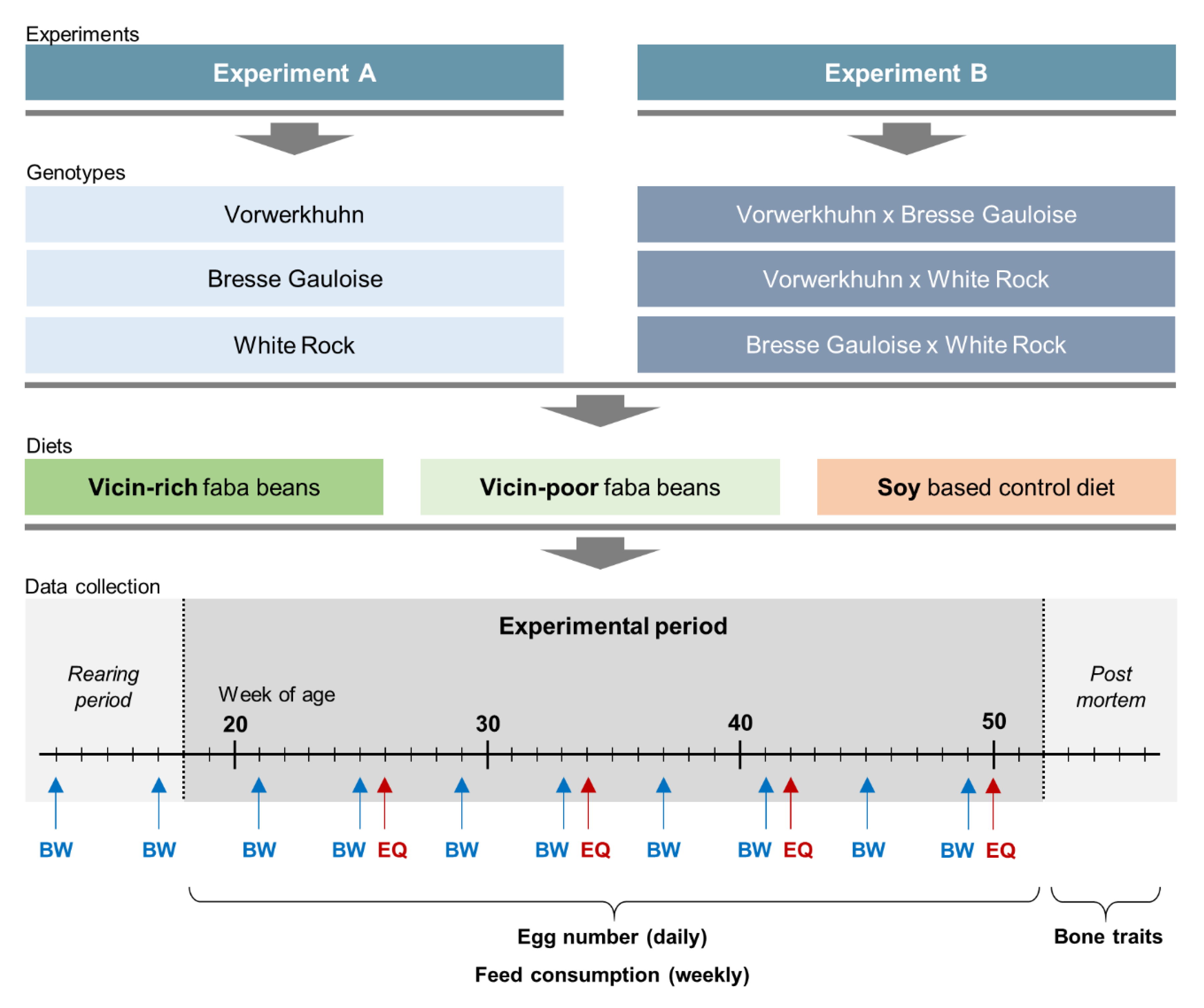
Two consecutive experiments were conducted. In experiment A, purebred hens of the VH, BG and WR breeds were tested. Experiment B dealt with crossbreds thereof, which were generated by crossing cocks of VH and BG either with hens of BG or WR, resulting in three crossbreds: VH × BG (VBG), VH × WR (VWR) and BG × WR (BWR).
Hatching and rearing procedures were identical in both experiments. After being wing-tagged for identification, blood samples were taken from VH and BG chicks within the first week of life for sex determination via DNA analysis [
25]. Female WR chicks were provided by the breeding company. In the case of experiment B, blood sampling and molecular sexing was only done for BWR chicks, because VWR and VBG chicks could be sexed visually based on different plumage color. Commercially available complete feeding stuffs for chicks (until 6 weeks of age; 11.4 MJ AMEn/kg DM, 180.0 g/kg crude protein, 26.1 g/kg crude fat, 37.5 g/kg crude fiber, 56.0 g/kg crude ash, 7.8 g/kg calcium, 4.7 g/kg phosphorous) and pullets (from 7 to 17 weeks of age; 11.0 MJ AMEn/kg DM, 145.0 g/kg crude protein, 37.0 g/kg crude fat, 65.05 g/kg crude fiber, 59.0 g/kg crude ash, 10.0 g/kg calcium, 6.0 g/kg phosphorous), as well as water were offered ad libitum. A standard lighting program was applied to the birds, where day length increased gradually from 8 h (8th weeks of age) to 14 h (23rd week of age). After hatch at the Institute of Animal Welfare and Animal Husbandry of the Friedrich-Loeffler-Institut (Celle, Germany), the chicks were raised in a floor housing system. At 7 weeks of age, all birds of the respective experiment were transferred to floor pens at the Institute of Farm Animal Genetics of the Friedrich-Loeffler-Institut (Mariensee, Germany), which were later used as experimental sites. The pens of 12.5 m
2 were equipped with wood chips, feeding and drinking troughs, a wooden perch, a dust bath, and nine laying nests.
2.3. Experimental Procedure
The experimental setup is shown in
Figure 1. In both experiments, the testing period lasted from 18th until 52nd week of age. The hens were subjected to three different feeding treatments. While two diets contained faba beans as an alternative source of protein, the third diet was a soybean-based standard feed as control (Soy). In order to examine the effect of anti-nutritive substances on performance and bone characteristics, the experimental diets contained either 20% of the VC-rich faba bean variety Fuego (VC+) or 20% of the VC-poor variety Tiffany (VC−). To meet the nutritional requirements of the hens without soybean meal, 21% blue sweet lupines (
Lupinus angustifolius cv. Boruta) were added to all diets. The protein plants were produced GMO-free in Germany. The composition of the diets is specified in
Table 1. The changeover to the layer diets has been progressively implemented during the 17th week of life. From the beginning of the 18th week, all hens were fed exclusively with the respective layer diet. A total number of 756 hens entered both experiments. In experiment A, 120 purebred hens per genotype, i.e., a total of 360 hens, were allocated to 18 floor pens (2 × 9) of 20 hens each, whereas in experiment B there were 132 crossbred hens per genotype, resulting in 22 hens per pen and 396 in total. Given the three genotypes per experiment and the three different diets, nine groups of genotype × diet combinations were formed, resulting in 40 purebred or 44 crossbred hens for each experimental group (genotype × diet combination). The housing conditions were the same as described above for the rearing period.
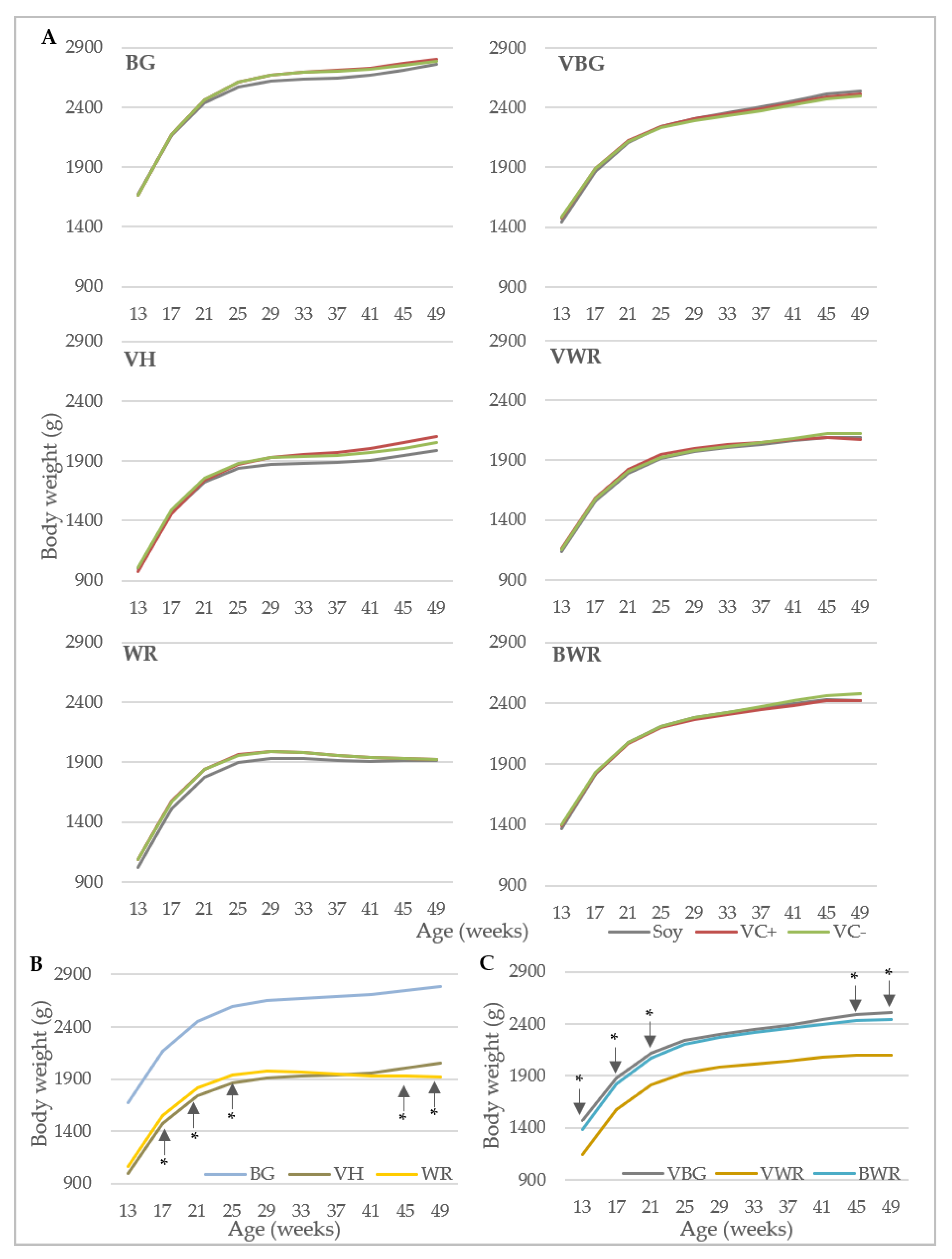
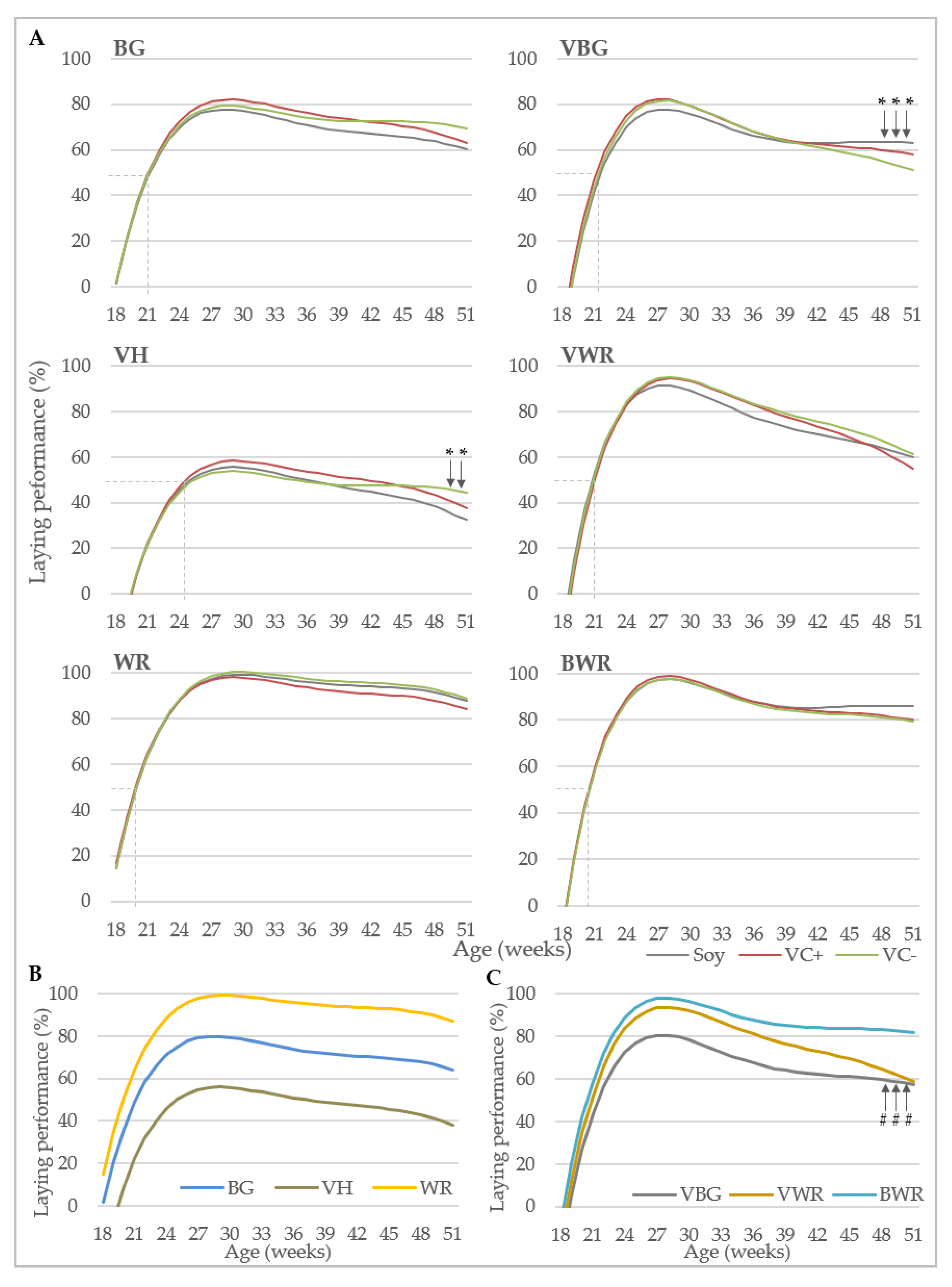
Data collection included performance traits and bone characteristics. Egg production was recorded daily at pen-level, with each observation representing the egg production of 40 (purebreds) or 44 hens (crossbreds). Laying performance was calculated by dividing the total number of eggs by the number of hens present at that day. Feed consumption (g) was recorded weekly at pen-level by weighing the remaining feedstuff. Starting at the 13th week of life, individual body weights (g) of the hens were recorded every four weeks using a digital table scale with a weighing accuracy of 0.1 g (CPA 16001S, Sartorius, Göttingen, Germany). Egg quality was analyzed at four points in time (week 26, 34, 42 and 50). At each point in time, 96 randomly selected eggs from four consecutive days were analyzed per genotype x diet combination. Egg weight (g) was recorded using a digital table scale (CPA 16001S, Sartorius, Goettingen, Germany). Eggshell breaking strength (N) was determined using a texture analyzer (TA.XTplus, Stable Micro Systems, Hamilton, MA, USA) equipped with a 50 N (Newton) load cell showing the maximum load in N that was required to break the eggshell. Eggshell weight (g) was determined after emptying the egg with a spoon and drying the shell for 30 s in a microwave (800 watt). After removing the shell membranes, equatorial eggshell thickness (mm) was measured using a caliper with an accuracy of 0.01 mm.
All hens were sacrificed by carbon dioxide inhalation during the 52nd week of age. The tibiotarsi of both sides and the left humerus were dissected and relieved from muscles and tendons. Bone weight (g), length (mm) and thickness (mm) were recorded and the bones were vacuum-packed and stored frozen (−20 °C) until further examination. Bone mineral density was examined by dual energy X-ray absorptiometry (GE Lunar
iDXA scanner, GE Healthcare, Solingen, Germany) as described by Jansen et al. [
9]. Bone breaking strength (N) of left tibiotarsus and humerus were assessed at the mid-diaphyseal region via three-point bending test (Instron Materials Testing System, Instron Corporation, Canton, MA, USA) using a 5 kN load cell. The span length was 40 mm (humerus) or 80 mm (tibiotarsus). The right tibiotarsus was used to measure diaphyseal cortical bone proportion (%) planimetrically [
26].
2.4. Statistical Analyses
The data were analyzed separately for experiments A and B using the statistical program SAS (SAS 9.3, SAS Institute Inc., Cary, NC, USA). The separate analysis was chosen, because the two experiments were performed in consecutive years, and an overlap between year- and genotype-effect was not safe to exclude. Therefore, it was also not possible to distinguish between heterosis and year effect.
For the analysis of hen body weight and laying performance, a polynomial growth function was applied according to the model
where
is the body weight respectively of laying performance,
is the overall mean,
is the fixed effect of the genotype (
= 1 to 3),
is the fixed effect of the diet (
= 1 to 3),
is the interaction of genotype x diet,
are the fixed regression coefficients up to the fourth polynomial degree of age (
) for body weight and up to the fifth polynomial degree of age
for laying performance,
are the fixed regression coefficients of the interaction between genotype and age,
are the fixed regression coefficients of the interaction between diet and age,
are the fixed regression coefficients of the interaction between genotype, diet and age,
is the random effect of the pen and
is the random error. For the polynomial analysis the MIXED procedure of SAS was used. Akaike’s information criterion (AIC) was used to determine the model with the best fit according to Koehn et al. [
27]. Sample sizes for body weight are listed in the
Supplementary Material (Table S1).
For the analysis of daily feed consumption, the experiment was split up into 3 periods (Period 1: week 18–30; Period 2: week 31–39; Period 3: week 40–51), because the variability of the daily feed use between the single weeks was rather high. The statistical model was similar to that described below for the egg quality traits (Equation (2). Results are presented in
Supplementary Material S1 (Figure S1).
Data for egg weight, eggshell breaking strength and eggshell thickness were analyzed with a linear mixed model as following:
where
is the respective parameter,
is the overall mean,
is the fixed effect of the genotype (
= 1 to 3),
is the fixed effect of the diet (
= 1 to 3),
is the fixed effect of the age in weeks,
,
,
and
are the interactions of the respective variables,
is the random effect of the pen and
is the random error.
As shell weight and egg weight are highly correlated [
28], shell weight was calculated with and without including egg weight as a covariate in the analysis. The applied model was the same as described above for the other egg traits (Equation (2). To verify the correlation of egg weight and shell weight in the experimental data, Pearson’s correlation coefficients (
) between egg and shell weight were calculated. They were
0.70 in experiment A and
0.74 in experiment B. The calculation of the least squares means (LS-means) and testing of significant differences was carried out as described in Nolte et al. [
29]. The calculation of daily feed intake and egg parameters was performed with the GLIMMIX procedure of SAS.
In the first experiment, an infestation with the northern fowl mite (
Ornithonyssus sylviarum) took place in the barn and during this period a massive discrepancy in the data compared to the time before and after the infestation was realized. For that reason, the affected data from week 31–39 were excluded from the final analysis. In the case of body weight and laying performance a calculation with stepwise exclusion of data were applied to model the growth and laying curves. The full data, analyzed with a linear mixed model together with the final curves is presented in
Supplementary Material Figures S2 (Body weight) and S3 (Laying performance).
The bone characteristics were analyzed using the GLIMMIX procedure of SAS according to the following model:
where
is the respective bone characteristic,
is the overall mean,
is the genotype (
= 1 to 3),
is the diet (
= 1 to 3),
is their interaction,
is the random effect of the sire and
is the random error. Since bone weight attributed a relatively large effect on bone strength [
12], this factor was considered as a covariate for the analysis of bone breaking strength. Sample sizes for bone characteristics are listed in the
Supplementary Material (Table S1).